Enhanced Performance of Inverted Perovskite Solar Cells Employing NiOx and Cu-Doped NiOx Nanoparticle Hole Transport Layers
Abstract
1. Introduction
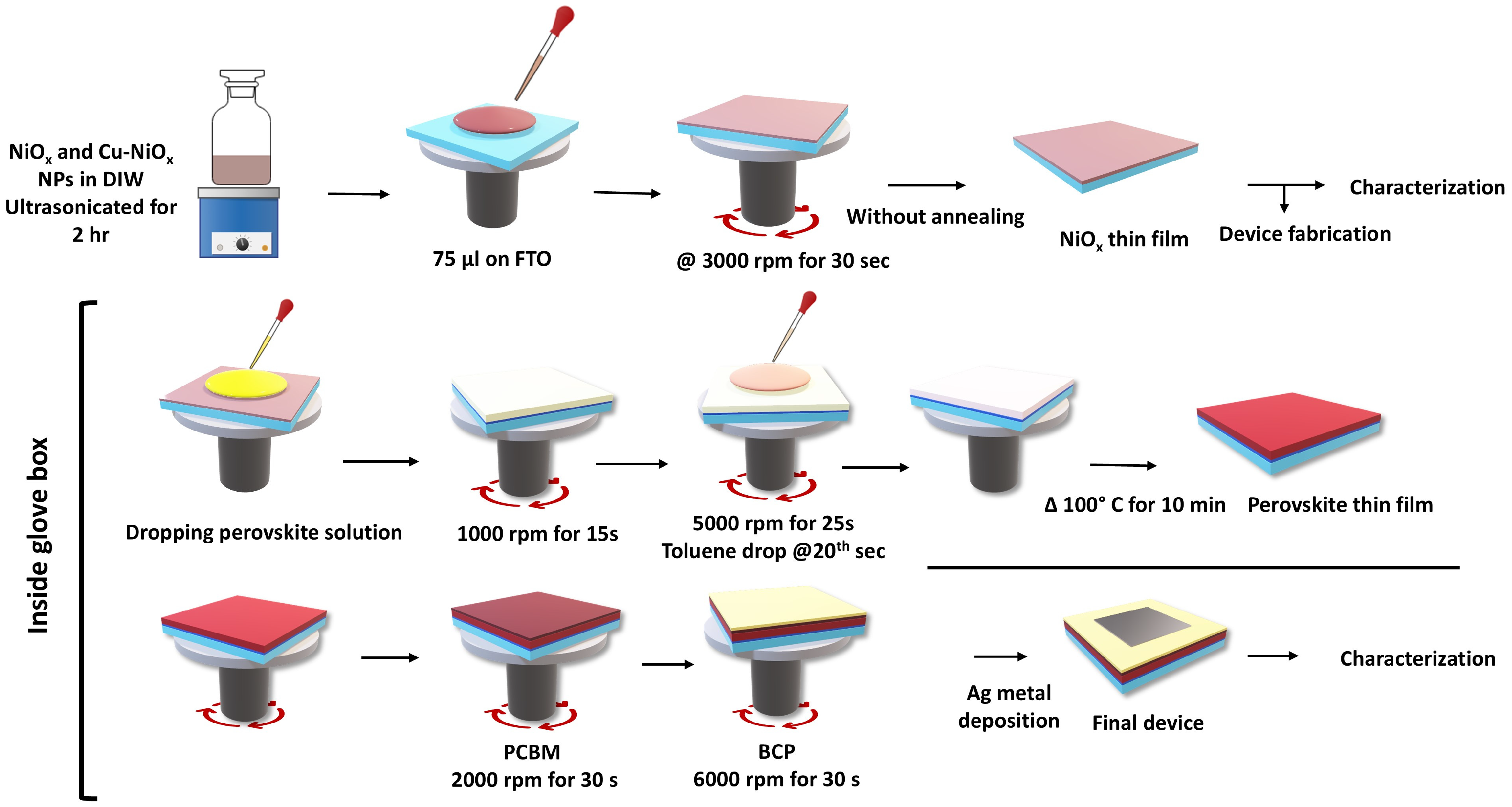
2. Experimental Details
2.1. Synthesis of NiO and Cu-Doped NiO NPs
2.2. PSC Device Fabrication
2.3. Characterization
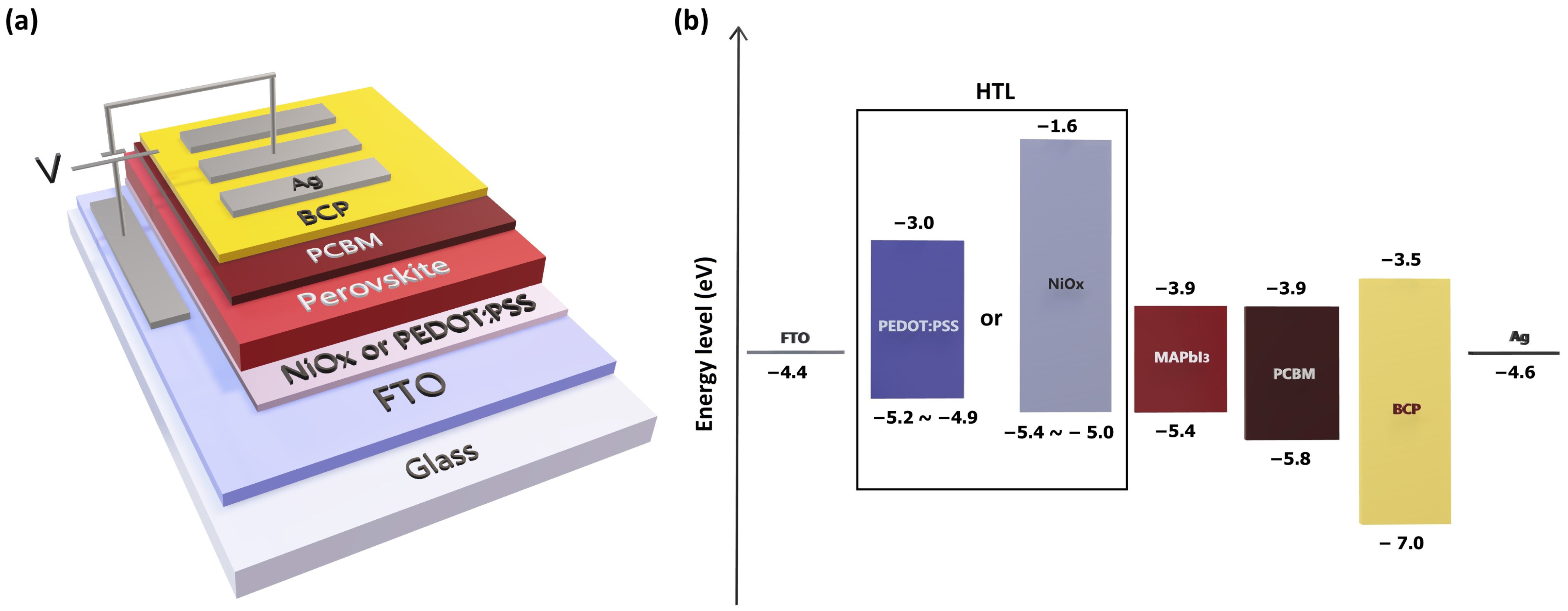
3. Results and Discussion
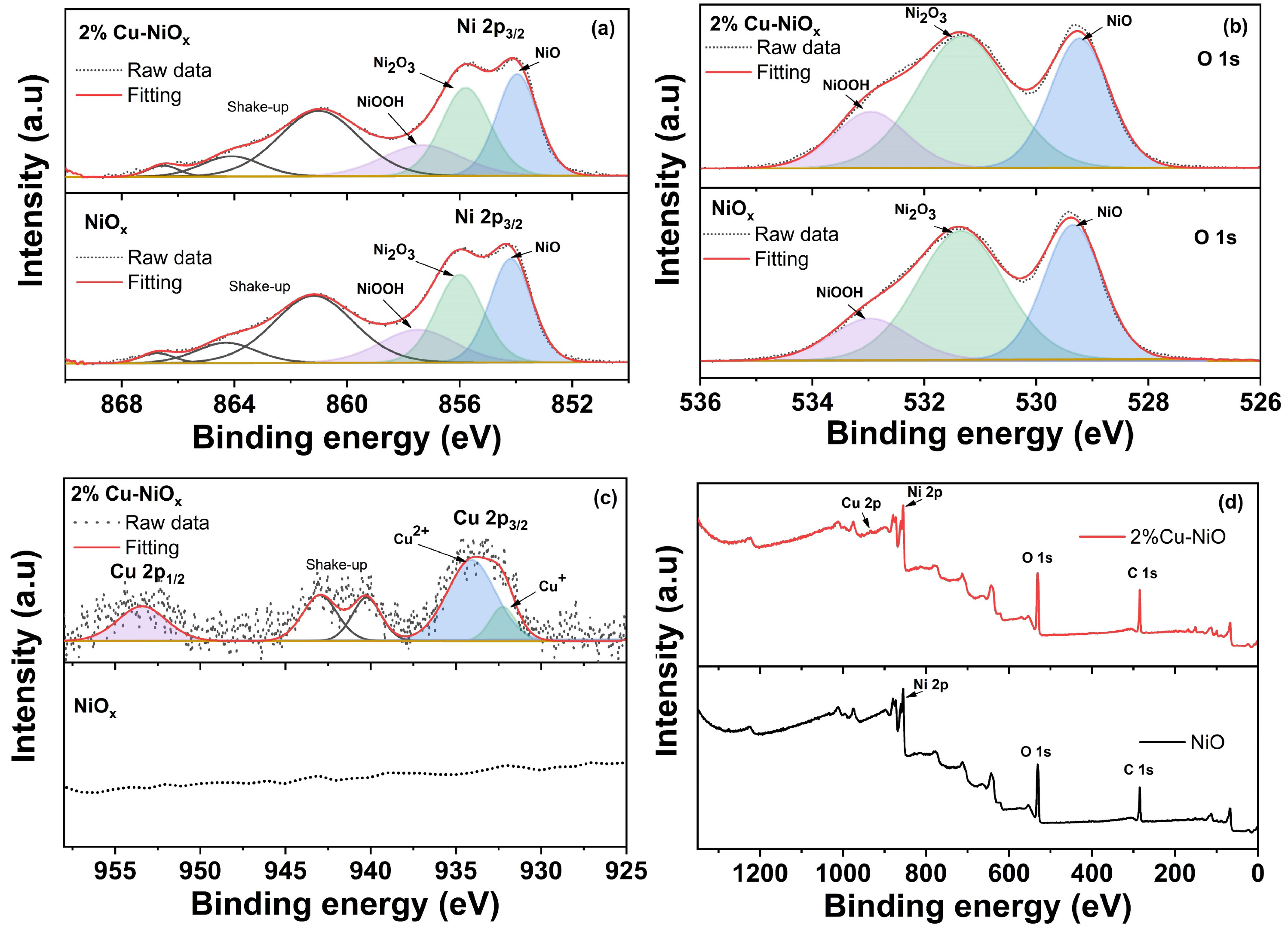
3.1. Chemical Reaction Mechanism of NiOx and Cu-NiOx NPs
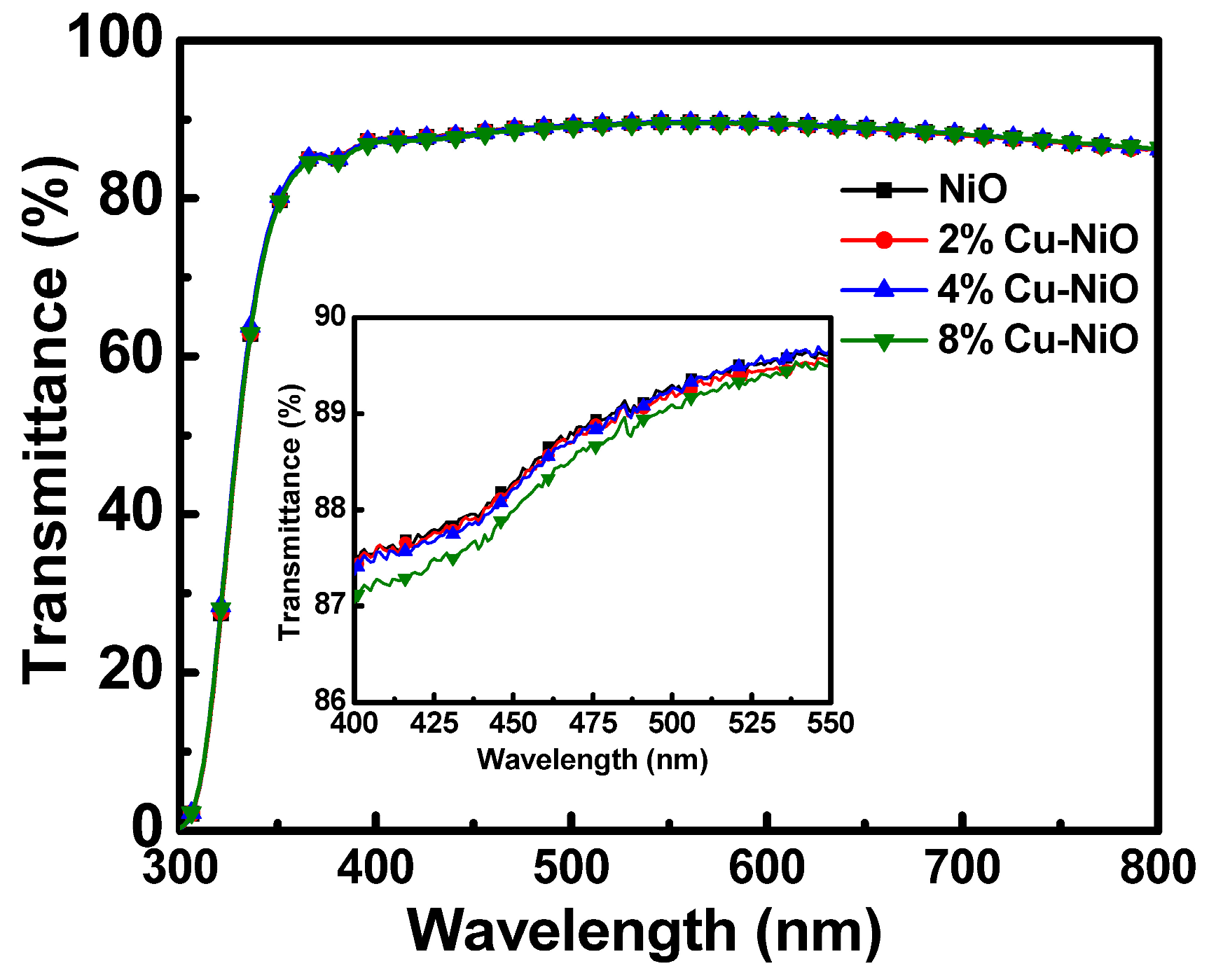
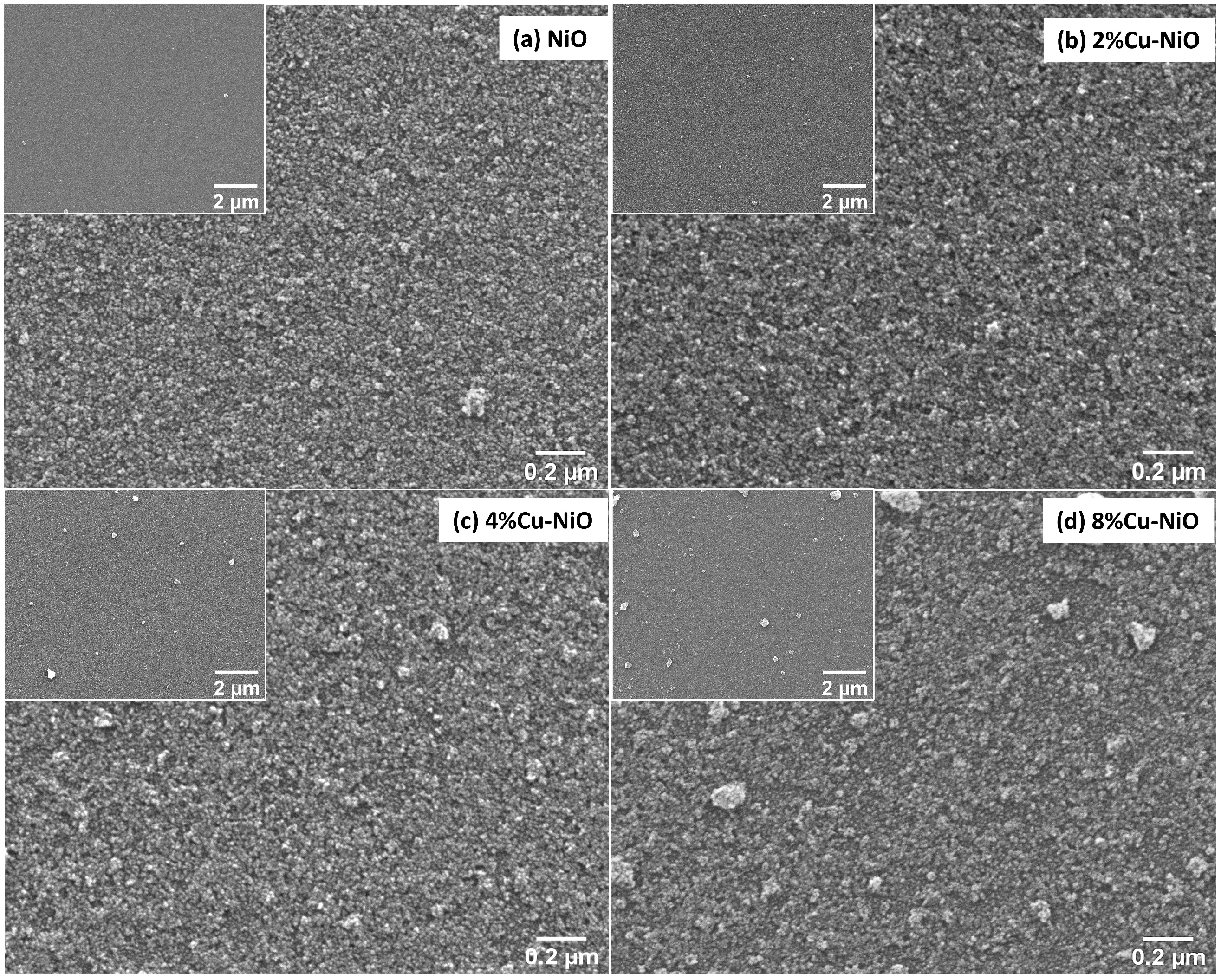
3.2. Characterization of the HTLs
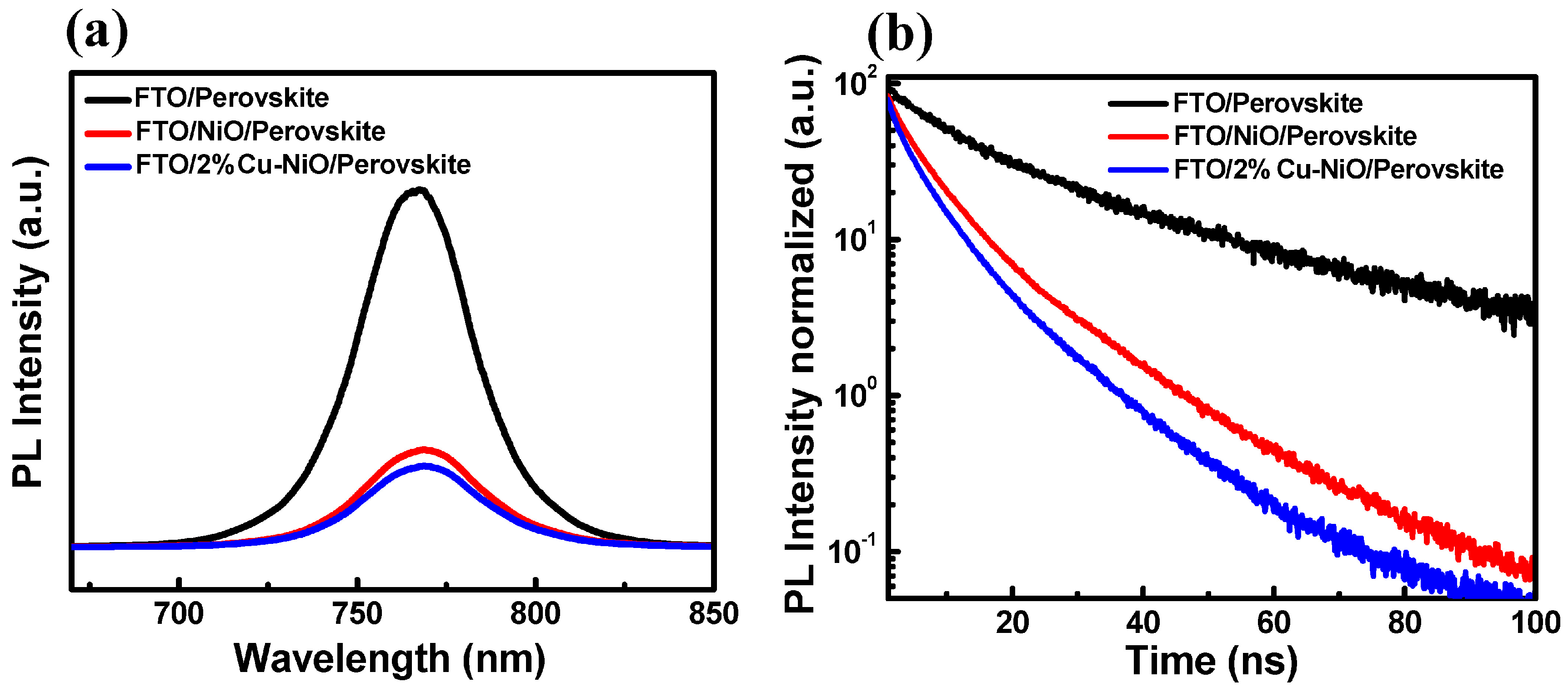
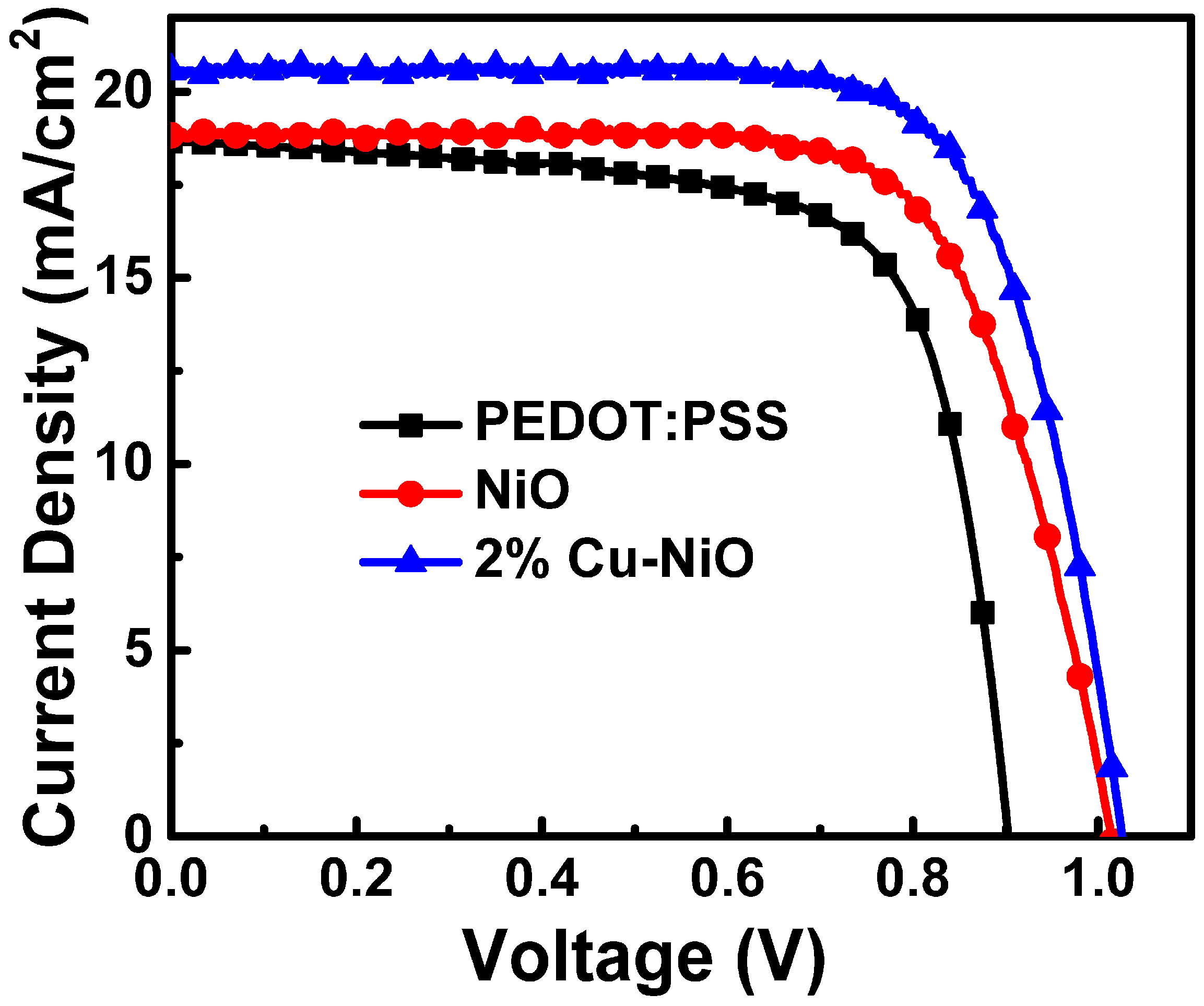
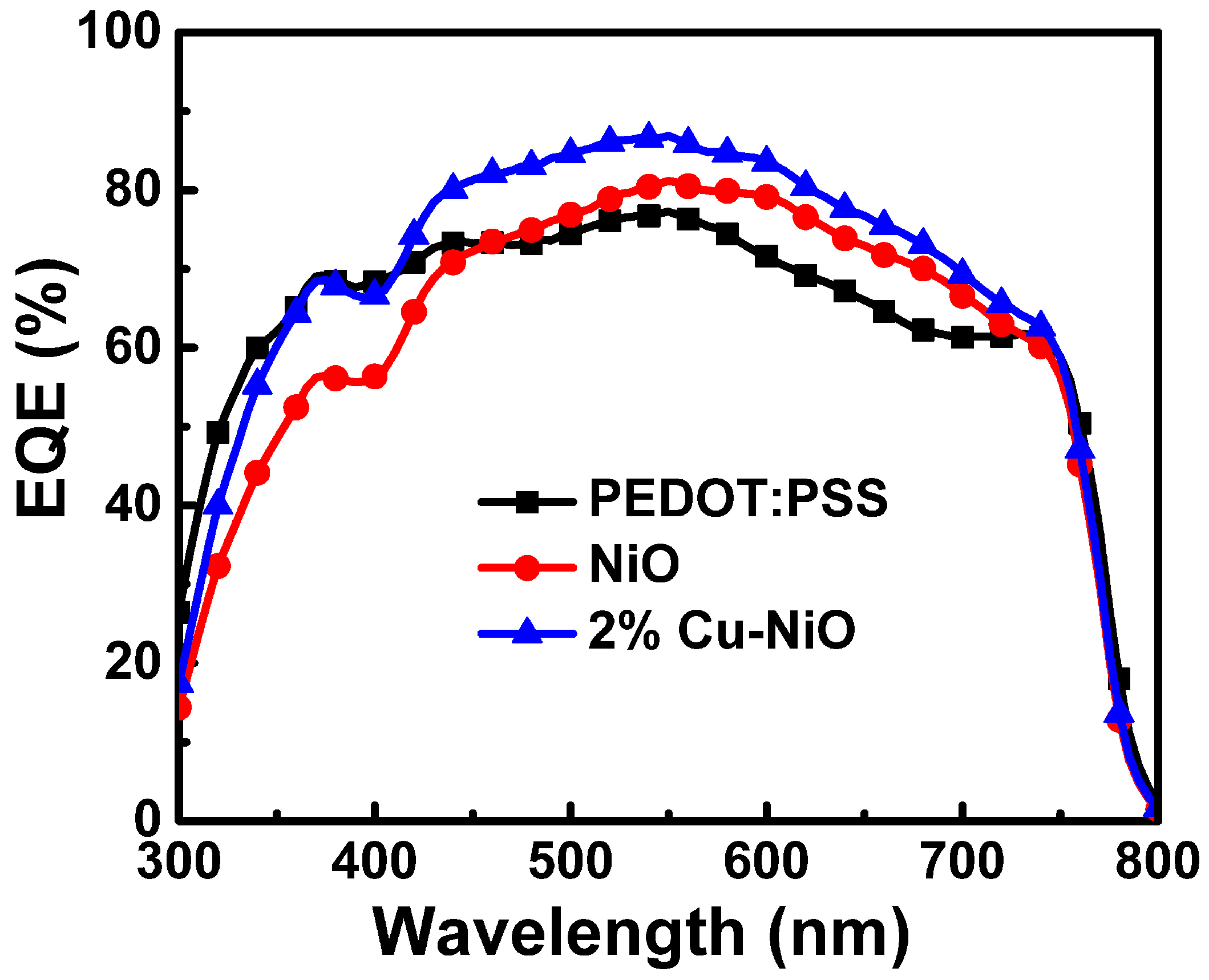
3.3. Characterization of the PSC Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masri, M.; B, G.K.; Hezam, A.; Alkanad, K.; Prashantha, K.; Manjunath, S.H.; Udayabhanu; Masri, F.; Qahtan, T.F.; Byrappa, K. Metal Halide Perovskite-Based Photocatalysts for Organic Pollutants Degradation: Advances, Challenges, and Future Directions. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133387. [Google Scholar] [CrossRef]
- Juhi, J.; Saski, M.; Kochaniec, M.K.; Wieczorek, W.; Dominko, R.; Lewiński, J. Exploring Metal Halide Perovskites as Active Architectures in Energy Storage Systems. J. Mater. Chem. A 2025. [Google Scholar] [CrossRef]
- Shooshtari, M.; Kim, S.-Y.; Pahlavan, S.; Rivera-Sierra, G.; Través, M.J.; Serrano-Gotarredona, T.; Bisquert, J.; Linares-Barranco, B. Advancing Logic Circuits with Halide Perovskite Memristors for Next-Generation Digital Systems. SmartMat 2025, 6, e70032. [Google Scholar] [CrossRef]
- Afre, R.A.; Pugliese, D. Perovskite Solar Cells: A Review of the Latest Advances in Materials, Fabrication Techniques, and Stability Enhancement Strategies. Micromachines 2024, 15, 192. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Mei, A.; Han, H. Applications of Metal Oxide Charge Transport Layers in Perovskite Solar Cells. Small Sci. 2023, 3, 2300020. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Wang, C.-Y.; Yang, S.-H. Exploration and Optimization of the Polymer-Modified NiOx Hole Transport Layer for Fabricating Inverted Perovskite Solar Cells. Nanomaterials 2024, 14, 1054. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Chen, K.C.; Chiang, T.Y.; Lin, P.Y.; Tsai, T.D.; Chang, Y.C.; Guo, T.F.; Chen, P.; Wen, T.C.; Hsu, Y.J. Nickel Oxide Electrode Interlayer in CH3NH3PbI3 Perovskite/PCBM Planar-Heterojunction Hybrid Solar Cells. Adv. Mater. 2014, 26, 4107–4113. [Google Scholar] [CrossRef]
- Alghamdi, A.R.M.; Yanagida, M.; Shirai, Y.; Andersson, G.G.; Miyano, K. Surface Passivation of Sputtered NiOx Using a SAM Interface Layer to Enhance the Performance of Perovskite Solar Cells. ACS Omega 2022, 7, 12147–12157. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, Y.; Wang, Y.; Feng, M.; Zhao, Y. Progress of Solution-Processed Metal Oxides as Charge Transport Layers towards Efficient and Stable Perovskite Solar Cells and Modules. Mater. Today Nano 2022, 20, 100252. [Google Scholar] [CrossRef]
- Aristizábal-Duarte, L.; González-Hernández, M.; Reyes, S.E.; Ramírez-Rincón, J.A.; Ortiz, P.; Cortés, M.T. Enhanced Charge Transport in Inverted Perovskite Solar Cells via Electrodeposited La-Modified NiOx Layers. Energies 2025, 18, 3590. [Google Scholar] [CrossRef]
- Yuk, D.; Roe, J.; Lee, Y.; Kim, J.; Seo, J.; Yeop, J.; Song, T.; Kim, J.Y. Improving the Optoelectrical Properties of a Nickel Oxide Hole Transport Layer by Hydrogen Peroxide Treatment for Efficient Organic Solar Cells. ACS Appl. Energy Mater. 2024, 7, 4558–4564. [Google Scholar] [CrossRef]
- Tang, G.; Chen, L.; Cao, X.; Wang, Y.; Zhang, H.; Tai, Q. A Review on Recent Advances in Flexible Perovskite Solar Cells. Sol. RRL 2025, 9, 2400844. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Wu, Y.-W.; Yang, S.-H.; Abdulhalim, I. Preparation of Nickel Oxide Nanoflakes for Carrier Extraction and Transport in Perovskite Solar Cells. Nanomaterials 2022, 12, 3336. [Google Scholar] [CrossRef] [PubMed]
- Reyes, S.E.; González-Hernández, M.; Cortés, M.T.; Ortiz, P. Effect of NH4F Surface Treatment of Electrodeposited NiOx on the Properties and Reproducibility of Inverted Perovskite Solar Cells. Surf. Interfaces 2025, 56, 105654. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, X.; Zhong, X.; Lu, C.; Lu, Y.; Li, X.; Tu, S. Construction of Novel D-A Type Self-Assembled Molecules Together with NiOx for Flexible Perovskite Solar Cells. Tetrahedron 2025, 176, 134541. [Google Scholar] [CrossRef]
- Noman, M.; Khan, Z.; Jan, S.T. A Comprehensive Review on the Advancements and Challenges in Perovskite Solar Cell Technology. RSC Adv. 2024, 14, 5085–5131. [Google Scholar] [CrossRef]
- Tutundzic, M.; Zhang, X.; Lammar, S.; Singh, S.; Marchezi, P.; Merckx, T.; Aguirre, A.; Moons, E.; Aernouts, T.; Kuang, Y.; et al. Toward Efficient and Fully Scalable Sputtered NiOx-Based Inverted Perovskite Solar Modules via Co-Ordinated Modification Strategies. Sol. RRL 2023, 8, 2300862. [Google Scholar] [CrossRef]
- Kim, J.W.; Cho, E.; Lee, H.; Kwon, S.; Park, J.; Kim, M.; Kim, D.; Na, S.; Lee, S. Enhancing Efficiency of Inverted Perovskite Solar Cells by Sputtered Nickel Oxide Hole Transport Layers. Sol. RRL 2023, 8, 2300933. [Google Scholar] [CrossRef]
- Yadav, K.; Samanta, A.; Ankita; Tarun; Das, S.; Kanjilal, A.; Pandey, U.K. Tailoring Sputter Grown NiOx Layer by Synergistic Surface Passivation for Efficient Perovskite Solar Cells. Sol. Energy 2025, 288, 113286. [Google Scholar] [CrossRef]
- Feng, M.; Wang, Y.; Liu, F.; Ren, M.; Wang, H.; Guo, J.; Chen, Y.; Miao, Y.; Zhao, Y. Rational Buried Interface Engineering of Inorganic NiOx Layer toward Efficient Inverted Perovskite Solar Cells. Sol. RRL 2023, 8, 2300712. [Google Scholar] [CrossRef]
- Lu, X.; Fan, X.; Zhang, H.; Xu, Q.; Ijaz, M. Review on Preparation of Perovskite Solar Cells by Pulsed Laser Deposition. Inorganics 2024, 12, 128. [Google Scholar] [CrossRef]
- Akalin, S.A.; Erol, M.; Uzunbayir, B.; Oguzlar, S.; Yildirim, S.; Choi, F.P.G.; Gunes, S.; Menda, U.D.Y.; Mendes, M.J. Physically-Deposited Hole Transporters in Perovskite PV: NiOx Improved with Li/Mg Doping. Adv. Mater. Technol. 2024, 9, 2301760. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, K. Rapid Advances Enabling High-Performance Inverted Perovskite Solar Cells. Nat. Rev. Mater. 2024, 9, 399–419. [Google Scholar] [CrossRef]
- Mann, D.S.; Kwon, S.-N.; Patil, P.; Na, S.-I. Revivification of Nickel Oxide-Perovskite Interfaces via Nickel Nitrate to Boost Performance in Perovskite Solar Cells. Nano Energy 2022, 106, 108062. [Google Scholar] [CrossRef]
- Song, D.; Shin, S.W.; Wu, H.-P.; Diau, E.W.-G.; Correa-Baena, J.P. Toward Maximizing Hole Selection with Self-Assembled Monolayers in Sn-Based Perovskite Solar Cells. ACS Energy Lett. 2025, 10, 1292–1312. [Google Scholar] [CrossRef]
- Soopy, A.K.K.; Parida, B.; Aravindh, S.A.; Al Ghaithi, A.O.; Qamhieh, N.; Amrane, N.; Benkraouda, M.; Liu, S.; Najar, A. Towards High Performance: Solution-Processed Perovskite Solar Cells with Cu-Doped CH3NH3PbI3. Nanomaterials 2024, 14, 172. [Google Scholar] [CrossRef]
- Verma, A.P.; Yadav, P.; Nalwa, K.S.; Kumar, M. Interface Engineering and Band Alignment Studies of Cu Doped NiO as a Hole Transport Layer for Triple Cationic Perovskite Solar Cells. Small 2025, 21, 2504237. [Google Scholar] [CrossRef]
- Jin, Y.; Feng, H.; Li, Y.; Zhang, H.; Chen, X.; Zhong, Y.; Zeng, Q.; Huang, J.; Weng, Y.; Yang, J.; et al. Recrystallizing Sputtered NiOx for Improved Hole Extraction in Perovskite/Silicon Tandem Solar Cells. Adv. Energy Mater. 2024, 15, 2403911. [Google Scholar] [CrossRef]
- Yu, S.; Xiong, Z.; Zhou, H.; Zhang, Q.; Wang, Z.; Ma, F.; Qu, Z.; Zhao, Y.; Chu, X.; Zhang, X.; et al. Homogenized NiOx Nanoparticles for Improved Hole Transport in Inverted Perovskite Solar Cells. Science 2023, 382, 1399–1404. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Shen, H.; Wang, L.; Zhao, Q.; Zheng, J.; Li, H.; Xie, Z.; Chen, A.; Miao, S.; et al. Highly Stable Inverted Perovskite Solar Cells with All-Inorganic Selective Contact Using Iron-Doped Zinc Oxide. J. Chem. Phys. 2025, 162, 094703. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Z.; Sun, J.; Liu, F.; Chen, J.; Ding, L.; Chen, C. Perovskite Solar Cells with NiOx Hole-Transport Layer. J. Semicond. 2023, 44, 100201. [Google Scholar] [CrossRef]
- Valenzuela-López, J.Y.; Millán-Franco, M.A.; Suárez-Campos, G.; Camacho-Cáceres, J.; Rodríguez-Castañeda, C.A.; Moreno-Romero, P.M.; Torres-Herrera, D.M.; Sotelo-Lerma, M.; Hu, H. Influence of Nickel Precursor Solutions on Nickel Oxide Thin Film Formation and Photovoltaic Properties of Air-Processed Inverted Perovskite Solar Cells. Opt. Mater. 2024, 149, 115082. [Google Scholar] [CrossRef]
- Madani, S.; Tesfamichael, T.; Wang, H.; Motta, N. Study of Pb-Based and Pb-Free Perovskite Solar Cells Using Cu-Doped Ni1-XO Thin Films as Hole Transport Material. Ceram. Int. 2022, 48, 15207–15217. [Google Scholar] [CrossRef]
- Liu, S.; Biju, V.P.; Qi, Y.; Chen, W.; Liu, Z. Recent Progress in the Development of High-Efficiency Inverted Perovskite Solar Cells. NPG Asia Mater. 2023, 15, 27. [Google Scholar] [CrossRef]
- Luo, X.; Liu, X.; Lin, X.; Wu, T.; Wang, Y.; Han, Q.; Wu, Y.; Segawa, H.; Han, L. Recent Advances of Inverted Perovskite Solar Cells. ACS Energy Lett. 2024, 9, 1487–1506. [Google Scholar] [CrossRef]
- Andrade, P.H.M.; Volkringer, C.; Loiseau, T.; Tejeda, A.; Hureau, M.; Moissette, A. Band Gap Analysis in MOF Materials: Distinguishing Direct and Indirect Transitions Using UV–Vis Spectroscopy. Appl. Mater. Today 2024, 37, 102094. [Google Scholar] [CrossRef]
- Barkaoui, S.; Wang, Y.; Zhang, Y.; Gu, X.; Li, Z.; Wang, B.; Baiker, A.; Li, G.; Zhao, Z. Critical Role of NiO Support Morphology for High Activity of Au/NiO Nanocatalysts in CO Oxidation. iScience 2024, 27, 110255. [Google Scholar] [CrossRef]
- de Souza Caldas, L.; Prieto, M.J.; Tănase, L.C.; Tiwari, A.; Schmidt, T.; Cuenya, B.R. Correlative in Situ Spectro-Microscopy of Supported Single CuO Nanoparticles: Unveiling the Relationships between Morphology and Chemical State during Thermal Reduction. ACS Nano 2024, 18, 13714–13725. [Google Scholar] [CrossRef]
- Baraneedharan, P.; Sekar, S.; Murugesan, S.; Ahamada, D.; Mohamed, S.A.B.; Lee, Y.; Lee, S. Recent Advances and Remaining Challenges in Perovskite Solar Cell Components for Innovative Photovoltaics. Nanomaterials 2024, 14, 1867. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, H.; Zhang, J.; Zhang, J.; Lu, L.; Zhu, X.; Li, D. NiOx Thickness Dependent Improvement of NiOx/Perovskite Interface for Inverted Planar Perovskite Solar Cells. Opt. Mater. 2022, 132, 112774. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, Y.; Li, S.; Jia, X.; Luo, D.; Zhang, W.; Snaith, H.J.; Gong, Q.; Zhu, R. The Promise and Challenges of Inverted Perovskite Solar Cells. Chem. Rev. 2024, 124, 10623–10700. [Google Scholar] [CrossRef]
- Ye, Z.; Xia, J.; Zhang, D.; Duan, X.; Xing, Z.; Jin, G.; Cai, Y.; Xing, G.; Chen, J.; Ma, D. Efficient Quasi-2D Perovskite Light-Emitting Diodes Enabled by Regulating Phase Distribution with a Fluorinated Organic Cation. Nanomaterials 2022, 12, 3495. [Google Scholar] [CrossRef]
- Miah, M.H.; Khandaker, M.U.; Aminul Islam, M.; Nur-E-Alam, M.; Osman, H.; Ullah, M.H. Perovskite Materials in X-Ray Detection and Imaging: Recent Progress, Challenges, and Future Prospects. RSC Adv. 2024, 14, 6656–6698. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Fan, J.; Djurišić, A.B.; Liu, F.; Tam, H.W.; Ng, A.; Surya, C.; Chan, W.K.; Wang, D.; et al. Understanding the Doping Effect on NiO: Toward High-Performance Inverted Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703519. [Google Scholar] [CrossRef]
- Nyiekaa, E.A.; Aika, T.A.; Orukpe, P.E.; Akhabue, C.E.; Danladi, E. Development on Inverted Perovskite Solar Cells: A Review. Heliyon 2024, 10, e24689. [Google Scholar] [CrossRef] [PubMed]
- Diethelm, M.; Lukas, T.; Smith, J.; Dasgupta, A.; Caprioglio, P.; Futscher, M.; Hany, R.; Snaith, H.J. Probing Ionic Conductivity and Electric Field Screening in Perovskite Solar Cells: A Novel Exploration through Ion Drift Currents. Energy Environ. Sci. 2025, 18, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Aliyaselvam, O.V.; Mustafa, A.N.; Azam, M.A.; Chelvanathan, P.; Islam, M.A.; Mahalingam, S.; Arith, F. Unveiling the Prospect of Copper Iodide as Hole Transporting Layer in Perovskite Solar Cell by Selective Dopant Strategy: A Review. Mater. Sci. Semicond. Process. 2025, 197, 109679. [Google Scholar] [CrossRef]
- Chin, Y.-C.; Daboczi, M.; Henderson, C.; Luke, J.; Kim, J.-S. Suppressing PEDOT:PSS Doping-Induced Interfacial Recombination Loss in Perovskite Solar Cells. ACS Energy Lett. 2022, 7, 560–568. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, W.; Chen, Y.; Zhao, H.; Wei, Y.; Zhou, R.; Wan, H.; Deng, J.; Fan, D.; Luo, Y.; et al. Multifunctional Amine Salt Additive for Enhancing the Performance of Perovskite and Perovskite/Silicon Tandem Solar Cells. Chem. Eng. J. 2025, 515, 163967. [Google Scholar] [CrossRef]
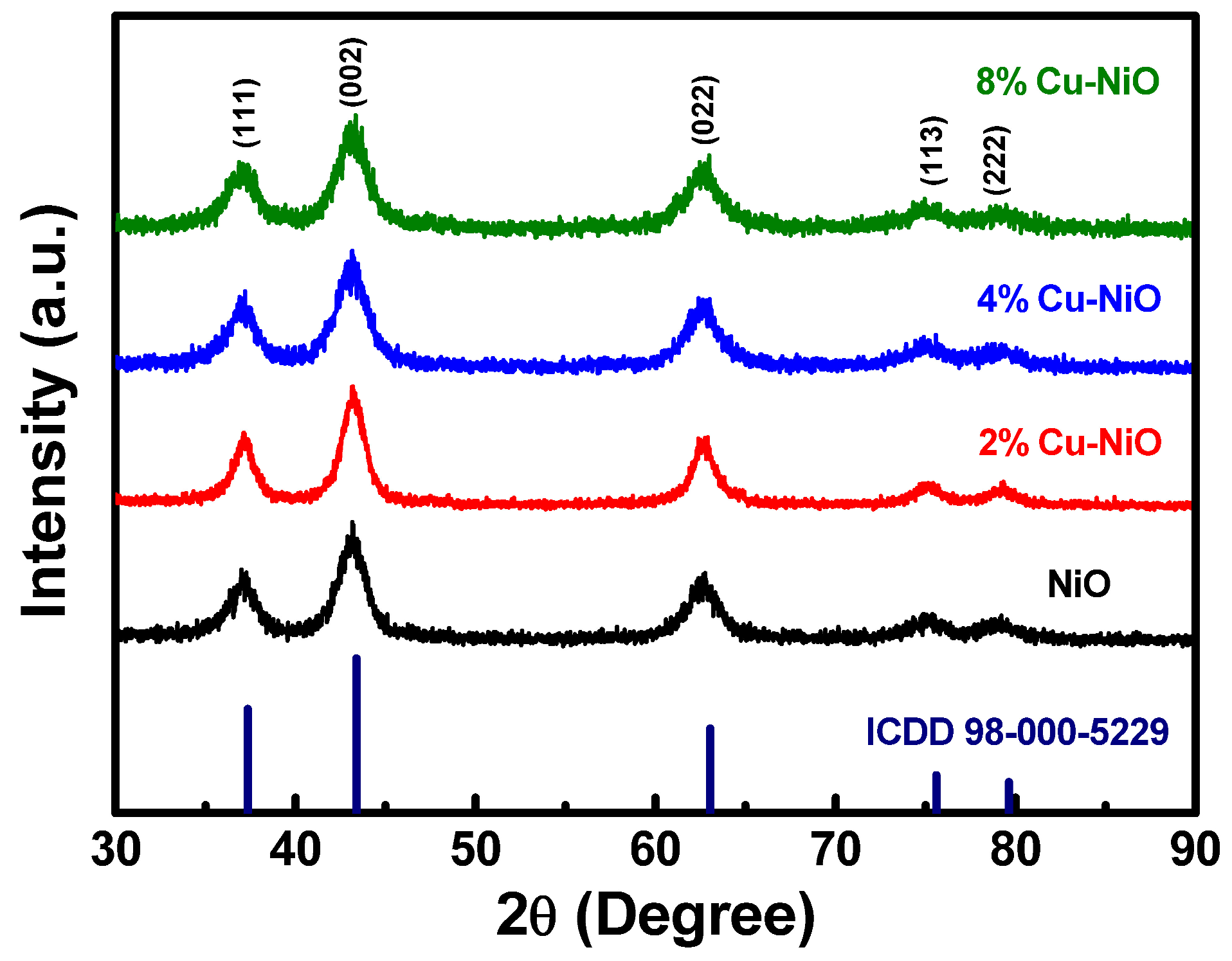



| Sample | 2θ | (hkl) | Interplanar Distance (Å) | FWHM (β) (radians) | Crystallite Size (D) (nm) | Dislocation Density (1/D2) (×1016) | Microstrain (β/4tanθ) | Lattice Parameter (a) |
|---|---|---|---|---|---|---|---|---|
| NiOx | 37 | (111) | 2.43 | 0.03 | 4.94 | 4.12 | 1.27 | 4.21 |
| 43.09 | (002) | 2.1 | 0.04 | 4.73 | 4.49 | 1.15 | 4.2 | |
| 62.54 | (022) | 1.49 | 0.04 | 4.52 | 4.92 | 0.85 | 4.21 | |
| 2% Cu-NiOx | 37.09 | (111) | 2.43 | 0.03 | 6.51 | 2.37 | 0.96 | 4.2 |
| 43.16 | (002) | 2.1 | 0.03 | 5.67 | 3.13 | 0.96 | 4.2 | |
| 62.59 | (022) | 1.49 | 0.03 | 6.25 | 2.57 | 0.62 | 4.2 | |
| 4% Cu-NiOx | 36.96 | (111) | 2.44 | 0.03 | 4.91 | 4.17 | 1.28 | 4.22 |
| 43.07 | (002) | 2.11 | 0.04 | 4.21 | 5.65 | 1.29 | 4.21 | |
| 62.55 | (022) | 1.49 | 0.04 | 4.06 | 6.07 | 0.95 | 4.2 | |
| 8% Cu-NiOx | 36.96 | (111) | 2.44 | 0.04 | 4.53 | 4.88 | 1.39 | 4.22 |
| 43.11 | (002) | 2.1 | 0.04 | 4.28 | 5.49 | 1.27 | 4.2 | |
| 62.57 | (022) | 1.49 | 0.05 | 3.98 | 6.34 | 0.97 | 4.2 | |
| ICDD 98-000-5229 | 37.25 | (111) | 2.42 | |||||
| 43.28 | (002) | 2.09 | ||||||
| 62.86 | (022) | 1.48 |
| Sample | A1 (%) | τ1 (ns) | A2 (%) | τ2 (ns) | τavg (ns) |
|---|---|---|---|---|---|
| FTO/PSK | 61.8 | 9.6 | 38.2 | 37.6 | 29.4 |
| FTO/NiOx/PSK | 64.9 | 3.3 | 35.1 | 11.4 | 8.6 |
| FTO/2% Cu-NiOx/PSK | 55.9 | 2.2 | 44.1 | 8.6 | 7.0 |
| HTL | VOC (V) | JSC (mA/cm2) | Fill Factor | PCE (%) | |
|---|---|---|---|---|---|
| PEDOT:PSS | Average | 0.900 ± 0.020 | 17.919 ± 0.863 | 0.697 ± 0.015 | 11.239 ± 0.464 |
| Best | 0.903 | 18.66 | 0.71 | 11.93 | |
| NiO | Average | 1.015 ± 0.019 | 19.014 ± 0.456 | 0.702 ± 0.011 | 13.539 ± 0.239 |
| Best | 1.015 | 18.83 | 0.71 | 13.72 | |
| 2% Cu-NiO | Average | 1.028 ± 0.003 | 20.172 ± 0.273 | 0.744 ± 0.005 | 15.417 ± 0.118 |
| Best | 1.026 | 20.57 | 0.74 | 15.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiruchelvan, P.S.; Lai, C.-C.; Tsai, C.-H. Enhanced Performance of Inverted Perovskite Solar Cells Employing NiOx and Cu-Doped NiOx Nanoparticle Hole Transport Layers. Appl. Sci. 2025, 15, 11449. https://doi.org/10.3390/app152111449
Thiruchelvan PS, Lai C-C, Tsai C-H. Enhanced Performance of Inverted Perovskite Solar Cells Employing NiOx and Cu-Doped NiOx Nanoparticle Hole Transport Layers. Applied Sciences. 2025; 15(21):11449. https://doi.org/10.3390/app152111449
Chicago/Turabian StyleThiruchelvan, Ponmudi Selvan, Chien-Chih Lai, and Chih-Hung Tsai. 2025. "Enhanced Performance of Inverted Perovskite Solar Cells Employing NiOx and Cu-Doped NiOx Nanoparticle Hole Transport Layers" Applied Sciences 15, no. 21: 11449. https://doi.org/10.3390/app152111449
APA StyleThiruchelvan, P. S., Lai, C.-C., & Tsai, C.-H. (2025). Enhanced Performance of Inverted Perovskite Solar Cells Employing NiOx and Cu-Doped NiOx Nanoparticle Hole Transport Layers. Applied Sciences, 15(21), 11449. https://doi.org/10.3390/app152111449







