Abstract
Objectives: This study aimed to evaluate the effect of a superior ankle traction manipulation on the strength and electrical activity (surface EMG) of the peroneus longus, gluteus medius, and tensor fasciae latae muscles in healthy young adults. Methods: In total, 30 healthy participants (26 men and 4 women) were enrolled in a prospective, randomized, double-blind, controlled study. Participants were randomly assigned to a Manipulation or Sham group. Muscle activity was recorded using surface EMG, and isometric strength was assessed with a Biodex dynamometer. EMG signals were normalized to session-specific maximal voluntary isometric contractions (MVIC) and expressed as %MVIC for amplitude and median frequency. Baseline differences were examined with Welch’s t-tests. The primary analysis used analysis of covariance (ANCOVA) on POST values adjusted for PRE, with partial eta squared (η2p) as an effect size. Change-score comparisons (Δ = POST − PRE) and Hedges-corrected Cohen’s d were reported as sensitivity analyses. False discovery rate (FDR) correction was applied across outcomes. Results: No significant between-group differences were observed after adjustment for baseline in any %MVIC amplitude or median frequency outcome (p > 0.05, all FDR-adjusted q > 0.05). Within-group analyses showed small, nonsignificant changes in both groups, with the Manipulation group tending toward slightly greater increases in peroneus longus %MVIC amplitude (Δ = +3.1%, p = 0.033, d = 0.79, not significant after FDR correction). Descriptive data indicated similar PRE and POST values across groups for all muscles. Conclusions: When EMG activity is expressed relative to MVIC and baseline differences are controlled, a single superior ankle traction manipulation does not produce statistically significant acute changes in peroneus longus, gluteus medius, or tensor fasciae latae activity compared with a sham procedure. These findings suggest that previously reported differences may have reflected unadjusted baseline variability rather than true intervention effects.
1. Introduction
Correct biomechanics of the ankle joint is crucial for normal everyday activities and sports activities. And the resulting limitation may affect climbing and descending stairs, crouching, jumping or running [1]. The resulting biomechanical disorders may change the input signals to the central nervous system and, consequently, affect neuromuscular control not only of the ankle joint, but also of other knee and hip joints [2]. The correct positioning of the talus in the ankle joint creates a mechanism that connects the upper subtalar joint with external rotation in the hip joint [3]. This may have a significant impact on the correct posture [4], leading to balance disorders or impaired coordination between the ankle and hip joints [5]. Limitations in proper neuromuscular control in the ankle joint may be caused by reduced activity of the peroneal muscles [6] and in the hip joint of the abductors, in particular, the gluteus medius muscle [7,8].
Mobilization and manipulation involve applying a high velocity, low amplitude (HVLA) force to the joint and surrounding structures, which can reduce pain [9] and modulate neuromechanical responses believed to underlie various physiological and clinical effects [10]. Ankle manipulation, in particular, has shown potential in enhancing hip abductor strength in individuals with ankle sprains, although it may simultaneously result in weakening of the tensor fascia lata (TFL) muscle, as reported by [11]. Additionally, ankle manipulation has demonstrated benefits in improving dorsiflexion range of motion and treating conditions such as ankle equinus [1,2]. However, the inconsistency in findings across studies highlights the need for more standardized research protocols to better evaluate its efficacy and guide clinical applications [3]. While HVLA manipulation has shown potential for pain reduction and functional improvement, the results remain variable. For instance, Fryer et al. [4] found no significant increase in dorsiflexion range of motion in asymptomatic individuals following a single HVLA thrust to the talocrural joint. In contrast, Weerasekara et al. [5] reported immediate improvements in dynamic balance and short-term dorsiflexion range in individuals with chronic ankle sprains. Notably, a manual manipulation approach for treating ankle equinus produced substantial immediate increases in dorsiflexion, outperforming a six-month daily stretching program [1].
Synergetic muscles cooperate with each other and influence each other, which is why their joint assessment in electromyographic tests is so important [12]. This repetitive function requires precisely coordinated activity of both muscles, resulting in a heterogeneous surface EMG (sEMG) signal [13]. Electromyography is often used to assess muscle activity occurring during movement or exercise to determine the potential for muscle-strengthening effects [14]. On ankle joint, studies have shown that ankle positioning can significantly influence quadriceps activation and torque output during isometric tasks, with dorsiflexion being particularly effective for enhancing strength [6]. Additionally, muscle activity has been observed to increase proportionally with walking speed and manual resistance, highlighting the importance of neuromuscular demand in functional tasks [7]. Analyzing the interaction between synergistic muscles allows for the detection of compensatory patterns and imbalances, guiding rehabilitation and performance strategies. This is especially relevant when assessing the impact of therapeutic interventions, such as joint manipulation, which may induce both local and systemic neuromuscular adaptations [8].
Although multiple-session manipulation protocols are often used clinically, the acute single-dose approach adopted in this study was deliberately chosen to investigate the immediate neuromechanical and neurophysiological responses to a standardized high-velocity, low-amplitude (HVLA) ankle traction. Examining a single manipulation allows for isolation of the direct, short-term neural and muscular effects without the confounding influence of cumulative treatment exposure or adaptation. Previous research has demonstrated that even a single HVLA manipulation can transiently increase muscle strength and corticospinal or corticomotor drive in lower-limb muscles of healthy or athlete populations [9,10].
The potential clinical implications of these findings include enhanced stability of the ankle and hip joints during locomotion, which may help prevent the development of osteoarthritis. Additionally, they may support improved hip joint function in postoperative rehabilitation and promote a better understanding of the biomechanical relationship between the ankle and hip joints and the activation of associated muscles. The primary objective of this study was to examine the immediate effects of a single ankle manipulation on the strength of the peroneal and hip abductor muscles in healthy individuals. The outcomes of this analysis may support the use of joint manipulation as a therapeutic approach in conditions involving hip abductor muscle imbalance, whether in athletic populations or in the context of degenerative musculoskeletal disorders.
2. Materials and Methods
2.1. Participants
Thirty-one healthy volunteers were recruited. The study group consisted of 26 men and 4 women. Basic anthropometric characteristics and division for the groups are shown in Table 1. Volunteers were qualified on the basis of inclusion criteria: (i) healthy person aged 19–26. The exclusion criteria included: (i) surgery of the lower limb and lumbar spine, lower limb injury within the last 6 months, (ii) pain in the ankle, knee or hip joint, (iii) hypermobility of the ankle joint, (iv) rheumatic diseases, (v) neurological diseases, (vi) neoplastic diseases, (vii) connective tissue diseases, (viii) symptoms of spinal root compression, sciatica, spinal canal stenosis, (ix) manipulation in the past, (x) manipulation in the history of treatment (Figure 1). This study was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk (Resolution No. NKBBN/866/2022-2023). The content of this study was explained in detail to the participants with an easy-to-understand description of the study protocol, and written informed consent was obtained from the volunteer before entering this study. Participants were asked to limit their physical activity to usual activities before this study.

Table 1.
Mean and standard deviation regarding anthropometric characteristics of the experimental and placebo groups.
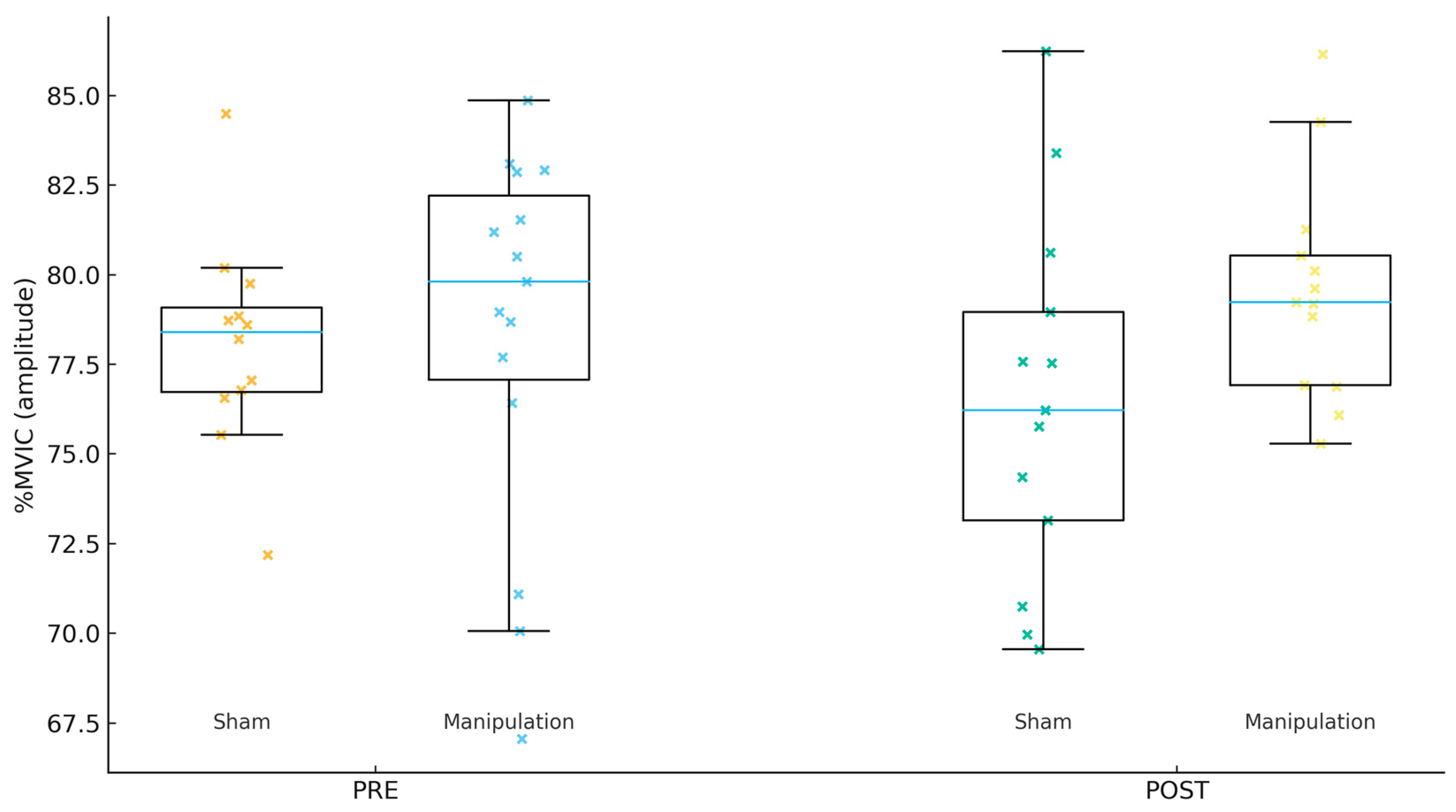
Figure 1.
Gluteus medius (Gmed) %MVIC by group and time.
2.2. Study Design
A prospective, randomized, double-blind study design was used to evaluate the effects of traction manipulation of the upper ankle joint on the peroneus longus (PL), gluteus medius (Gmed), and tensor fascia lata (TFL) muscles. This research was carried out at the Department of Biomechanics and Sports Engineering, AWFiS in Gdańsk, in the Physical Exercise Laboratory. Before this study, participants were asked to limit their physical activity to usual activities. The selected limb was selected by a random “coin toss” method. The volunteers were assessed for muscle activity using surface EMG.
2.3. Assessment of Muscle Strength
The strength of the peroneus longus (PL) and hip abductors (gluteus medius–Gmed, and tensor fasciae latae–TFL) muscles was assessed using the Biodex System 4 device (Biodex Medical Systems Inc., Shirley, NY, USA). All muscles were assessed according to the manufacturer’s recommendations. PL strength assessment was performed in a seated position on an adjustable dynamometer seat and the test leg was raised using a support arm below the knee. The foot was placed on the footrest and secured with two Velcro straps. All subjects completed the test barefoot. The subtalar joint was placed in a neutral position by setting the axis of the dynamometer to intersect the sagittal axis of the joint. Additionally, standard diagonal Velcro straps were used to keep the torso stable, and a single strap secured the hip. The arms were crossed over the chest and the opposite leg was rested on a support arm attached to the chair. PL strength was measured in volunteers using sEMG. For the abductor examination, the volunteers lay on their sides. The lower limb being examined was attached to the arm of the device using leather straps, and the rotating shaft of the device was aligned with the anatomical axis of the hip joint to allow for movement in the frontal plane. The participants’ pelvis was stabilized using leather straps.
Each time during the PL, GMed, and TFL testing, each participant performed three 5 s maximum voluntary isometric contractions (MVIC). There was a 30 s break between each repetition, and during the performance, participants were verbally encouraged to maximize their effort. The highest peak torque (Nm) of the three tests was taken for further analyses. Peak torque was also normalized to individual body weight (Nm × kg−1) [15].
2.4. Assessment of Surface EMG
Surface EMG data were recorded for the peroneus longus (Per) during maximal voluntary isometric contraction (MVIC) inversion and for the tensor fasciae latae (TFL) and gluteus medius (GMed) during hip-abduction MVIC. The middle three seconds of each five-second MVIC were analyzed to minimize transient onset and offset effects. Signals were collected and differentially amplified at a gain of 500 using a TeleMyo DTS system (Noraxon, Scottsdale, AZ, USA) and disposable Ag/AgCl surface electrodes (1 cm2; Sorimex, Toruń, Poland). The raw sEMG signals were band-pass filtered between 15 and 500 Hz and sampled at 1500 Hz (16-bit resolution) using an analogue-to-digital converter. All recordings were archived and processed in MyoResearch 2.8 software (Noraxon).
Electrode placement and skin preparation followed the SENIAM protocol [11], with precise anatomical reference points to ensure inter-subject consistency. For the Per, electrodes were positioned along the line from the fibular head to the lateral malleolus at the proximal one-third of this distance, parallel to the muscle fibers, with a reference electrode over the fibular head. For the GMed, electrodes were centered midway between the iliac crest and the greater trochanter, aligned with the fiber orientation, with a reference on the iliac crest. For the TFL, electrodes were placed one-quarter of the distance from the anterior superior iliac spine (ASIS) to the lateral femoral condyle, with a reference electrode on the ASIS. Before electrode application, the skin was shaved, lightly abraded, and cleaned with 70% isopropyl alcohol to achieve an impedance below 5 kΩ. Inter-electrode distance was kept constant at 20 mm (center-to-center).
To minimize artifacts and noise, all EMG traces were visually inspected prior to analysis. Recordings with visible baseline drift, motion artifacts, or cable disturbances were discarded. When minor 50 Hz interference was observed, a narrow notch filter was applied. The system’s common-mode rejection ratio exceeded 100 dB, ensuring high signal quality. Signals were full-wave rectified and smoothed by the root-mean-square (RMS) method using a 300 ms moving window. The mean RMS amplitude (EMGRMS, µV) and the median frequency of the raw power spectrum (EMGMED, Hz) were extracted for each muscle as outcome measures.
To ensure reproducibility, all measurements were performed by the same examiner under identical environmental conditions. Electrode positions were marked on the skin to allow for accurate replacement between PRE and POST sessions. Each participant performed three five-second MVICs separated by 30 s of rest, and the highest RMS amplitude was used for normalization. In the lower limb, published MVIC sEMG protocols using similar processing (band-pass 15–500 Hz, RMS windows, frequency-domain analysis) show good–excellent repeatability for amplitude and spectral measures (commonly ICC > 0.80, often approaching ≥0.90 for some muscles/metrics), though exact values depend on muscle and feature [12]. Normalized EMG values were expressed as a percentage of the session-specific MVIC (%MVIC) for both amplitude and median frequency to enable comparisons across participants and sessions.
2.5. Intervention and Blinding Procedures
Participants were randomly allocated to either the Manipulation or Sham (placebo) group using a computer-generated random sequence (Microsoft Excel, simple randomization). Randomization was performed by an independent researcher not involved in data collection or analysis. Allocation codes were placed in sequentially numbered, opaque, sealed envelopes that were opened only after completion of all baseline measurements. Because of the small number of female volunteers (n = 4), randomization was not stratified by sex; however, women were evenly distributed between groups (two per group).
2.5.1. Blinding
Both participants and the researcher responsible for EMG and strength measurements were blinded to group allocation. Only the treating therapist knew the assignment in order to perform the appropriate technique; this therapist had no role in outcome recording or analysis. All participants received identical explanations (“You will feel a quick pulling motion around your ankle”) and identical body positioning, contact, and session duration to preserve blinding.
2.5.2. Manipulation Group (Experimental)
All participants were placed in a supine position with the upper and lower limbs aligned along the torso and the ankle joint just beyond the edge of the plinth. Participants received the intervention using a high-speed, low-amplitude, short-duration traction manipulation. Participants were informed about how the intervention would be carried out. The volunteer was in a supine position with the upper and lower limbs placed along the torso. The therapist, positioned on the foot side, wraps both hands around the neck of the talus bone and extends the ankle joint. Manipulation of the ankle joint was performed, moving it parallel to the lower limb in the lower limb axis with the maximum range of tissue tension. Reaching the maximum tissue tension, the therapist made sure that the volunteer did not feel any discomfort by stopping at the maximum capsular tension and then performed manipulations increasing the tissue tension. The technique was performed only once for each subject. Participants who received the placebo intervention were also informed about how the intervention would be performed. The volunteer was in a supine position with the upper and lower limbs placed along the torso. The therapist, positioned on the foot side, covers the distal part of the lower leg above the ankles with both hands, thus bypassing the upper ankle joint, while extending the ankle joint. Manipulation of the ankle joint was performed, moving it parallel to the lower limb in the lower limb axis with the maximum range of tissue tension. Reaching the maximum tissue tension, the therapist made sure that the volunteer did not feel any discomfort by stopping at the maximum capsular tension and then performed manipulations increasing the tissue tension. The technique was performed only once for each subject [16].
2.5.3. Sham Group (Placebo)
For the placebo condition, all preparatory steps were identical—participant position, hand contact, ankle extension, and verbal instruction. However, to ensure the procedure was non-therapeutic, the therapist placed both hands over the distal lower leg just above the malleoli, deliberately bypassing the talocrural joint and avoiding joint play engagement. Gentle, steady traction was applied for approximately 2–3 s and then slowly released without thrust, acceleration, or cavitation. The total contact time, positioning, and verbal cues were identical to those in the manipulation group to preserve participant blinding and expectation. The sham method thus replicated the tactile and kinesthetic sensations of the intervention while withholding the critical HVLA mechanical component.
2.5.4. Timeline of Assessments
The experimental session followed a consistent timeline for all participants. After arrival, participants rested for 10 min and underwent baseline (PRE) measurements of EMG and maximal voluntary isometric contraction (MVIC). The assigned intervention (manipulation or sham) was then performed immediately afterward. Within 2 min post-intervention (POST), all EMG and MVIC tests were repeated in the same sequence as baseline to capture the immediate neuromuscular responses while minimizing fatigue or recovery effects.
2.5.5. Therapist Standardization
All procedures were delivered by licensed physiotherapists with postgraduate manual-therapy training. Before the experiment, therapists attended a two-hour calibration session to standardize both the HVLA and sham techniques, ensuring identical hand placement, verbal instructions, and timing between groups.
2.5.6. Manipulation-Check
Immediately after the post-intervention measurements, participants answered a brief question: “Do you believe you received the real joint manipulation or a simulated one?” (options: real, simulated, unsure). The proportion of correct guesses did not differ from chance (53%, χ2 = 0.27, p = 0.60), confirming that participant blinding was effective.
2.6. Sample Size
The planned sample size was established a priori on the basis of published recommendations for pilot and mechanistic studies in the absence of reliable variance estimates for EMG outcomes. Following Julious [13] and Eldridge et al. [14], a minimum of 12 participants per arm is typically sufficient to estimate the standard deviation and feasibility parameters for a future definitive trial. We therefore targeted 30 participants (15 per group) to provide stable variance estimates while maintaining logistical feasibility for the intensive EMG protocol.
To quantify the magnitude of effects detectable with reasonable power, we conducted an a priori analysis in G*Power 3 for a two-tailed independent-samples t test (α = 0.05, 1–β = 0.80). With n1 = n2 = 15, this design has 80% power to detect a standardized mean difference in d ≈ 1.06 (large effect), as obtained from G*Power’s two-sample mean comparison module [15]. When baseline values are included as a covariate in an ANCOVA, statistical efficiency improves in proportion to the squared correlation between baseline and follow-up; specifically, the residual variance is reduced by approximately 1, so that if the pre–post correlation is r ≈ 0.70, the variance reduction is ≈0.51 and the detectable standardized difference improves to about d ≈ 0.75. This efficiency result and its use in planning are standard for ANCOVA [17]. These calculations indicate the present study was adequately powered for large acute effects, but underpowered for small-to-moderate effects; accordingly, the absence of significant between-group differences supports the interpretation of no large immediate neuromuscular changes, rather than evidence of no effect. For planning a confirmatory trial targeting a moderate effect (d = 0.50) at α = 0.05 and 1–β = 0.80, approximately 64 participants per group are required using the same two-sample framework in G*Power [15].
2.7. Statistical Procedures
Data were analyzed using IBM SPSS Statistics for Windows, version 29.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics (mean and standard deviation) were computed for each variable. The normality of the data distribution was assessed using the Shapiro–Wilk test. Levene’s test was applied to verify the homogeneity of variances between groups.
Before analysis, all electromyographic (EMG) signals were normalized to session-specific maximal voluntary isometric contractions (MVIC). Each EMG outcome was expressed as a percentage of MVIC (%MVIC), computed for amplitude and median frequency measures separately. This normalization procedure allowed for comparisons between participants and across sessions while minimizing inter-individual variability in raw signal amplitude.
Baseline (PRE) differences between groups were tested using Welch’s t-tests, which do not assume equal variances. When significant or relevant baseline differences were identified, the main analyses of intervention effects were performed using an analysis of covariance (ANCOVA) model, with the POST value as the dependent variable, group (Manipulation vs. Sham) as the fixed factor, and the corresponding PRE value as a covariate. This model provides adjusted group effects accounting for baseline variability, as recommended for pre–post experimental designs.
As a sensitivity analysis, unadjusted between-group differences in change (Δ = POST − PRE) were also evaluated using Welch’s t-tests. For each test, Cohen’s d (Hedges-corrected for small samples) was computed as an effect-size estimate, and 95% confidence intervals (CIs) were reported for within- and between-group changes [17]. In the ANCOVA models, partial eta squared (η2p) was reported to quantify the magnitude of the adjusted group effect.
To control for inflation of Type I error due to multiple outcomes, p-values from the ANCOVA family were adjusted using the Benjamini–Hochberg false discovery rate (FDR) procedure. Both raw and adjusted (q) values are presented.
Descriptive statistics are reported as mean ± standard deviation (SD) for each group at PRE and POST. Distribution plots were produced to visualize data spread. All results are accompanied by exact p-values, confidence intervals, and effect sizes to allow for full interpretation of both statistical and practical significance.
3. Results
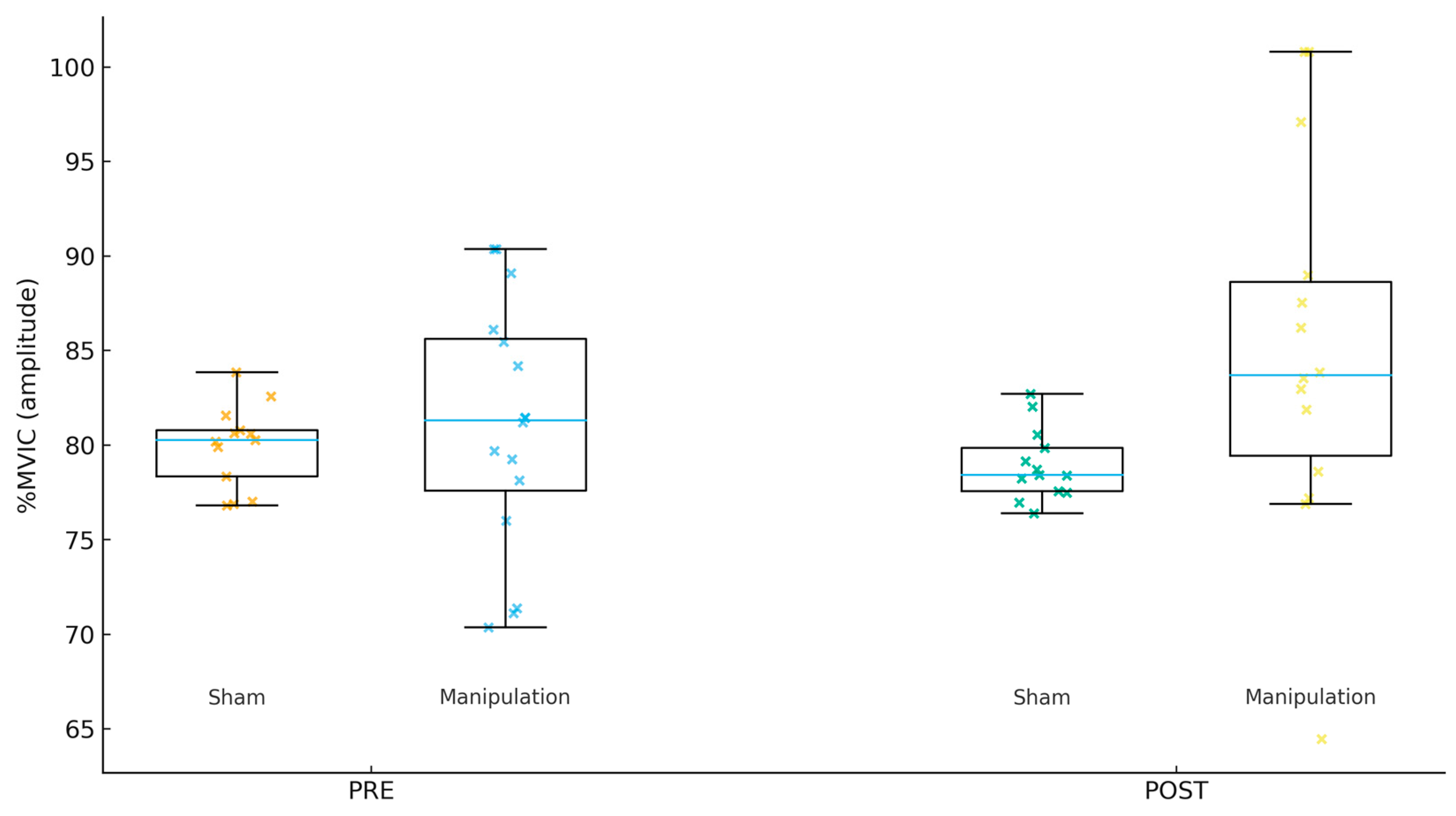
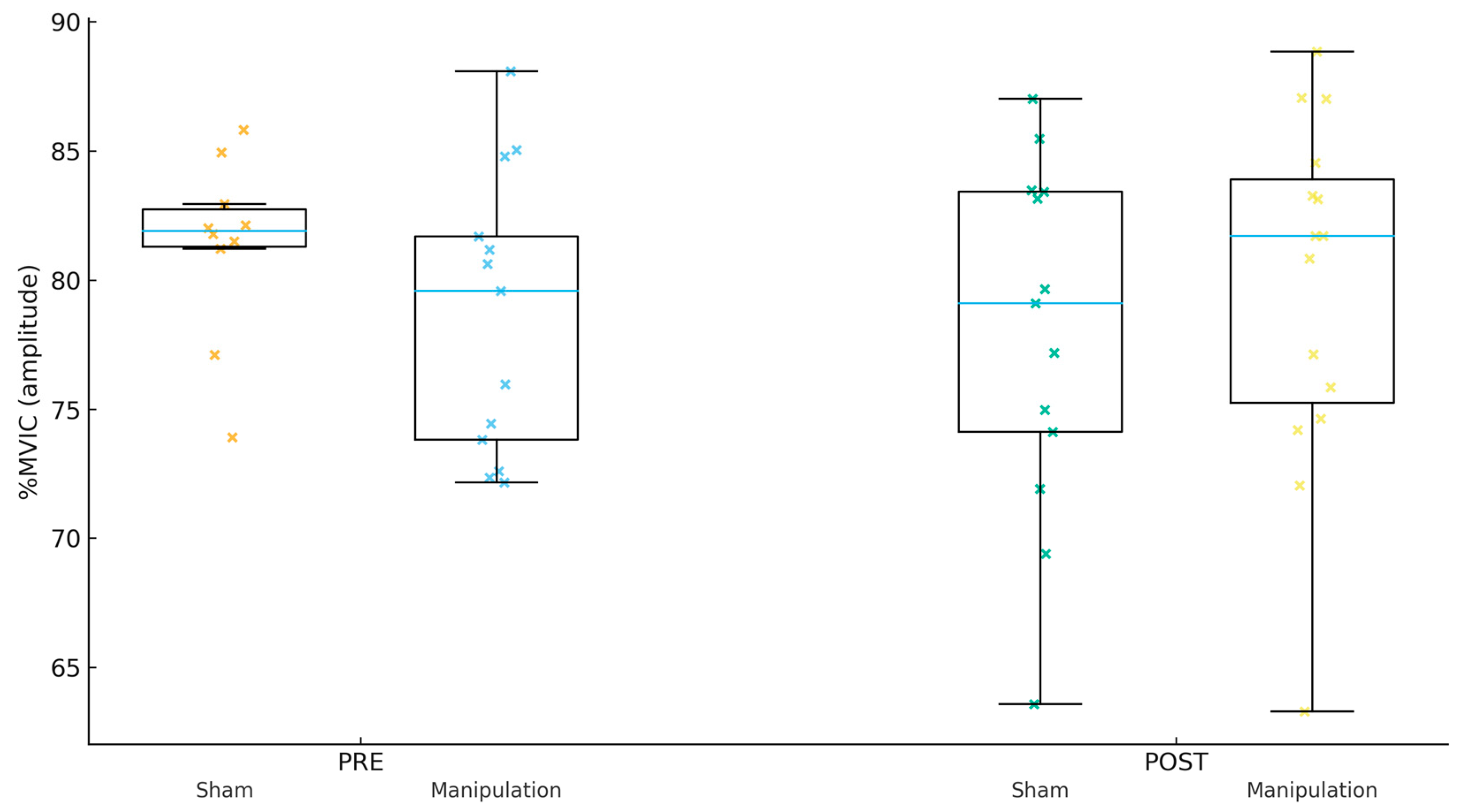
For Gmed %MVIC (amplitude, Figure 1), the PRE mean ± SD was 80.22 ± 6.67 in Sham and 77.53 ± 6.24 in Manipulation (p = 0.267). For TFL %MVIC (amplitude, Figure 2), the PRE mean ± SD was 81.38 ± 5.75 in Sham and 80.97 ± 6.53 in Manipulation (p = 0.856). For Per %MVIC (amplitude, Figure 3), the PRE mean ± SD was 81.39 ± 14.64 in Sham and 73.53 ± 12.18 in Manipulation (p = 0.125). Table 2 shows the descriptive statistics of study variables by experimental and control groups at pre- and post-intervention.
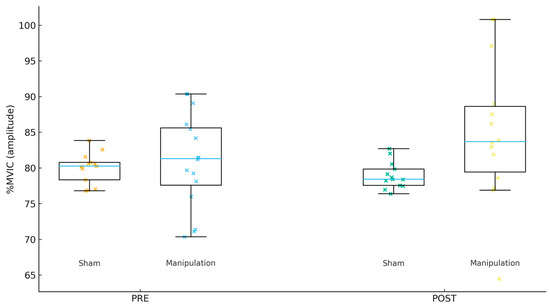
Figure 2.
Tensor fasciae latae (TFL) %MVIC by group and time.
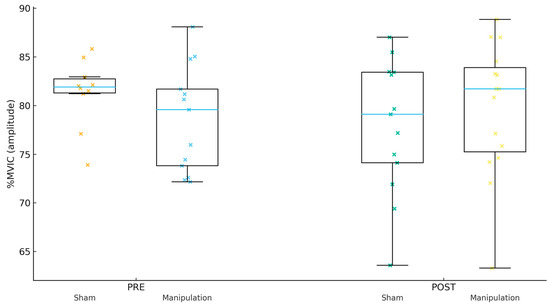
Figure 3.
Peroneus longus (Per) %MVIC by group and time.

Table 2.
Descriptive statistics (means and standard deviations) of study variables by experimental and control groups at pre- and post-intervention.
For Gmed %MVIC (median frequency), the PRE mean ± SD was 26.49 ± 19.92 in Sham and 36.18 ± 29.52 in Manipulation (p = 0.297). For TFL %MVIC (median frequency), the PRE mean ± SD was 18.54 ± 10.20 in Sham and 25.32 ± 13.71 in Manipulation (p = 0.133). For Per %MVIC (median frequency), the PRE mean ± SD was 29.12 ± 9.23 in Sham and 44.71 ± 30.22 in Manipulation (p = 0.065).
For Gmed %MVIC (amplitude), the adjusted group effect was not significant (p = 0.907; q = 0.907; partial η2 = 0.001; R2 = 0.013). For TFL %MVIC (amplitude), the adjusted group effect was not significant (p = 0.059; q = 0.176; partial η2 = 0.126; R2 = 0.128). For Per %MVIC (amplitude), the adjusted group effect was not significant (p = 0.159; q = 0.239; partial η2 = 0.072; R2 = 0.083).
For Gmed %MVIC (median frequency), the adjusted group effect was not significant (p = 0.118; q = 0.354; partial η2 = 0.088; R2 = 0.149). For TFL %MVIC (median frequency), the adjusted group effect was not significant (p = 0.659; q = 0.659; partial η2 = 0.007; R2 = 0.742). For Per %MVIC (median frequency), the adjusted group effect was not significant (p = 0.366; q = 0.549; partial η2 = 0.030; R2 = 0.042).
For Gmed %MVIC (amplitude), the within-group change was 36.34 [−50.69, 123.38] in Sham and 38.71 [−12.88, 90.29] in Manipulation. The between-group difference in change was 2.36 with a 95% CI of [−95.20, 99.93] (p = 0.960; d = 0.02). For TFL %MVIC (amplitude), the within-group change was −2.00 [−5.05, 1.06] in Sham and 13.90 [−1.95, 29.75] in Manipulation. The between-group difference in change was 15.90 with a 95% CI of [−0.15, 31.94] (p = 0.052; d = 0.70). For Per %MVIC (amplitude), the within-group change was −6.30 [−15.81, 3.21] in Sham and 8.06 [−1.90, 18.01] in Manipulation. The between-group difference in change was 14.36 with a 95% CI of [1.21, 27.51] (p = 0.033; d = 0.79).
For Gmed %MVIC (median frequency), the within-group change was 35.92 [−7.82, 79.66] in Sham and 0.94 [−6.77, 8.65] in Manipulation. The between-group difference in change was −34.98 with a 95% CI of [−79.15, 9.18] (p = 0.111; d = −0.65). For TFL %MVIC (median frequency), the within-group change was 1.56 [−1.80, 4.91] in Sham and −0.41 [−4.29, 3.48] in Manipulation. The between-group difference in change was −1.96 with a 95% CI of [−6.87, 2.94] (p = 0.419; d = −0.29). For Per %MVIC (median frequency), the within-group change was 16.55 [−14.56, 47.67] in Sham and −8.46 [−21.72, 4.80] in Manipulation. The between-group difference in change was −25.01 with a 95% CI of [−58.00, 7.98] (p = 0.129; d = −0.59).
4. Discussion
The present study aimed to examine the immediate effects of a single superior ankle traction manipulation on the strength and electrical activity of the peroneus longus, gluteus medius, and tensor fasciae latae muscles in healthy individuals. Contrary to our initial expectations, did not reveal any statistically significant between-group differences following the manipulation. Both amplitude and median frequency expressed as %MVIC showed no significant group effect after adjustment, indicating that a single manipulation did not acutely alter neuromuscular activation in the assessed muscles compared with the sham procedure.
Although no adjusted group differences emerged, descriptive and exploratory analyses revealed small, non-significant increases in peroneus longus %MVIC amplitude in the Manipulation group (Δ = +3.1%), while the Sham group remained stable or slightly decreased. This pattern, although not statistically significant after false-discovery-rate (FDR) correction, may suggest a subtle tendency toward enhanced recruitment or sustained activation of the peroneal musculature immediately after manipulation. Such a response could reflect transient changes in proprioceptive input or spinal excitability rather than robust strength adaptations. However, given the small magnitude and lack of significance after correction, these findings should be interpreted cautiously and regarded as preliminary.
Our adjusted analyses do not support an acute increase in GMed activation after a single ankle-region manipulation in healthy participants. By comparison, Lawrence and colleagues [18] reported increased hip abductor force and immediate increases in GMed activation following a series of high-velocity, low-amplitude manipulations applied to the talocrural/subtalar/proximal-distal tibiofibular complex, but in individuals with prior ankle sprain and unilateral TFL weakness rather than a healthy, unselected sample. The discrepancy likely reflects differences in population (clinical deficits vs. healthy volunteers), manipulation dosage (multiple ankle-region thrusts in the involved limb), and analysis approach; in impaired cohorts, greater neuromuscular plasticity or baseline inhibition may permit larger post-manipulation effects that are not observed in healthy controls. A plausible neurophysiological mechanism for remote muscle facilitation is transient modulation of central drive and reflex pathways after manipulation, as seen in studies where spinal manipulation increased strength and corticospinal excitability of ankle plantar flexors in elite athletes [9] and altered motor unit behavior acutely [19]. Within the broader framework of regional interdependence, remote joint interventions may influence neuromuscular output via neurophysiological rather than purely biomechanical routes, although effects can be subtle and context-dependent [20].
We observed small, non-significant increases in PL %MVIC amplitude in the manipulation group that did not survive correction, suggesting at most a modest and transient tendency toward enhanced peroneal activation in healthy participants. In contrast, tibiofibular joint manipulation has been shown to acutely facilitate fibularis (peroneus) longus and soleus activation in individuals with chronic ankle instability (CAI), a population with known arthrokinematic and neuromuscular impairments [21]. Over multi-week periods, proximal or distal tibiofibular manipulation has also been associated with improvements in ankle dorsiflexion range and function in CAI, implying that repeated dosing and clinical deficits may be important moderators of response [22]. Altered PL activation patterns have been documented in individuals with functional ankle instability during gait, underscoring the role of peroneal muscles in dynamic stability and the potential headroom for change in impaired populations that is absent in healthy participants [23]. Transient increases in corticospinal excitability and voluntary activation after spinal manipulation provide a biologically plausible pathway for short-term facilitation of distal muscles, although effects may be task-, muscle-, and population-specific [10].
The TFL showed no significant between-group differences in %MVIC amplitude or median frequency after adjustment. Prior work that reported hip abductor facilitation after ankle-region manipulation targeted participants with unilateral TFL weakness during muscle testing and prior ankle sprain, again pointing to the importance of baseline impairment and targeted selection for observing clinically meaningful effects [18]. Given TFL’s role in frontal-plane control and its interplay with GMed, the absence of an effect in our healthy participants is consistent with a ceiling effect and with evidence that manipulation-induced changes in muscle output are more detectable when pre-existing inhibition or motor control deficits are present [19].
This study presents several limitations that should be considered when interpreting the findings. First, the relatively small sample size of healthy participants may have limited statistical power to detect subtle differences between groups, particularly after applying false-discovery-rate corrections. The inclusion of predominantly male participants (26 men and 4 women) also restricts the generalizability of the results, as sex-related differences in hip and ankle neuromuscular control have been widely documented in previous research. Women tend to demonstrate distinct lower-limb kinematics, neuromuscular recruitment patterns, and proprioceptive responses compared with men, which may influence the effects of manipulative interventions. Consequently, the current results should primarily be interpreted as representative of young, healthy males. A covariate analysis including sex was considered but not performed due to the small and unbalanced female subsample (n = 4), which would produce unstable estimates and violate the assumptions of ANCOVA. Instead, we acknowledge this imbalance as a limitation and recommend that future studies ensure adequate female representation or stratify randomization by sex to permit meaningful between-sex comparisons.
Moreover, the experimental design assessed only the immediate post-manipulation effects, precluding conclusions about the persistence or cumulative nature of potential neuromuscular adaptations over time. The use of a single manipulation session may have been insufficient to elicit measurable central or peripheral adaptations, as repeated high-velocity, low-amplitude (HVLA) manipulations are more likely to produce detectable neuroplastic effects [10].
An additional limitation concerns the fixed order of intervention administration, as the ankle manipulation was consistently performed after the baseline measurements. This introduces the potential for order or learning effects, in which repeated maximal voluntary contractions could produce mild neuromuscular adaptation or fatigue, possibly influencing post-test EMG amplitudes or torque values independent of the manipulation itself. However, several methodological aspects likely minimized this risk. All participants performed identical testing sequences under the same conditions, with fixed 30 s rest intervals between MVICs, and the order was the same for both groups; thus, any non-specific temporal or repetition effects would have influenced both groups equally, reducing systematic bias. Furthermore, EMG data were normalized to each session’s maximal contraction (%MVIC), which helps correct for intra-session recruitment changes related to repetition or familiarization. Nevertheless, we acknowledge that randomizing the order of real and sham manipulations or employing a crossover design with an appropriate washout period would provide stronger control for potential order effects, and this refinement is planned for future research.
Additionally, although EMG normalization to MVIC improves inter-subject comparability, small variations in electrode placement or participant effort may still contribute to measurement variability. The outcomes selected—namely, isometric muscle strength and surface EMG amplitude/frequency—provide precise indicators of neuromuscular activation but do not directly assess the functional or behavioral implications of ankle manipulation. No dynamic or performance-based measures (e.g., balance, gait, or functional reach) were included, limiting the ecological validity of the findings. Future investigations should integrate objective functional tests, such as the Y-Balance Test or single-leg hop assessments, alongside electrophysiological measurements to determine whether acute neuromuscular changes translate into improved dynamic stability, balance control, or performance in daily and athletic tasks. Finally, because this study involved healthy individuals with no underlying dysfunction, ceiling effects may have limited the observable impact of the manipulation compared with clinical populations where baseline inhibition or instability is present.
From a clinical standpoint, these findings suggest that a single superior ankle traction manipulation does not induce meaningful immediate changes in hip abductor or peroneal muscle activation when assessed under normalized and baseline-adjusted conditions in healthy adults. The lack of significant group differences underscores the importance of cautious interpretation of unadjusted pre–post findings. Clinicians should therefore view ankle manipulation as a potentially complementary, rather than primary, intervention for neuromuscular enhancement in asymptomatic populations. However, subtle trends toward increased peroneal activation in the manipulation group, although not statistically significant, may have clinical relevance in populations with chronic ankle instability, where proprioceptive deficits and motor inhibition are common. Consequently, future clinical applications and research should consider the dosage, population characteristics, and combination with functional rehabilitation exercises when integrating ankle manipulation into therapeutic programs aimed at improving lower-limb stability and neuromuscular performance.
5. Conclusions
When electromyographic activity is normalized to maximal voluntary isometric contraction and baseline differences are statistically controlled, a single superior ankle traction manipulation does not produce significant acute changes in the electrical activity or isometric strength of the peroneus longus, gluteus medius, or tensor fasciae latae muscles in healthy individuals. The minor, non-significant trends toward increased peroneal activation observed in the manipulation group suggest that any neuromuscular influence of a single intervention is likely subtle and transient. These findings emphasize the importance of rigorous normalization, baseline adjustment, and correction for multiple comparisons in studies examining manual therapy–induced neuromuscular effects. Further research should explore repeated interventions, clinical populations, and longer-term outcomes to determine whether ankle manipulation can meaningfully enhance neuromuscular function through regional interdependence mechanisms.
Author Contributions
R.S.: Conceptualization, data curation, investigation, methodology, project administration, writing—original draft, and writing—review and editing; P.W.: data curation, investigation, and methodology; P.A.: methodology, data curation, writing—review and editing, R.L.: formal analysis, writing—original draft, and writing—review and editing, P.Ł.: formal analysis, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk (Resolution No. NKBBN/866/2022-2023), accessed date 17 December 2022. Participants received a detailed explanation of this study, including a simplified description of the protocol. Before participating, they provided written informed consent, confirming their voluntary participation and understanding that they could withdraw from this study at any time without penalty. This study adhered to the ethical principles outlined in the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data can be provided upon reasonable request to the corresponding author.
Acknowledgments
The materials obtained during this study are secured and are in the possession of the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dananberg, H.J. Manipulation of the Ankle as a Method of Treatment for Ankle and Foot Pain. J. Am. Podiatr. Med. Assoc. 2004, 94, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Dananberg, H.; Shearstone, J.; Guillano, M. Manipulation Method for the Treatment of Ankle Equinus. J. Am. Podiatr. Med. Assoc. 2000, 90, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R. Effectiveness of High-Velocity Low-Amplitude Manipulation in the Treatment of Ankle Joint Dysfunction: A Comprehensive Review. Reabil. Moksl. Slauga Kineziter. Ergoter. 2024, 1, 4–14. [Google Scholar] [CrossRef]
- Fryer, G.A.; Mudge, J.M.; McLaughlin, P.A. The Effect of Talocrural Joint Manipulation on Range of Motion at the Ankle. J. Manip. Physiol. Ther. 2002, 25, 384–390. [Google Scholar] [CrossRef]
- Weerasekara, I.; Osmotherly, P.; Snodgrass, S.; Marquez, J.; de Zoete, R.; Rivett, D.A. Clinical Benefits of Joint Mobilization on Ankle Sprains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1395–1412.e5. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, J.-H.; Yu, S.-M.; An, C.-M. The Effects of Ankle Position on Torque and Muscle Activity of the Knee Extensor During Maximal Isometric Contraction. J. Sport Rehabil. 2020, 29, 37–42. [Google Scholar] [CrossRef]
- Perry, J.; Ireland, M.L.; Gronley, J.; Hoffer, M.M. Predictive Value of Manual Muscle Testing and Gait Analysis in Normal Ankles by Dynamic Electromyography. Foot Ankle 1986, 6, 254–259. [Google Scholar] [CrossRef]
- Lehman, G.J.; McGill, S.M. The Importance of Normalization in the Interpretation of Surface Electromyography: A Proof of Principle. J. Manip. Physiol. Ther. 1999, 22, 444–446. [Google Scholar] [CrossRef]
- Christiansen, T.L.; Niazi, I.K.; Holt, K.; Nedergaard, R.W.; Duehr, J.; Allen, K.; Marshall, P.; Türker, K.S.; Hartvigsen, J.; Haavik, H. The Effects of a Single Session of Spinal Manipulation on Strength and Cortical Drive in Athletes. Eur. J. Appl. Physiol. 2018, 118, 737–749. [Google Scholar] [CrossRef]
- Navid, M.S.; Niazi, I.K.; Lelic, D.; Amjad, I.; Kumari, N.; Shafique, M.; Holt, K.; Rashid, U.; Drewes, A.M.; Haavik, H. Chiropractic Spinal Adjustment Increases the Cortical Drive to the Lower Limb Muscle in Chronic Stroke Patients. Front. Neurol. 2022, 12, 747261. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Fauth, M.L.; Petushek, E.J.; Feldmann, C.R.; Hsu, B.E.; Garceau, L.R.; Lutsch, B.N.; Ebben, W.P. Reliability of Surface Electromyography During Maximal Voluntary Isometric Contractions, Jump Landings, and Cutting. J. Strength Cond. Res. 2010, 24, 1131–1137. [Google Scholar] [CrossRef]
- Julious, S.A. Sample Size of 12 per Group Rule of Thumb for a Pilot Study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A. CONSORT 2010 Statement: Extension to Randomised Pilot and Feasibility Trials. BMJ 2016, 355, i5239. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.G.A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Altman, D.G. Statistics Notes: Analysing Controlled Trials with Baseline and Follow up Measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxfordshire, UK, 2013. [Google Scholar]
- Lawrence, M.A.; Raymond, J.T.; Look, A.E.; Woodard, N.M.; Schicker, C.M.; Swanson, B.T. Effects of Tibiofibular and Ankle Joint Manipulation on Hip Strength and Muscle Activation. J. Manip. Physiol. Ther. 2020, 43, 406–417. [Google Scholar] [CrossRef]
- Niazi, I.K.; Kamavuako, E.N.; Holt, K.; Janjua, T.A.M.; Kumari, N.; Amjad, I.; Haavik, H. The Effect of Spinal Manipulation on the Electrophysiological and Metabolic Properties of the Tibialis Anterior Muscle. Healthcare 2020, 8, 548. [Google Scholar] [CrossRef]
- Sueki, D.G.; Cleland, J.A.; Wainner, R.S. A Regional Interdependence Model of Musculoskeletal Dysfunction: Research, Mechanisms, and Clinical Implications. J. Man. Manip. Ther. 2013, 21, 90–102. [Google Scholar] [CrossRef]
- Grindstaff, T.L.; Beazell, J.R.; Sauer, L.D.; Magrum, E.M.; Ingersoll, C.D.; Hertel, J. Immediate Effects of a Tibiofibular Joint Manipulation on Lower Extremity H-Reflex Measurements in Individuals with Chronic Ankle Instability. J. Electromyogr. Kinesiol. 2011, 21, 652–658. [Google Scholar] [CrossRef]
- Beazell, J.R.; Grindstaff, T.L.; Sauer, L.D.; Magrum, E.M.; Ingersoll, C.D.; Hertel, J. Effects of a Proximal or Distal Tibiofibular Joint Manipulation on Ankle Range of Motion and Functional Outcomes in Individuals with Chronic Ankle Instability. J. Orthop. Sports Phys. Ther. 2012, 42, 125–134. [Google Scholar] [CrossRef]
- Santilli, V.; Frascarelli, M.A.; Paoloni, M.; Frascarelli, F.; Camerota, F.; De Natale, L.; De Santis, F. Peroneus Longus Muscle Activation Pattern during Gait Cycle in Athletes Affected by Functional Ankle Instability. Am. J. Sports Med. 2005, 33, 1183–1187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).