Assessment of Continuous Flow-Dependent Red Cell Aggregation Using a Microfluidic Chip
Featured Application
Abstract
1. Introduction
2. Materials and Methods
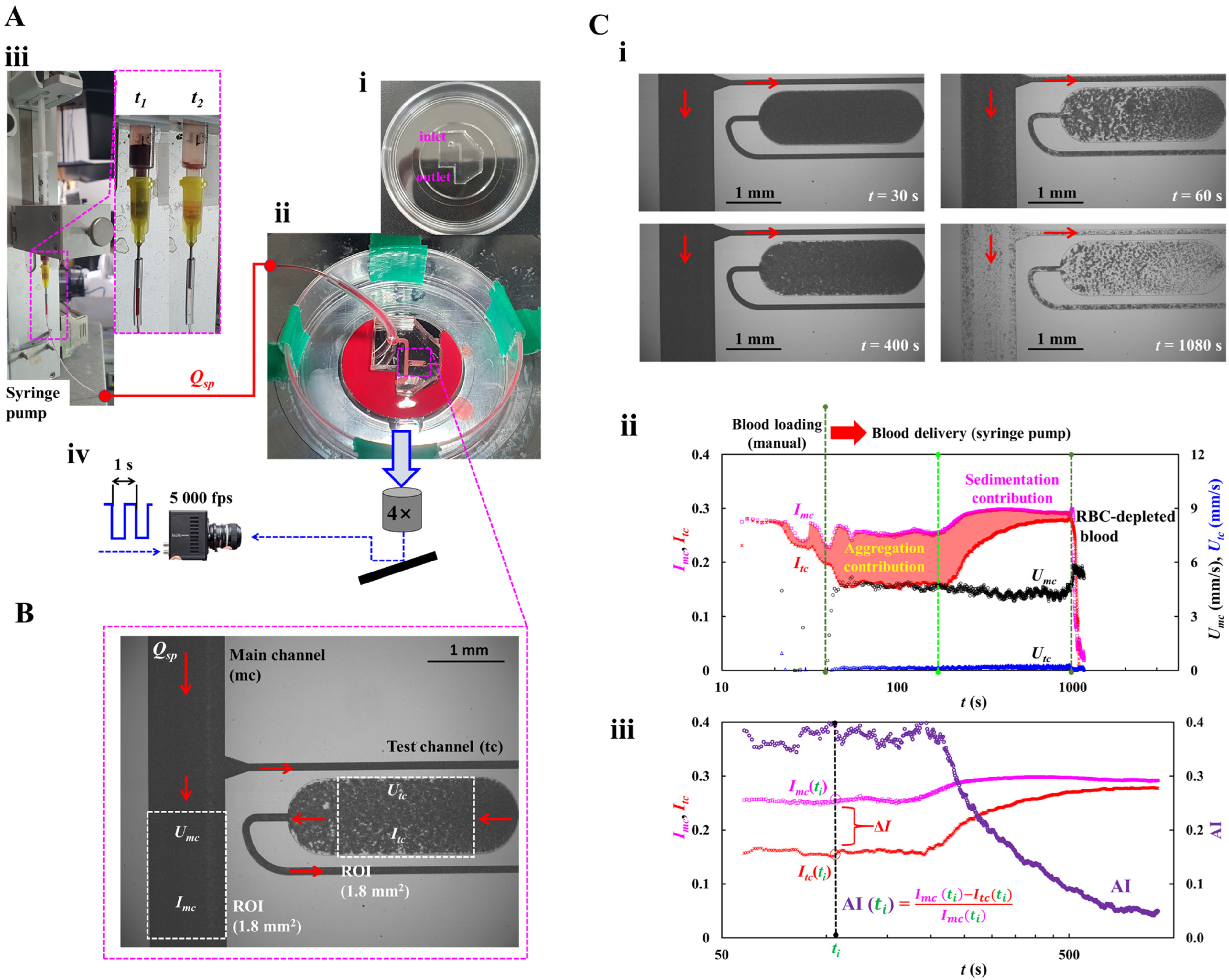
2.1. Microfluidic Chip and Experimental Setup
2.2. Blood Velocity and Blood Imaging Intensity in Main and Test Channels
2.3. Quantification of RBC Aggregation Index (AI)
2.4. Blood Sample Preparation for Quantifying RBC Aggregation
3. Results and Discussion
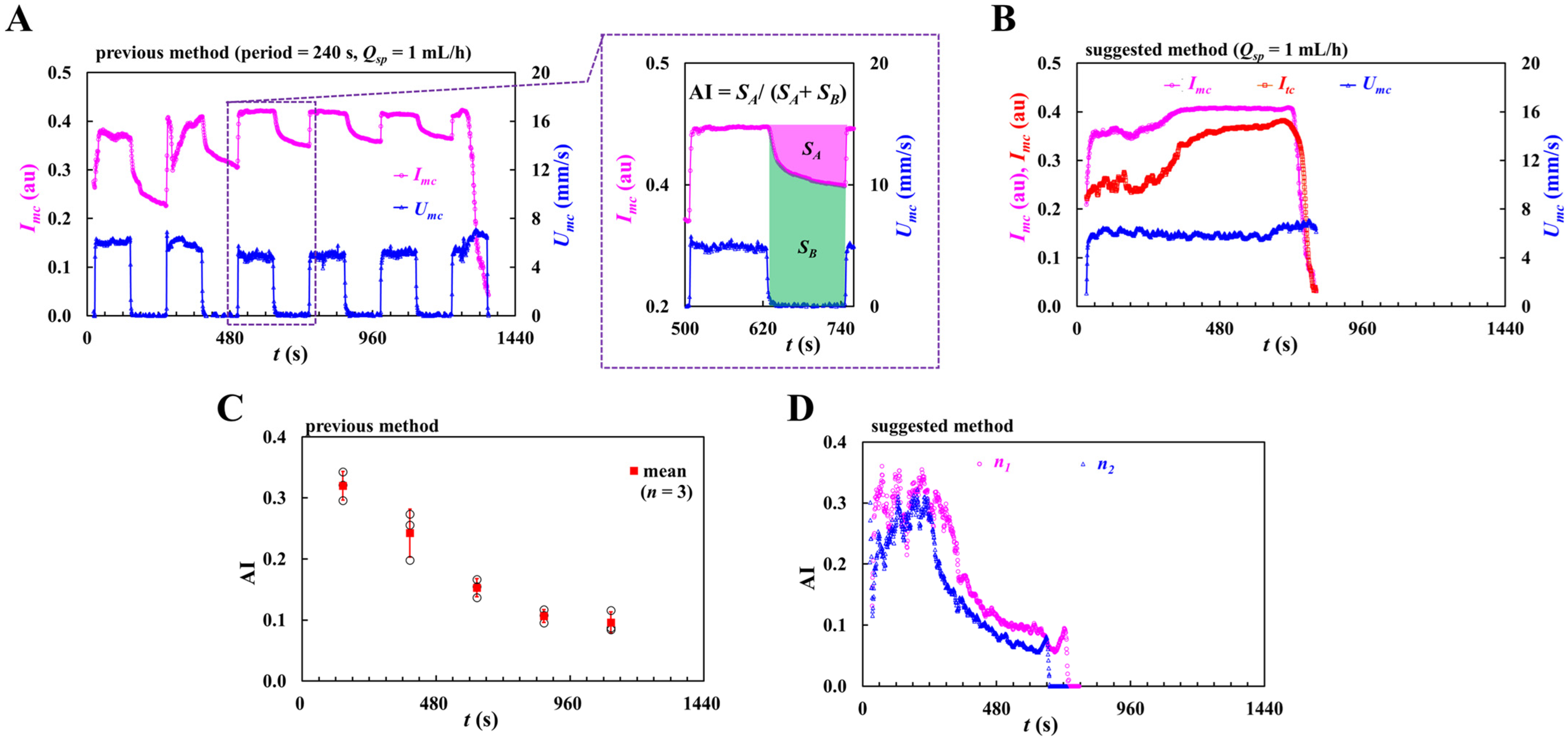
3.1. Mathematical Model and Its Validation of a Proposed Microfluidic System
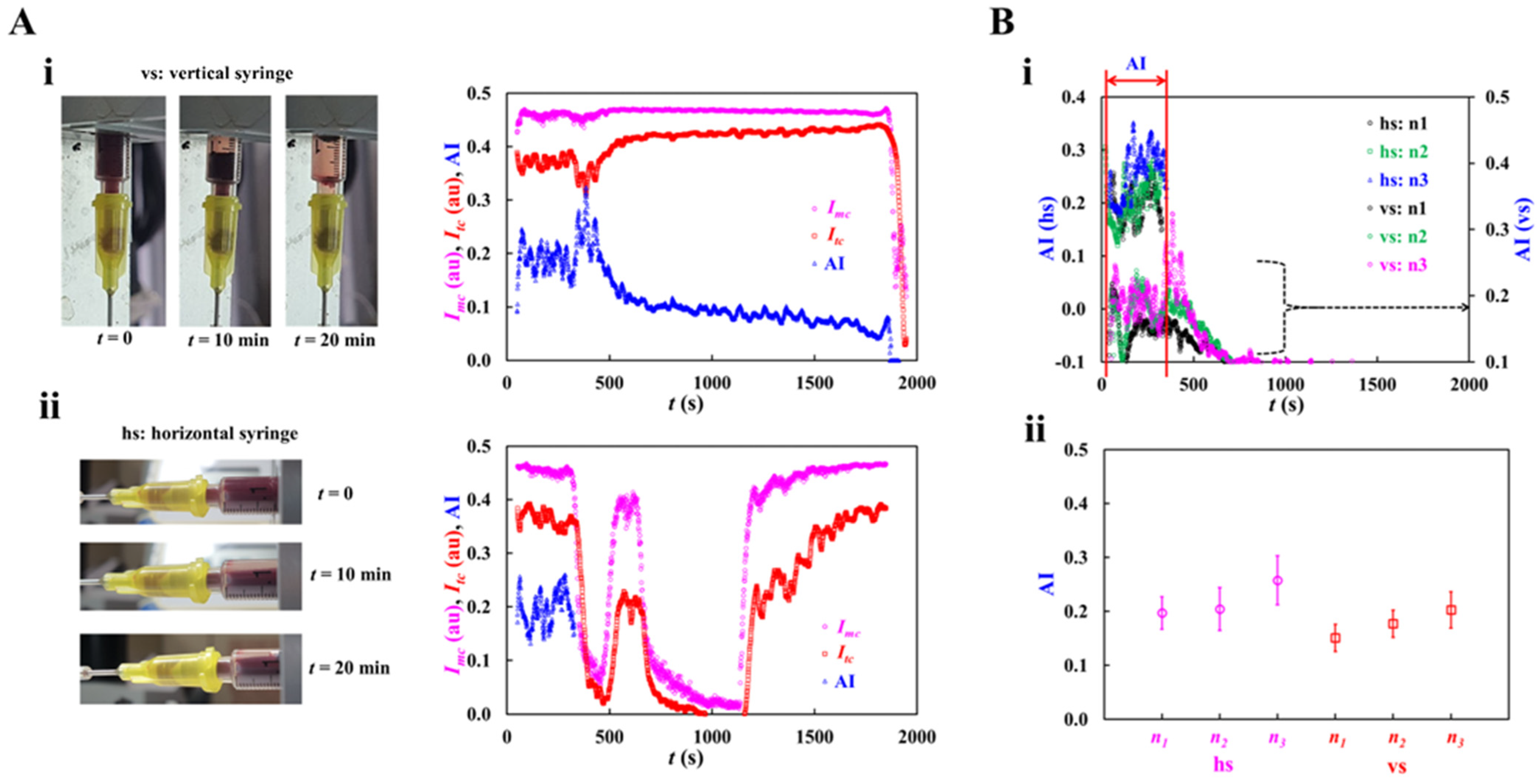
3.2. Contribution of Syringe Installation to RBC Aggregation
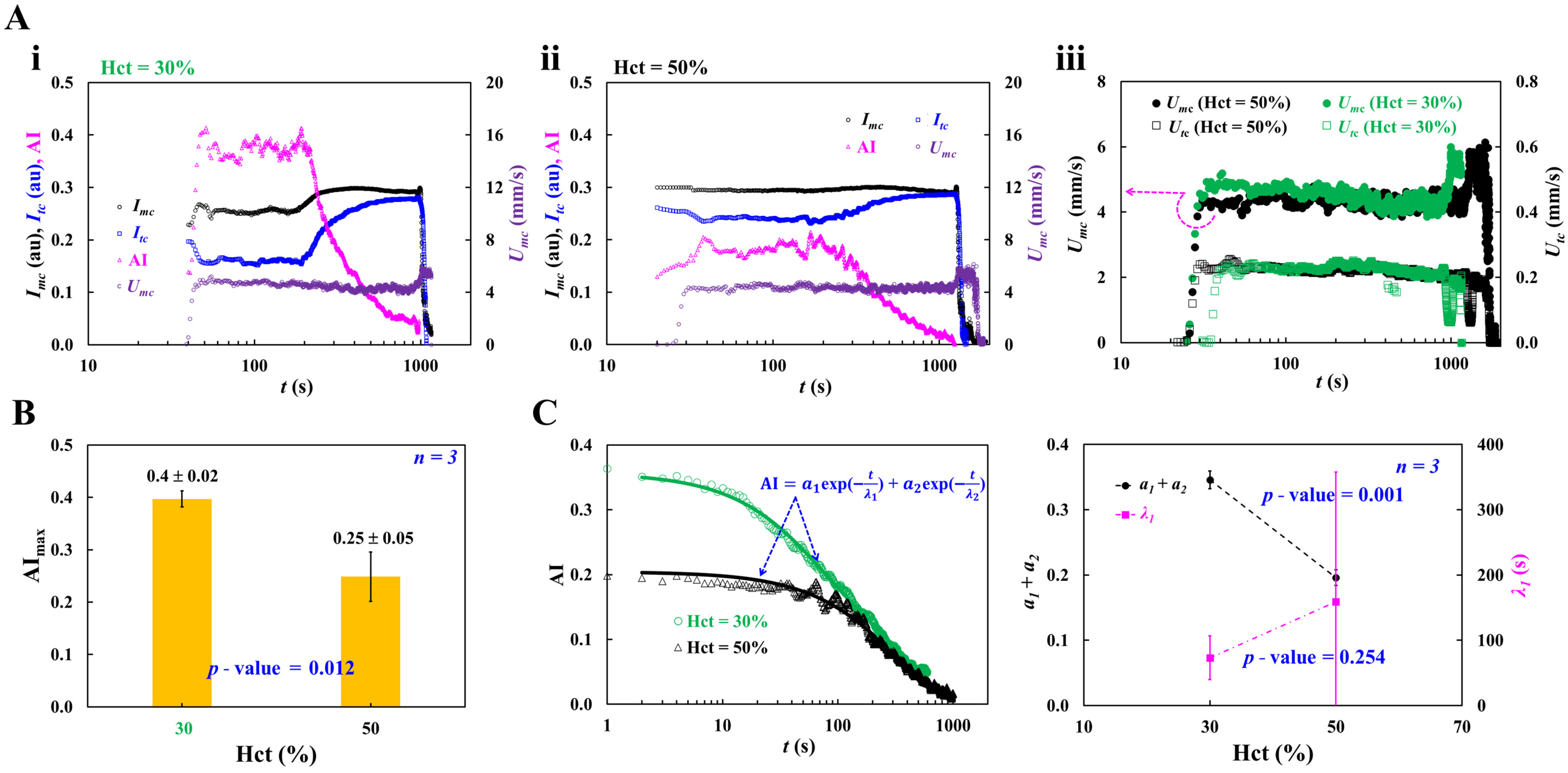
3.3. Contribution of Hematocrit to RBC Aggregation
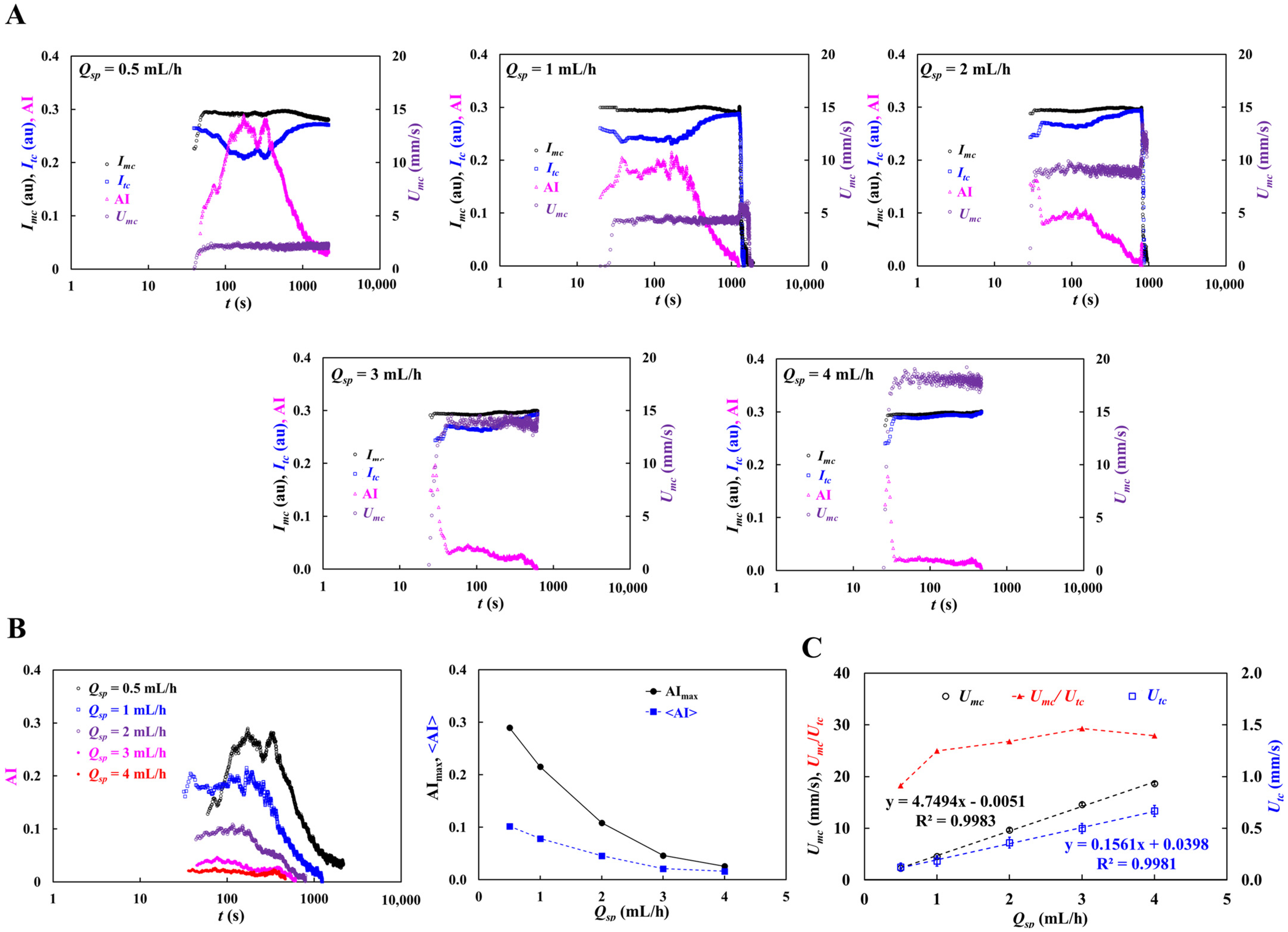
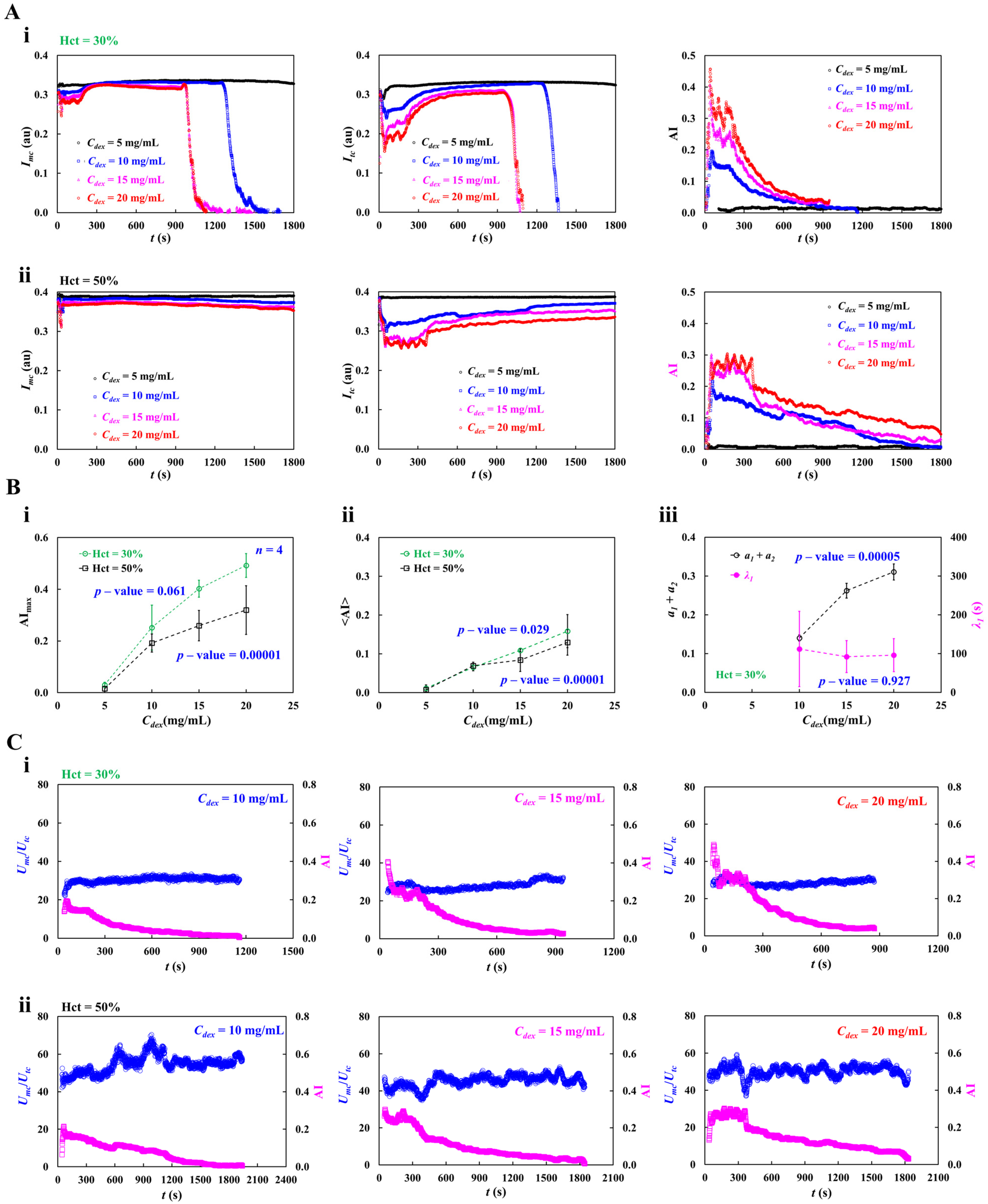
3.4. Contribution of Dextran Solution and Hematocrit to RBC Aggregation
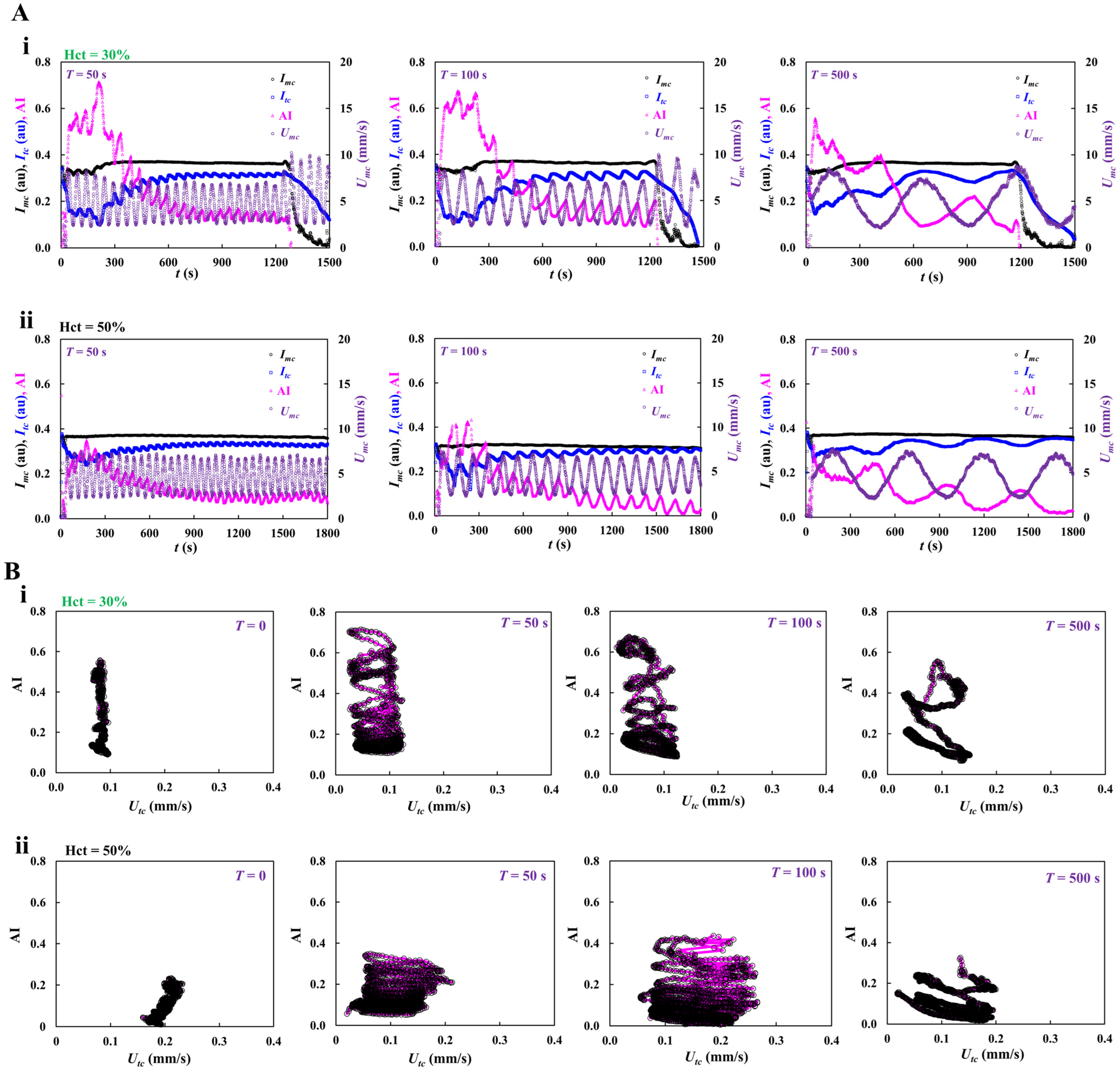
3.5. Contribution of Pulsatile Infusion Flow Pattern to Time-Lapse AI
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Moreno, N.; Korneev, K.; Semenov, A.; Topuz, A.; John, T.; Lettinga, M.P.; Ellero, M.; Wagner, C.; Fedosov, D.A. Aggregation and disaggregation of red blood cells: Depletion versus bridging. Biophys. J. 2025, 124, 1285–1297. [Google Scholar] [CrossRef]
- Deng, Y.; Papageorgiou, D.P.; Li, X.; Perakakis, N.; Mantzoros, C.S.; Dao, M.; Karniadakis, G.E. Quantifying fibrinogen-dependent aggregation of red blood cells in type 2 diabetes mellitus. Biophys. J. 2020, 119, 900–912. [Google Scholar] [CrossRef]
- Barath, B.; Somogyi, V.; Tanczos, B.; Varga, A.; Bereczky, Z.; Nemeth, N.; Deak, A. Examination of the relation between red blood cell aggregation and hematocrit in human and various experimental animals. Clin. Hemorheol. Microcirc. 2021, 78, 187–198. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Farley, R.A.; Meiselman, H.J. Erythrocyte aggregation tendency and cellular properties in horse, human, and rat: A comparative study. Am. J. Physiol. Heart Circ. Physiol. 1997, 273, H2604–H2612. [Google Scholar] [CrossRef]
- Yalcin, O.; Aydin, F.; Ulker, P.; Uyuklu, M.; Gungor, F.; Armstrong, J.K.; Meiselman, H.J.; Baskurt, O.K. Effects of red blood cell aggregation on myocardial hematocrit gradient using two approaches to increase aggregation. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H765–H771. [Google Scholar] [CrossRef]
- Higuchi, M.; Watanabe, N. A rapid and accurate method for estimating the erythrocyte sedimentation rate using a hematocrit-corrected optical aggregation index. PLoS ONE 2022, 17, e0270977. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, L.; Chen, Z. Effect of erythrocyte aggregation on hematocrit measurement using spectral-domain optical coherence tomography. IEEE Trans. Biomed. Eng. 2008, 55, 2753–2758. [Google Scholar] [CrossRef]
- Daousani, C.; Kostić, N.; Malenović, A.; Rmandić, M.; Dotsikas, Y. On the Use of Image Analysis for Hematocrit Evaluation in Dried Blood Spots. Appl. Sci. 2024, 14, 9860. [Google Scholar] [CrossRef]
- Melepattu, M.P.; Maitrejean, G.; Wagner, C.; Podgorski, T. Influence of erythrocyte density on aggregability as a marker of cell age: Dissociation dynamics in extensional flow. J. Biomech. 2025, 183, 112603. [Google Scholar] [CrossRef]
- Maurer, F.; John, T.; Makhro, A.; Bogdanova, A.; Minetti, G.; Wagner, C.; Kaestner, L. Continuous percoll gradient centrifugation of erythrocytes-explanation of cellular bands and compromised age separation. Cells 2022, 11, 1296. [Google Scholar] [CrossRef] [PubMed]
- Ermolinskiy, P.; Lugovtsov, A.; Yaya, F.; Lee, K.; Kaestner, L.; Wagner, C.; Priezzhev, A. Effect of red clood cell aging In vivo on their aggregation properties in vitro: Measurements with laser tweezers. Appl. Sci. 2020, 10, 7581. [Google Scholar] [CrossRef]
- Zhao, Y.; Brandon-Coatham, M.; Yazdanbakhsh, M.; Mykhailova, O.; William, N.; Osmani, R.; Kanias, T.; Acker, J.P. Cold storage surpasses the impact of biological age and donor characteristics on red blood cell morphology classified by deep machine learning. Sci. Rep. 2025, 15, 7735. [Google Scholar] [CrossRef] [PubMed]
- Matteoli, P.; Nicoud, F.; Mendez, S. Impact of the membrane viscosity on the tank-treading behavior of red blood cells. Phys. Rev. Fluids 2021, 6, 043602. [Google Scholar] [CrossRef]
- Gürbüz, A.; Pak, O.S.; Taylor, M.; Sivaselvan, M.V.; Sachs, F. Effects of membrane viscoelasticity on the red blood cell dynamics in a microcapillary. Biophys. J. 2023, 122, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Johnson, P.C.; Popel, A.S. Red blood cell aggregation and dissociation in shear flows simulated by lattice boltzmann method. J. Biomech. 2008, 41, 47–55. [Google Scholar] [CrossRef]
- Lazari, D.; Leal, J.K.F.; Brock, R.; Bosman, G. The relationship between aggregation and deformability of red blood cells in health and disease. Front. Physiol. 2020, 11, 288. [Google Scholar] [CrossRef]
- Weber-Fishkin, S.; Seidner, H.S.; Gunter, G.; Frame, M.D. Erythrocyte aggregation in sudden flow arrest is linked to hyperthermia, hypoxemia, and band 3 availability. J. Thromb. Haemost. 2022, 20, 2284–2292. [Google Scholar] [CrossRef]
- Çolak, E.; Ekici, Ö.; Erdener, Ş.E. In silico investigation of the RBC velocity fluctuations in ex vivo capillaries. Appl. Sci. 2025, 15, 7796. [Google Scholar] [CrossRef]
- Reinhart, W.H.; Piety, N.Z.; Shevkoplyas, S.S. Influence of red blood cell aggregation on perfusion of an artificial microvascular network. Microcirculation 2017, 24, e12317. [Google Scholar] [CrossRef]
- Porro, B.; Conte, E.; Zaninoni, A.; Bianchi, P.; Veglia, F.; Barbieri, S.; Fiorelli, S.; Eligini, S.; Di Minno, A.; Mushtaq, S.; et al. Red blood cell morphodynamics: A new potential marker in high-risk patients. Front. Physiol. 2020, 11, 603633. [Google Scholar] [CrossRef]
- Peshkova, A.D.; Rednikova, E.K.; Khismatullin, R.R.; Kim, O.V.; Muzykantov, V.R.; Purohit, P.K.; Litvinov, R.I.; Weisel, J.W. Red blood cell aggregation within a blood clot causes platelet-independent clot shrinkage. Blood Adv. 2025, 9, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Hadengue, A.L.; Del-Pino, M.; Simon, A.; Levenson, J. Erythrocyte disaggregation shear stress, sialic acid, and cell aging in humans. Hypertension 1998, 32, 324–330. [Google Scholar] [CrossRef]
- Kiełtyka-Słowik, A.; Teległów, A.; Gradek, J. Rheological and morphological properties of blood vs. vibration exercises (Smovey®)—A preliminary study on elderly women. Appl. Sci. 2025, 15, 5058. [Google Scholar] [CrossRef]
- McCarthy, R.P.; LaDisa, J.F., Jr. Influence of boundary conditions and blood rheology on indices of wall shear stress from IVUS-based patient-specific stented coronary artery simulations. Sci. Rep. 2025, 15, 15868. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, P.; Yan, Z.; Liu, Z.; Ma, Q.; Zhang, Z.; Wang, Y.; Su, Y. The relationship between erythrocytes and diabetes mellitus. J. Diabetes Res. 2021, 2021, 6656062. [Google Scholar] [CrossRef]
- Chung, S.M.; Oh, J.H.; Moon, J.S.; Kim, Y.K.; Yoon, J.S.; Won, K.C.; Lee, H.W. Critical shear stress is associated with diabetic kidney disease in patients with type 2 diabetes. Sci. Rep. 2018, 8, 908. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, U.; Choi, K.U.; Nam, J.H.; Lee, J.H.; Lee, C.H.; Son, J.W.; Park, J.S.; Shin, D.G.; Won, K.C.; et al. Hemorheologic alterations in patients with type 2 diabetes mellitus presented with an acute myocardial infarction. Diabetes Metab. J. 2018, 42, 155–163. [Google Scholar] [CrossRef]
- Lominadze, D.; Joshua, I.G.; Schuschke, D.A. Increased erythrocyte aggregation in spontaneously hypertensive rats. Am. J. Hypertens. 1998, 11, 784–789. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef] [PubMed]
- Del, F. Giudice A Review of Microfluidic Devices for Rheological Characterisation. Micromachines 2022, 13, 167. [Google Scholar]
- Antonova, N.; Khristov, K. Microrheological and Microfluidic Approaches for Evaluation of the Mechanical Properties of Blood Cells. Appl. Sci. 2025, 15, 8291. [Google Scholar] [CrossRef]
- Kim, H.; Zhbanov, A.; Yang, S. Microfluidic systems for blood and blood cell characterization. Biosensors 2022, 13, 13. [Google Scholar] [CrossRef]
- Trejo-Soto, C.; Lazaro, G.R.; Pagonabarraga, I.; Hernandez-Machado, A. Microfluidics Approach to the Mechanical Properties of Red Blood Cell Membrane and Their Effect on Blood Rheology. Membranes 2022, 12, 217. [Google Scholar] [CrossRef]
- Grigorev, G.V.; Lebedev, A.V.; Wang, X.; Qian, X.; Maksimov, G.V.; Lin, L. Advances in Microfluidics for Single Red Blood Cell Analysis. Biosensors 2023, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, G.; Doll-Nikutta, K.; Mueller, M.; Berg, P.; Glasmacher, B. Development and characterization of a Dextran/CaCl(2)-based blood-mimicking fluid: A comparative study of rheological and mechanical properties in artificial erythrocyte suspensions. Soft Matter 2025, 21, 3101–3116. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, W.; Giri, N.V.; Qin, N.; Zhao, P.; Phan, C.-M.; Haines, L.; Jones, L.; Ren, C.L. A novel microfluidic viscometer for measuring viscosity of ultrasmall volumes of Newtonian and non-Newtonian liquids. J. Micromech. Microeng. 2025, 35, 055005. [Google Scholar] [CrossRef]
- Kang, Y.J. Simultaneous viscosity measurement of suspended blood and plasma separated by an ultrasonic transducer. Appl. Sci. 2023, 13, 3574. [Google Scholar] [CrossRef]
- Kang, Y.J. A microfluidic-based blood viscometer. Phys. Fluids 2025, 37, 091904. [Google Scholar] [CrossRef]
- Du, J.; Lee, S.; Sinha, S.; Solberg, F.S.; Ho, D.L.L.; Sampson, J.P.; Wang, Q.; Tam, T.; Skylar-Scott, M.A. A visual, in-expensive, and wireless capillary rheometer for characterizing wholly-cellular bioinks. Small 2024, 20, e2304778. [Google Scholar] [CrossRef]
- Liang, M.; Ming, D.; Zhong, J.; Shannon, C.S.; Rojas-Carabali, W.; Agrawal, K.; Ai, Y.; Agrawal, R. Pathophysiological associations and measurement techniques of red blood cell deformability. Biosensors 2025, 15, 566. [Google Scholar] [CrossRef]
- Yang, T.; Bragheri, F.; Minzioni, P. A comprehensive review of optical stretcher for cell mechanical characterization at single-cell level. Micromachines 2016, 7, 90. [Google Scholar] [CrossRef]
- Guo, Q.; Duffy, S.P.; Matthews, K.; Santoso, A.T.; Scott, M.D.; Ma, H. Microfluidic analysis of red blood cell deformability. J. Biomech. 2014, 47, 1767–1776. [Google Scholar] [CrossRef]
- Stathoulopoulos, A.; Passos, A.; Kaliviotis, E.; Balabani, S. Partitioning of dense RBC suspensions in single microfluidic bifurcations: Role of cell deformability and bifurcation angle. Sci. Rep. 2024, 14, 535. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lizarralde-Iragorri, M.A.; Roman, J.; Ghasemi, R.; Lefevre, J.P.; Martincic, E.; Brousse, V.; Francais, O.; El Nemer, W.; Le Pioufle, B. Characterization of red blood cell microcirculatory parameters using a bioimpedance microfluidic device. Sci. Rep. 2020, 10, 9869. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Liu, Z.; Luo, S.; Huang, J.; Chen, C.; Chen, R.; Li, F. A Microfluidic Approach for Assessing the Rheological Properties of Healthy Versus Thalassemic Red Blood Cells. Micromachines 2025, 16, 957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rao, H.; Zhang, H.; Wang, M.; Wu, Y.; Wu, Y.; Han, C.; Yan, C.; Zhang, L.; Chen, W.; et al. Efficient characterization of red blood cell rheological properties using a multichannel microfluidic chip and optical tweezers. Mater. Today Adv. 2024, 24, 100545. [Google Scholar] [CrossRef]
- Isiksacan, Z.; D’Alessandro, A.; Wolf, S.M.; McKenna, D.H.; Tessier, S.N.; Kucukal, E.; Gokaltun, A.A.; William, N.; Sandlin, R.D.; Bischof, J.; et al. Assessment of stored red blood cells through lab-on-a-chip technologies for precision transfusion medicine. Proc. Natl. Acad. Sci. USA 2023, 120, e2115616120. [Google Scholar] [CrossRef]
- Kang, Y.J. Microfluidic chip for quantitatively assessing hemorheological parameters. Micromachines 2025, 16, 567. [Google Scholar] [CrossRef]
- Namgung, B.; Lee, T.; Tan, J.K.S.; Poh, D.K.H.; Park, S.; Chng, K.Z.; Agrawal, R.; Park, S.Y.; Leo, H.L.; Kim, S. Vibration motor-integrated low-cost, miniaturized system for rapid quantification of red blood cell aggregation. Lab Chip 2020, 20, 3930–3937. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, Y.S.; Zhbanov, A.; Yang, S. A physiometer for simultaneous measurement of whole blood viscosity and its determinants: Hematocrit and red blood cell deformability. Analyst 2019, 144, 3144–3157. [Google Scholar] [CrossRef]
- Passos, A.; Louka, M.; Vryonidis, C.; Inglezakis, A.; Loizou, C.; Nikiphorou, E.; Psarelis, S.; Kaliviotis, E. Red blood cell sedimentation rate measurements in a high aspect ratio microchannel. Clin. Hemorheol. Microcirc. 2022, 82, 313–322. [Google Scholar] [CrossRef]
- Zhbanov, A.; Yang, S. Effects of aggregation on blood sedimentation and conductivity. PLoS ONE 2015, 10, e0129337. [Google Scholar] [CrossRef]
- Melepattu, M.P.; Maîtrejean, G.; Podgorski, T. Dissociation of red blood cell aggregates in extensional flow. Phys. Rev. Fluids 2024, 9, L071101. [Google Scholar] [CrossRef]
- Isiksacan, Z.; Erel, O.; Elbuken, C. A portable microfluidic system for rapid measurement of the erythrocyte sedimentation rate. Lab Chip 2016, 16, 4682–4690. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Yang, Y.; Suh, J.S. Measurement of erythrocyte aggregation in a microchip stirring system by light transmission. Clin. Hemorheol. Microcirc. 2009, 41, 197–207. [Google Scholar] [CrossRef]
- Kang, Y.J. Biomechanical investigation of red cell sedimentation using blood shear stress and blood flow image in a capillary chip. Micromachines 2023, 14, 1594. [Google Scholar] [CrossRef]
- Mehri, R.; Mavriplis, C.; Fenech, M. Red blood cell aggregates and their effect on non-Newtonian blood viscosity at low hematocrit in a two-fluid low shear rate microfluidic system. PLoS ONE 2018, 13, e0199911. [Google Scholar] [CrossRef]
- Sebastian, B.; Dittrich, P.S. Microfluidics to mimic bood flow in health and disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504. [Google Scholar] [CrossRef]
- Isiksacan, Z.; Serhatlioglu, M.; Elbuken, C. In vitro analysis of multiple blood flow determinants using red blood cell dynamics under oscillatory flow. Anayst 2020, 145, 5996–6005. [Google Scholar] [CrossRef] [PubMed]
- Lezzar, D.L.; Lam, F.W.; Huerta, R.; Mukhamedshin, A.; Lu, M.; Shevkoplyas, S.S. A high-throughput microfluidic device based on controlled incremental filtration to enable centrifugation-free, low extracorporeal volume leukapheresis. Sci. Rep. 2022, 12, 13798. [Google Scholar] [CrossRef]
- Nam, K.H.; Yeom, E.; Lee, S.J. Extracorporeal bypass model of blood circulation for the study of microvascular hemodynamics. Microvasc. Res. 2012, 83, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Thielicke, W.; Stamhuis, E.J. PIVlab—Towards user-friendly, affordable and accurate digital particle image velocimetry in MATLAB. J. Open Res. Softw. 2014, 2, e30. [Google Scholar] [CrossRef]
- Kang, Y.J. Blood viscometer using capillary blood flow under disposable compliance pump. Int. J. Mech. Sci. 2024, 277, 109456. [Google Scholar] [CrossRef]
- Kang, Y.J. Red blood cell sedimentation index using shear stress of blood flow in microfluidic channel. Biosensors 2022, 12, 547. [Google Scholar] [CrossRef]
- Schmid-Schonbein, H.; Gaehtgens, P.; Hirsch, H. On the shear rate dependence of red cell aggregation in vitro. J. Clin. Investig. 1968, 47, 1447–1454. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 2012, 12, 515–545. [Google Scholar] [CrossRef]
- Riera-Llobet, C.; Méndez-Mora, L.; Cabello-Fusarés, M.; Hernández-Machado, A. Altered blood rheology in multiwidth microchannels: Hematocrit and tonicity variation. Phys. Fluids 2023, 35, 082017. [Google Scholar] [CrossRef]
- Li, G.; Ye, T.; Yang, B.; Wang, S.; Li, X. Temporal-spatial heterogeneity of hematocrit in microvascular networks. Phys. Fluids 2023, 35, 021906. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.J. Assessment of Continuous Flow-Dependent Red Cell Aggregation Using a Microfluidic Chip. Appl. Sci. 2025, 15, 11481. https://doi.org/10.3390/app152111481
Kang YJ. Assessment of Continuous Flow-Dependent Red Cell Aggregation Using a Microfluidic Chip. Applied Sciences. 2025; 15(21):11481. https://doi.org/10.3390/app152111481
Chicago/Turabian StyleKang, Yang Jun. 2025. "Assessment of Continuous Flow-Dependent Red Cell Aggregation Using a Microfluidic Chip" Applied Sciences 15, no. 21: 11481. https://doi.org/10.3390/app152111481
APA StyleKang, Y. J. (2025). Assessment of Continuous Flow-Dependent Red Cell Aggregation Using a Microfluidic Chip. Applied Sciences, 15(21), 11481. https://doi.org/10.3390/app152111481






