Regenerative Applications and Performance of Periodontal Ligament Stem Cells: A Comprehensive Review of In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Search Strategy
2.3. Eligibility Criteria
- In vivo studies (animal or human) transplanting PDLSCs alone or compared to other oral cavity-derived stem cells.
- Analyses focused exclusively on PDLSC transplantation effects on periodontal tissues, particularly bone/soft tissue defects mimicking periodontitis.
- Studies using xenografts, autografts, or allografts that assessed regeneration of at least one key periodontal tissue (alveolar bone, periodontal ligament, cementum).
- Articles published in English or French.
- Studies combining PDLSC with non-oral stem cells in the same culture.
- Use of gene therapy with PDLSC transplantation.
- Use of PDLSC-derived exosomes.
2.4. Study Selection and Data Extraction
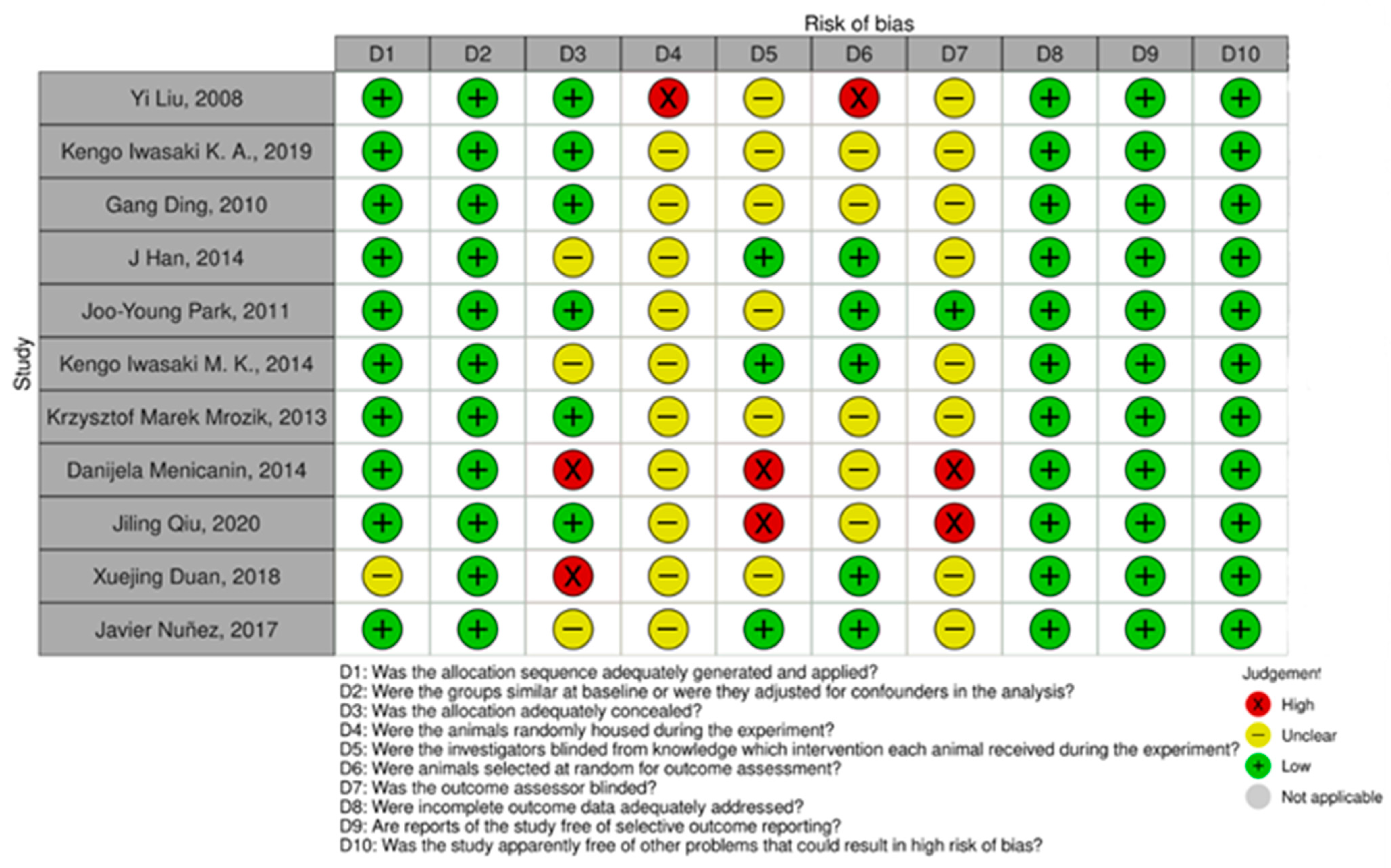
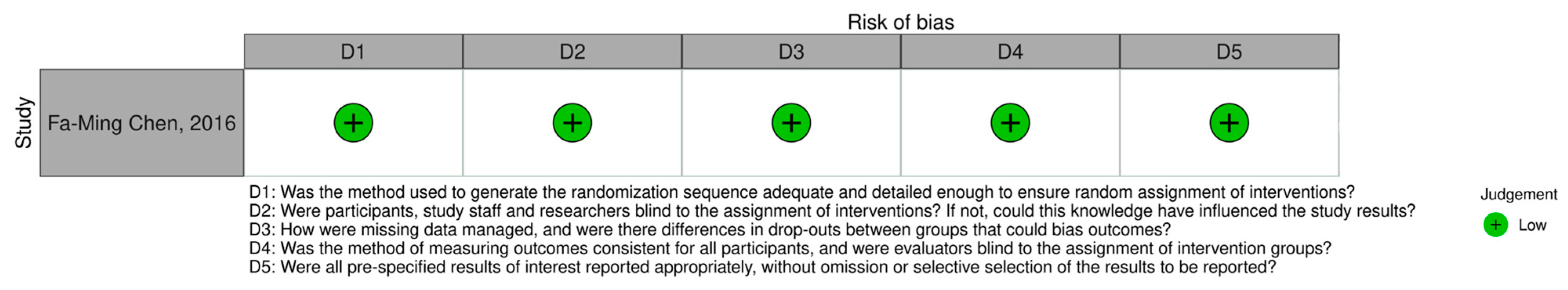
2.5. Risk of Bias Assessment
3. Results
3.1. Study Selection
3.2. Synthesis of Results
3.3. Risk of Bias Assessment
4. Discussion
4.1. Autologous PDLSC Transplantation
4.2. Allogeneic PDLSC Transplantation
4.3. Xenogeneic PDLSC Transplantation and Conditioned Media
4.4. Mechanistic Considerations
4.5. Methodological Limitations
4.6. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| a-PDLSC | Alveolar socket-derived periodontal ligament stem cell subtype |
| AL | Attachment loss; clinical measurement of periodontal tissue support |
| BMP | Bone morphogenetic protein; growth factor promoting osteogenic differentiation |
| CM | Conditioned medium; culture medium enriched with cell-secreted bioactive factors |
| Cochrane RoB 2 | Cochrane Risk of Bias 2 tool for human randomised studies |
| DPSC | Dental pulp stem cell |
| DFSC | Dental follicle stem cell |
| EGF | Epidermal growth factor; regulator of cell proliferation and wound healing |
| FGF | Fibroblast growth factor; family of proteins stimulating angiogenesis and tissue repair |
| GF-CM | Growth factor-conditioned medium |
| GMSC-CM | Gingival mesenchymal stem cell-conditioned medium |
| GMP | Good Manufacturing Practice; regulatory standard ensuring product quality and safety |
| HA/TCP | Hydroxyapatite/tricalcium phosphate; bioceramic scaffold for bone regeneration |
| HGF | Hepatocyte growth factor; cytokine involved in tissue regeneration and angiogenesis |
| IDO | Indoleamine 2,3-dioxygenase; enzyme mediating immunomodulatory effects |
| MEM | Minimum essential medium; basal nutrient medium for cell culture |
| MSC | Mesenchymal stem cell; multipotent stromal cell capable of differentiating into mesenchymal tissues |
| PAFSC | Periodontal alveolar follicle stem cell |
| PD | Probing depth; distance from gingival margin to base of periodontal pocket |
| PDGF | Platelet-derived growth factor; growth factor stimulating fibroblast and osteoblast activity |
| PDLC | Periodontal ligament cell (non-stem) |
| PDLSC | Periodontal ligament stem cell |
| PEG | Polyethylene glycol; synthetic polymer used in scaffold formulations |
| PGE2 | Prostaglandin E2; lipid mediator with anti-inflammatory and immunoregulatory roles |
| PICO | Population, Intervention, Comparison, Outcome; framework for clinical question design |
| PLGA | Poly(lactic-co-glycolic acid); biodegradable copolymer used for scaffold fabrication |
| PRF | Platelet-Rich Fibrin; autologous fibrin matrix enriched in platelets and growth factors |
| r-PDLSC | Root surface-derived periodontal ligament stem cell subtype |
| RCT | Randomised controlled trial |
| RoB 2 | Risk of Bias 2; tool for assessing methodological quality of randomised trials |
| SHED | Stem cells from human exfoliated deciduous teeth |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation; protocol for assessing bias in animal studies |
| TGF-β | Transforming growth factor-beta; cytokine promoting tissue repair and cell differentiation |
References
- Cui, T.L. Epidemiological and sociodemographic transitions of severe periodontitis incidence, prevalence, and disability-adjusted life years for 21 world regions and globally from 1990 to 2019: An age–period–cohort analysis. J. Periodontol. 2023, 94, 193–203. [Google Scholar] [CrossRef]
- Bouchard, P. Parodontologie et Dentisterie Implantaire; Quintessence International: Paris, France, 2015. [Google Scholar]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A multifaceted disease of tooth-supporting tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S.; et al. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef] [PubMed]
- Myon, L.F. Ingénierie du tissu osseux oro-maxillofacial par combinaison de biomatériaux, cellules souches, thérapie génique. Rev. Stomatol. Chir. Maxillofac. 2011, 112, 201–211. [Google Scholar] [CrossRef]
- Dissanayaka, W.A.; Zhang, C. Scaffold-based and scaffold-free strategies in dental pulp regeneration. J. Endod. 2020, 46, S81–S89. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Jin, Y. Periodontal tissue engineering and regeneration: Current approaches and expanding opportunities. Tissue Eng. Part B Rev. 2010, 16, 219–255. [Google Scholar] [CrossRef]
- Tsuruga, E.; Takita, H.; Itoh, H.; Miyauchi, M.; Mori, K.; Nakamura, T. Pore size of porous hydroxyapatite as a cell substrate controlling BMP-induced osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, C.A. Silk fibroin as a functional biomaterial for tissue engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Ruzaidi, D.A.; Jalaludin, M.A.; Zainal, Z. Electrically conductive scaffolds with low immunogenicity for biomedical applications: A review. Polymers 2021, 13, 3395. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Mondrinos, M.J.; Griffith, L.G.; Lee, P.C. Porogen-based solid freeform fabrication of polycaprolactone–calcium phosphate scaffolds. Biomaterials 2006, 27, 4399–4408. [Google Scholar] [CrossRef]
- Shimoaka, T.; Ohba, S.; Kawaguchi, H. Regulation of osteoblast, chondrocyte, and osteoclast functions by fibroblast growth factor-18 in comparison to FGF-2 and FGF-10. J. Biol. Chem. 2002, 277, 7493–7500. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- González, L.; Delgado, G.; Martínez, J. GH modulates hepatic epidermal growth factor signaling in the mouse. J. Endocrinol. 2009, 204, 299–309. [Google Scholar] [CrossRef]
- Martínez, G.Z.; Urrutia, D.C.; González, L. Smad3 regulates inflammatory and regulatory T cell differentiation. J. Biol. Chem. 2009, 284, 35283–35286. [Google Scholar] [CrossRef]
- Ross, R.; Bowen-Pope, D.F. Platelet-derived growth factor: Structure, function and role in disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1990, 327, 155–169. [Google Scholar] [CrossRef]
- Catros, S.; Guillemot, F.; Fricain, J.C. Bone tissue engineering in oral and maxillofacial surgery: Clinical applications. Med. Buccale Chir. Buccale 2010, 16, 227–237. [Google Scholar] [CrossRef]
- Biehl, J.K.; Russell, B. Introduction to stem cell therapy. J. Cardiovasc. Nurs. 2009, 24, 98–103. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Yao, S.; Huang, G.T.-J.; Shi, S.; Bartold, P.; Wang, C.-Y.; Shi, S. BMP-6 expression in dental follicle stem cells and its effect on osteogenic differentiation. Cells Tissues Organs 2013, 198, 438–447. [Google Scholar] [CrossRef]
- Dimassi, S.; Roussel, J.; Gauthier, P. Dental stem cells: Hope in neuroregenerative medicine? Ann. Biol. Clin. 2020, 78, 593–603. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wang, J.; Wang, C.-Y.; Shi, S.; Shi, S. Repair of human periodontal bone defects using autologous stem cells from inflammatory dental pulp. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Alencar, A.H.; Kitten, G.T.; Sousa-Neto, M.D. Mesenchymal stem cells in the dental tissues: A review. Braz. Dent. J. 2011, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.; Hynes, K.; Gronthos, S.; Bartold, P.M. Periodontal ligament-derived cells for periodontal regeneration in animal models: A systematic review. J. Periodontal Res. 2015, 50, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, R.P.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Ding, G.; Fang, D.; Zhang, C.; Bartold, P.M.; Gronthos, S.; Shi, S.; Wang, S. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 2008, 26, 1065–1071. [Google Scholar] [CrossRef]
- Iwasaki, K.; Akazawa, K.; Nagata, M.; Komaki, M.; Honda, I.; Morioka, C.; Yokoyama, N.; Ayame, H.; Yamaki, K.; Tanaka, Y.; et al. Fate of transplanted periodontal ligament stem cells in surgically created periodontal defects in rats. Int. J. Mol. Sci. 2019, 20, 192. [Google Scholar] [CrossRef]
- Ding, G.; Liu, Y.; Wang, W.; Wei, F.; Liu, D.; Fan, Z.; An, Y.; Zhang, C.; Wang, S. Allogeneic periodontal ligament stem cell therapy for periodontitis in swine. Stem Cells 2010, 28, 1829–1838. [Google Scholar] [CrossRef]
- Han, J.; Menicanin, D.; Marino, V.; Ge, S.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Assessment of regenerative potential of allogeneic periodontal ligament stem cells in rodents. J. Periodontal Res. 2014, 49, 333–345. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeon, S.H.; Choung, P.H. Efficacy of periodontal stem cell transplantation in advanced periodontitis. Cell Transplant. 2011, 20, 271–285. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Yokoyama, N.; Tanaka, Y.; Taki, A.; Honda, I.; Kimura, Y.; Takeda, M.; Akazawa, K.; Oda, S.; et al. Periodontal regeneration using PDLSC-transferred amnion. Tissue Eng. Part A 2014, 20, 693–704. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Wada, N.; Marino, V.; Richter, W.; Shi, S.; Wheeler, D.L.; Gronthos, S.; Bartold, P.M. Regeneration of periodontal tissues using allogeneic PDLSCs in ovine model. Regen. Med. 2013, 8, 711–723. [Google Scholar] [CrossRef]
- Menicanin, D.; Mrozik, K.M.; Wada, N.; Marino, V.; Shi, S.; Bartold, P.M.; Gronthos, S. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev. 2014, 23, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, X.; Zhou, H.; Zhang, C.; Wang, Y.; Huang, J.; Liu, M.; Yang, P.; Song, A. Enhancement of periodontal regeneration by PDLSC- and GMSC-derived conditioned media. Stem Cell Res. Ther. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Lin, Z.; Lin, X.; Wang, Z.; Wu, Y.; Ji, M.; Lu, W.; Wang, X.; Zhang, D. Study of PRF combined with PDLSCs in periodontal regeneration. J. Cell Mol. Med. 2018, 22, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, J.; Sanchez, N.; Vignoletti, F.; Sanz-Martin, I.; Caffesse, R.; Santamaria, S.; Garcia-Sanz, J.A.; Sanz, M. Cell therapy with allogenic canine periodontal ligament-derived cells in periodontal regeneration of critical-size defects. J. Clin. Periodontol. 2018, 45, 453–461. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2018, 19, 7–33. [Google Scholar] [CrossRef]

| No. | Reference | PDLSC Source | Host Model | Scaffold | Cell Dose | Incubation | Key Findings |
|---|---|---|---|---|---|---|---|
| 1 | Liu et al. [30] | Autologous | Miniature pigs | HA/TCP | 2 × 107 | 12 wk | Superior bone regeneration; reduced attachment loss. |
| 2 | Iwasaki et al. [31] | Xenogeneic | Rats | Amnion | 5 × 105 | 4 wk | Enhanced bone, cementum, and Sharpey’s fibres. |
| 3 | Ding et al. [32] | Allogeneic/Autologous | Pigs | HA/TCP | 2 × 106 | 12 wk | Full periodontal regeneration only in the PDLSC group. |
| 4 | Han et al. [33] | Allogeneic | Rats | Gelfoam | 1 × 106 | 28 d (4 wk) | Near-complete bone, cementum and Sharpey’s fibres regeneration. |
| 5 | Park et al. [34] | Autologous | Beagle dogs | – | 6 × 106 | 8 wk | Best regeneration outcomes in the PDLSC group. |
| 6 | Iwasaki et al. [35] | Xenogeneic | Rats | Amnion | – | 4 wk | Organised bone and Sharpey’s fibres in the PDLSC group. |
| 7 | Mrozik et al. [36] | Allogeneic | Sheep | Gelfoam | 1 × 107 | 4 wk | Highest cementum and fibre thickness in the PDLSC group |
| 8 | Menicanin et al. [37] | Autologous | Sheep | Gelfoam | 2 × 106 | 6 wk | Complete tissue regeneration in the PDLSC group |
| 9 | Qiu et al. [38] | Xenogeneic | Rats | Bio-Guide | – | 4 wk | Superior bone, Sharpey’s fibre, and cementum in the PDLSC group. |
| 10 | Duan et al. [39] | Allogeneic | Rats | PRF or Collagen | – | 12–24 d (2–4 wk) | Best bone, Sharpeys fibre, and cementum regeneration with PRF + PDLSC. |
| 11 | Nuñez et al. [40] | Allogeneic | Beagle dogs | Bio-Oss Collagen | 1.4 × 106 | 3 mo (12 wk) | Comparable outcomes with the scaffold alone. |
| 12 | Chen et al. [41] | Autologous | Humans | Bio-Oss | 1 × 107 | 3–12 mo (12–52 wk) | Greater clinical attachment gain in the PDLSC group. |
| No. | Study | Group | Time (Weeks) | PD (SD, mm) | AL (SD, mm) |
|---|---|---|---|---|---|
| 1 | Liu et al. [30] | PDLSC Autologous | 0 | 10 (0.4) | 12.1 (1.8) |
| 2 | Liu et al. [30] | PDLSC Autologous | 12 | 2.9 (0.3) | 3.3 (0.6) |
| 3 | Liu et al. [30] | HA/TCP | 0 | 10.2 (0.4) | 12.8 (1.4) |
| 4 | Liu et al. [30] | HA/TCP | 12 | 3.6 (0.8) | 5.3 (0.3) |
| 5 | Liu et al. [30] | Control | 0 | 10 (0.6) | 12.4 (1.7) |
| 6 | Liu et al. [30] | Control | 12 | 4.7 (0.4) | 6.3 (0.5) |
| 7 | Ding et al. [32] | Control | 0 | 10 (0.5) | 13.4 (1) |
| 8 | Ding et al. [32] | Control | 12 | 9 (0.4) | 12 (0.8) |
| 9 | Ding et al. [32] | HA/TCP | 0 | 10.2 (0.4) | 13.8 (1) |
| 10 | Ding et al. [32] | HA/TCP | 12 | 10.4 (0.5) | 14 (0.7) |
| 11 | Ding et al. [32] | PDLSC Autologous | 0 | 10 (0.4) | 13.5 (1.8) |
| 12 | Ding et al. [32] | PDLSC Autologous | 12 | 2.9 (0.3) | 3.3 (0.5) |
| 13 | Ding et al. [32] | PDLSC Allogeneic | 0 | 10 (0.4) | 12 (1.6) |
| 14 | Ding et al. [32] | PDLSC Allogeneic | 12 | 3 (0.3) | 3.5 (0.6) |
| 15 | Ding et al. [32] | PDLC | 0 | 10 (0.4) | 7 (1.5) |
| 16 | Ding et al. [32] | PDLC | 12 | 6 (0.3) | 4 (0.6) |
| 17 | Park et al. [34] | Control | 0 | 5 (0.5) | 7.9 (0.9) |
| 18 | Park et al. [34] | Control | 8 | 5.2 (0.1) | 8.3 (0.7) |
| 19 | Park et al. [34] | PDLSC Autologous | 0 | 5.05 (0.58) | 7.05 (0.76) |
| 20 | Park et al. [34] | PDLSC Autologous | 8 | 2.88 (0.75) | 4.03 (0.84) |
| 21 | Park et al. [34] | DPSC | 0 | 4.88 (0.53) | 6.6 (0.59) |
| 22 | Park et al. [34] | DPSC | 8 | 4.54 (0.41) | 6.15 (0.58) |
| 23 | Park et al. [34] | PAFSC | 0 | 5.04 (0.66) | 6.7 (0.8) |
| 24 | Park et al. [34] | PAFSC | 8 | 3.85 (0.8) | 5 (0.58) |
| No. | Cell Dose Range | Mass-Normalised Cell Dose Range (Cells·kg−1) | Number of Studies | Regeneration Range | Efficacy Rating |
|---|---|---|---|---|---|
| 1 | Low (5 × 105–1 × 106) | 1.7 × 106–3.3 × 106 (rats) | 2 [31,33] | 33–100% | High variability |
| 2 | Medium (1.4 × 106–2 × 106) | 4.0 × 104–5.7 × 104 (pigs) | 1 [32] | 34–36% | Low |
| 3 | High (6 × 106–2 × 107) | 2.5 × 105–6.0 × 105 (dogs and sheep) | 3 [34,36] | 50–95% | Moderate to high |
| No. | Study | Group | Regeneration (%) |
|---|---|---|---|
| 1 | Liu et al. [30] | PDLSC-HA/TCP Autologous | 80 |
| 2 | HA/TCP | 44 | |
| 3 | Control | 12 | |
| 4 | Iwasaki et al. [31] | PDLSC-Amnion Xenogeneic | 33 |
| 5 | Control | 3 | |
| 6 | Ding et al. [32] | PDLSC-HA/TCP Autologous | 36 |
| 7 | PDLSC-HA/TCP Allogeneic | 34 | |
| 8 | HA/TCP | 11 | |
| 9 | PDLC | 18 | |
| 10 | Control | 12 | |
| 11 | Han et al. [33] | PDLSC-Gelfoam Allogeneic | 100 |
| 12 | Gelfoam | 50 | |
| 13 | Park et al. [34] | PDLSC Autologous | 95 |
| 14 | PAFSC | 78 | |
| 15 | DPSC | 67 | |
| 16 | Control | 17 | |
| 17 | Iwasaki et al. [35] | PDLSC-Amnion Xenogeneic | 88 |
| 18 | Amnion | 75 |
| No. | Cell Source | Number of Studies | Host Models | Mass-Normalised Cell Dose Range (Cells·kg−1) | Follow-Up Range | Regeneration Range (%) | Advantage vs. Control | Safety Profile |
|---|---|---|---|---|---|---|---|---|
| 1 | Autologous | 5 | Pigs, Dogs, Sheep, Humans | 5.7 × 104– 5.7 × 105 | 6–52 weeks | 36–95 | +24% to 78% | No adverse effects reported |
| 2 | Allogeneic | 5 | Rats, Sheep, Dogs | 2.5 × 105– 3.3 × 106 | 2–12 weeks | 34–100 | +12% to 50% | No immunological rejection |
| 3 | Xenogeneic | 3 | Rats only | 1.7 × 106– NR | 4 weeks | 33–88 | +13% to 30% | No immune rejection observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podembski, R.; Barahona, I.; Izidoro, C.; Romero, A.; Mascarenhas, P. Regenerative Applications and Performance of Periodontal Ligament Stem Cells: A Comprehensive Review of In Vivo Studies. Appl. Sci. 2025, 15, 11444. https://doi.org/10.3390/app152111444
Podembski R, Barahona I, Izidoro C, Romero A, Mascarenhas P. Regenerative Applications and Performance of Periodontal Ligament Stem Cells: A Comprehensive Review of In Vivo Studies. Applied Sciences. 2025; 15(21):11444. https://doi.org/10.3390/app152111444
Chicago/Turabian StylePodembski, Romain, Isabel Barahona, Catarina Izidoro, Alexis Romero, and Paulo Mascarenhas. 2025. "Regenerative Applications and Performance of Periodontal Ligament Stem Cells: A Comprehensive Review of In Vivo Studies" Applied Sciences 15, no. 21: 11444. https://doi.org/10.3390/app152111444
APA StylePodembski, R., Barahona, I., Izidoro, C., Romero, A., & Mascarenhas, P. (2025). Regenerative Applications and Performance of Periodontal Ligament Stem Cells: A Comprehensive Review of In Vivo Studies. Applied Sciences, 15(21), 11444. https://doi.org/10.3390/app152111444








