The Effect of Pretreatment of Tetraselmis subcrodiformis (Wille) Butcher and Limnospira platensis (Gomont) Ciferri et Tiboni Biomass with Solidified Carbon Dioxide on the Efficiency of Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
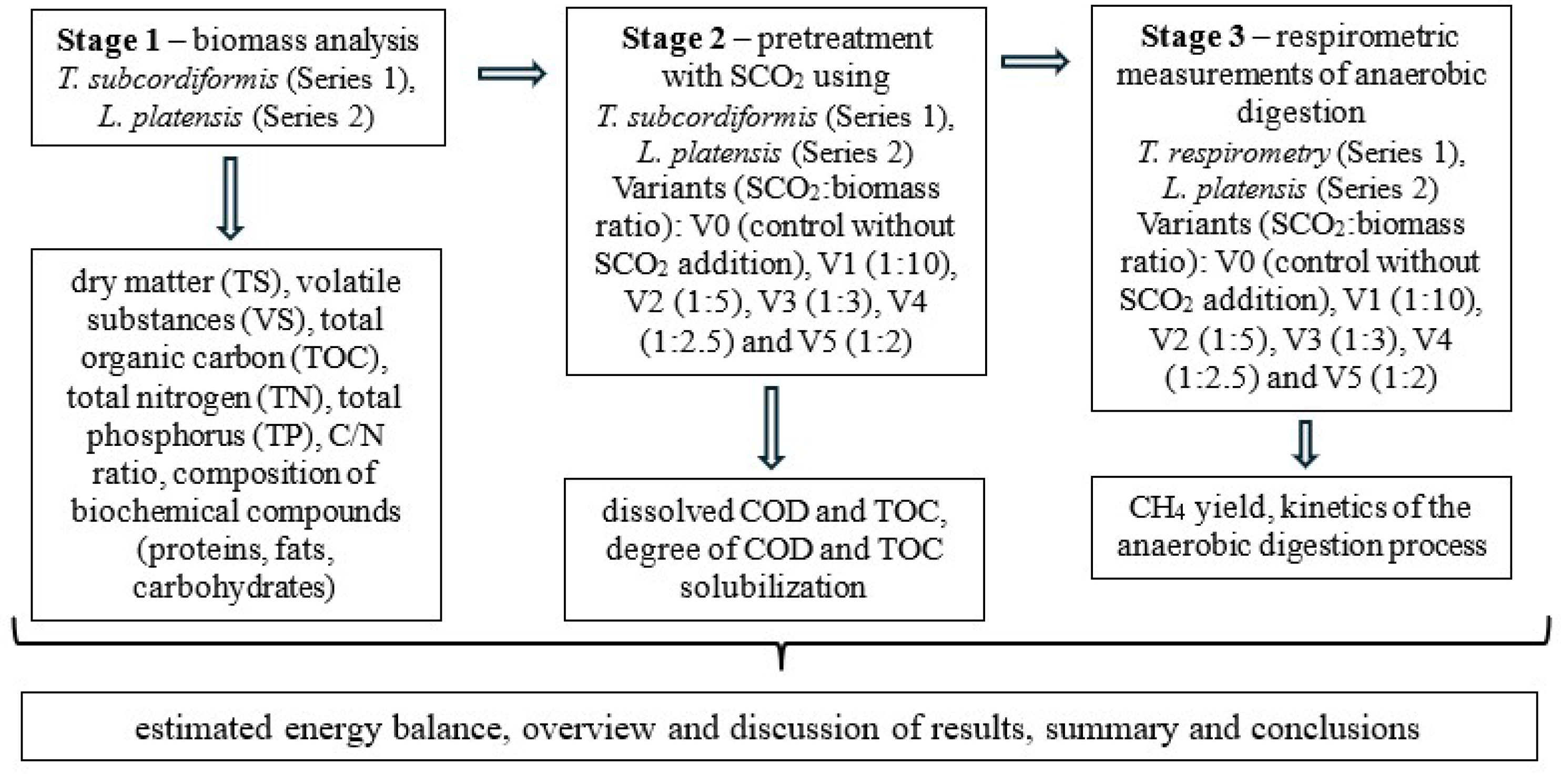
2.1. Organisation of the Experiment
2.2. Tetraselmis Subcordiformis and Limnospira platensis Biomass
2.3. Solid Carbon Dioxide—SCO2
2.4. Anaerobic Sludge Inoculum
2.5. Test Stations
2.5.1. Biomass Pretreatment with SCO2
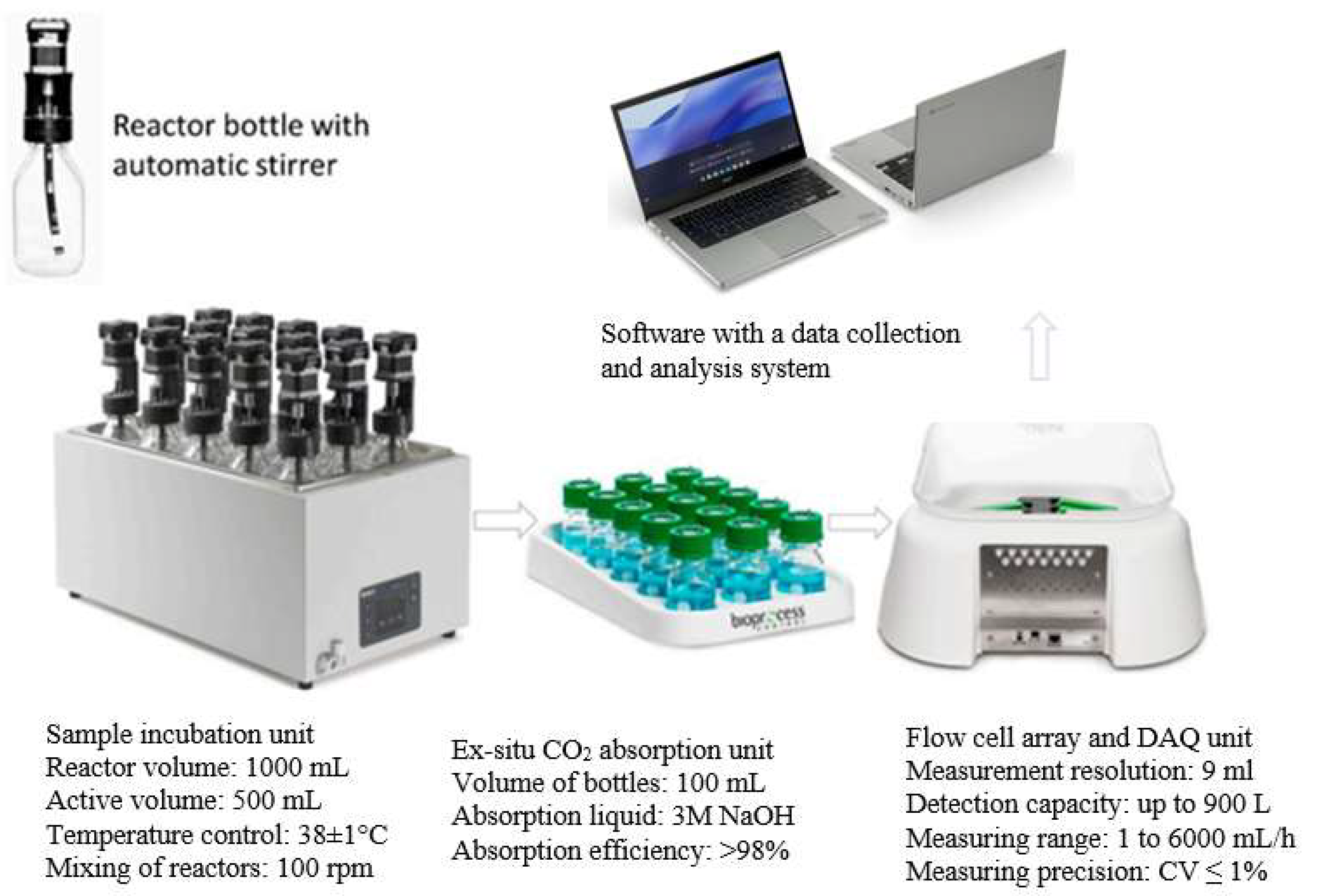
2.5.2. Respirometric Batch Fermentation Bioreactors
2.6. Analytical Methods
2.7. Computational and Statistical Methods
- -
- sCOD S0 and sTOC S0 are the initial concentrations of the soluble fractions of COD and TOC [mg/L], respectively, before pretreatment;
- -
- sCOD S1 and sTOC S1 are the concentrations of these fractions after the application of SCO2;
- -
- COD T0 and TOC T0 represent the total content of the analysed indicators in the biomass of microalgae and cyanobacteria before pre-treatment.
- -
- V(t)—cumulative CH4 quantity at time t, [mL/gVS];
- -
- Vmax—maximum asymptotic CH4 yield [mL/gVS];
- -
- Rmax—maximum CH4 production rate [mL/gVS/d];
- -
- λ—duration of the lag phase [d];
- -
- t—fermentation time [d];
- -
- e—Euler’s constant (≈2.71828).
- -
- P_SCO2—appliance power (W);
- -
- M_SCO2—mass of the SCO2 produced (kg);
- -
- Y_SCO2—efficiency of the appliance (kg/h).
- -
- Y_CH4—methane yield (L/kgVS, L/kgFM);
- -
- CV_CH4—calorific value of methane (Wh/L);
- -
- VS_biomass—VS of the T. subcordiformis/L. platensis biomass used (kgVS);
- -
- FM_biomass—FM of the T. subcordiformis/L. platensis biomass used (kgFM).
3. Results and Discussion
3.1. Characterisation of the Biomass of T. subcordiformis and L. platensis
3.2. Indirect Indicators of Pretreatment Efficiency
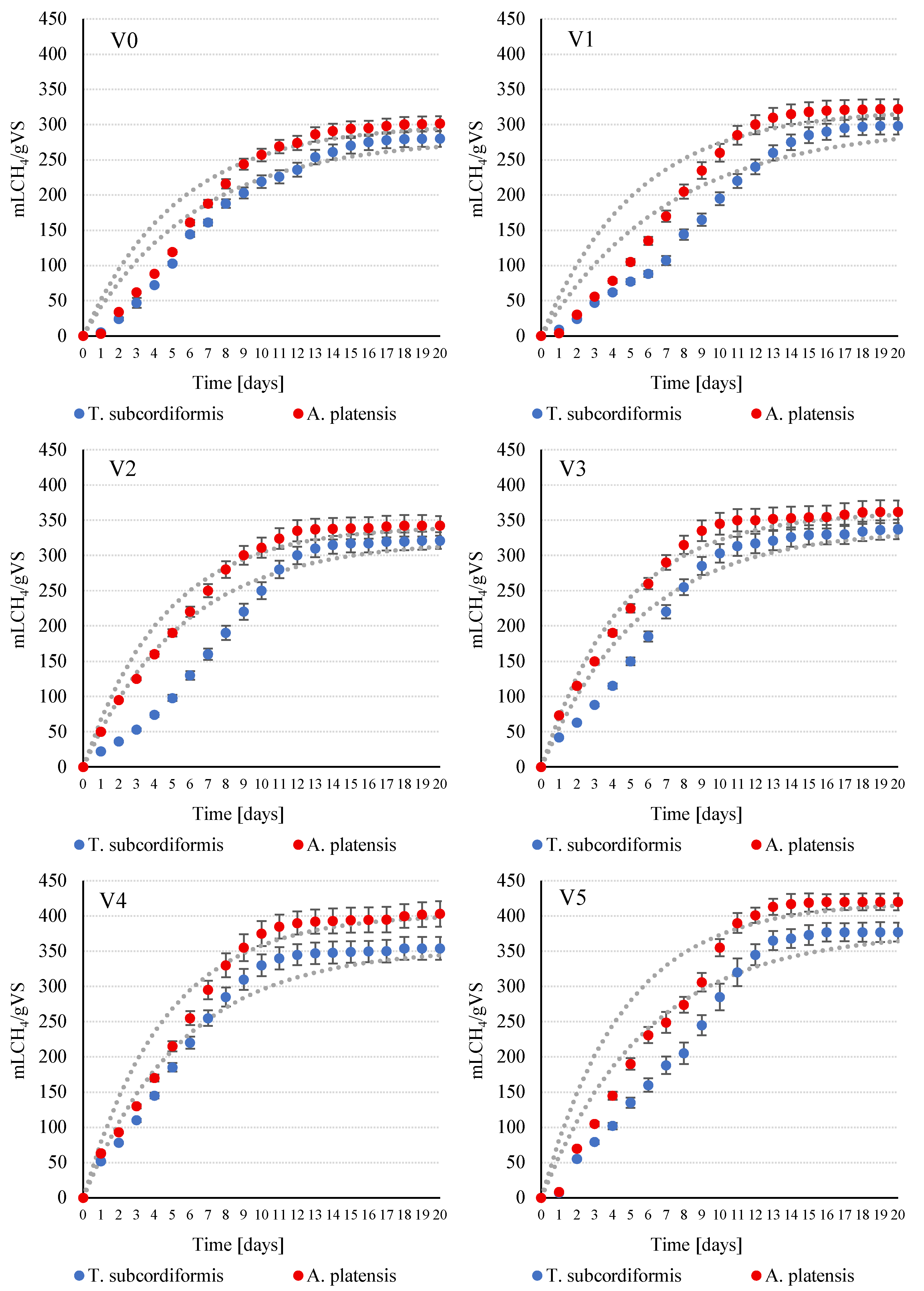
3.3. Anaerobic Respirometry Measurements
3.4. Energy Balance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jha, P.; Ghosh, S.; Panja, A.; Kumar, V.; Singh, A.K.; Prasad, R. Microalgae and biogas: A boon to energy sector. Environ. Sci. Pollut. Res. 2023, 32, 7411–7431. [Google Scholar] [CrossRef] [PubMed]
- Kowthaman, C.N.; Senthil Kumar, P.; Arul Mozhi Selvan, V.; Ganesh, D. A comprehensive insight from microalgae production process to characterization of biofuel for the sustainable energy. Fuel 2022, 310, 122320. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, R.P.; Rana, S.; Joshi, D.; Kumar, D.; Bhardwaj, N.; Gupta, R.K.; Kumar, A. Mechanisms of Stress Tolerance in Cyanobacteria under Extreme Conditions. Stresses 2022, 2, 531–549. [Google Scholar] [CrossRef]
- López-Hernández, J.-F.; Kean-Meng, T.; Asencio-Alcudia, G.-G.; Asyraf-Kassim, M.; Alvarez-González, C.-A.; Márquez-Rocha, F.-J. Sustainable Microalgae and Cyanobacteria Biotechnology. Appl. Sci. 2022, 12, 6887. [Google Scholar] [CrossRef]
- Begum, N.; Qi, F.; Yang, F.; Khan, Q.U.; Faizan; Fu, Q.; Li, J.; Wang, X.; Wang, X.; Wang, J.; et al. Nutritional Composition and Functional Properties of A. platensis-Derived Peptides: A Green and Sustainable Protein-Rich Supplement. Processes 2024, 12, 2608. [Google Scholar] [CrossRef]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Studies on the Impact of Selected Pretreatments on Protein Solubility of Arthrospira platensis Microalga. Agriculture 2023, 13, 221. [Google Scholar] [CrossRef]
- Pradhan, N.; Kumar, S.; Selvasembian, R.; Rawat, S.; Gangwar, A.; Senthamizh, R.; Yuen, Y.K.; Luo, L.; Ayothiraman, S.; Saratale, G.D.; et al. Emerging trends in the pretreatment of microalgal biomass and recovery of value-added products: A review. Bioresour. Technol. 2023, 369, 128395. [Google Scholar] [CrossRef]
- Elnajjar, E.; Purayil, S.T.P.; Alnuaimi, F.; Al Khawaja, H.; Shaikhoun, L.; Arnaoud, N.; Almutawa, S. Mechanistic investigation of efficient cell disruption methods for lipid extraction from various macro and micro species of algae. Bioresour. Technol. Rep. 2023, 22, 101482. [Google Scholar] [CrossRef]
- Xiao, C.; Liao, Q.; Fu, Q.; Huang, Y.; Xia, A.; Shen, W.; Chen, H.; Zhu, X. Exergy analyses of biogas production from microalgae biomass via anaerobic digestion. Bioresour. Technol. 2019, 289, 121709. [Google Scholar] [CrossRef]
- Greenstein, K.E.; Zamyadi, A.; Wert, E.C. Comparative Assessment of Physical and Chemical Cyanobacteria Cell Lysis Methods for Total Microcystin-LR Analysis. Toxins 2021, 13, 596. [Google Scholar] [CrossRef]
- Demuez, M.; Mahdy, A.; Tomás-Pejó, E.; González-Fernández, C.; Ballesteros, M. Enzymatic cell disruption of microalgae biomass in biorefinery processes. Biotechnol. Bioeng. 2015, 112, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gong, M.; Xu, C.; Bassi, A. Investigation of an alternative cell disruption approach for improving hydrothermal liquefaction of microalgae. Fuel 2017, 197, 138–144. [Google Scholar] [CrossRef]
- Waseem, M.; Khan, M.U.; Bokhary, A.; Ahring, B.K. Emerging trends in sewage sludge pretreatment: Enhancing treatment efficiency and sustainable waste management. Clean. Water 2025, 3, 100080. [Google Scholar] [CrossRef]
- Tan, H.T.; Khong, N.M.H.; Khaw, Y.S.; Ahmad, S.A.; Yusoff, F.M. Optimization of the Freezing-Thawing Method for Extracting Phycobiliproteins from Arthrospira sp. Molecules 2020, 25, 3894. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Lebiocka, M.; Rożej, A.; Zacharska, E.; Pawłowski, L. Freezing/thawing effects on anaerobic digestion of mixed sewage sludge. Bioresour. Technol. 2010, 101, 3466–3473. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef]
- Hasan, M.M.; Mofijur, M.; Uddin, M.N.; Kabir, Z.; Badruddin, I.A.; Khan, T.M.Y. Insights into Anaerobic Digestion of Microalgal Biomass for Enhanced Energy Recovery. Front. Energy Res. 2024, 12, 1355686. [Google Scholar] [CrossRef]
- Shivakumar, S.; Serlini, N.; Esteves, S.M.; Miros, S.; Halim, R. Cell Walls of Lipid-Rich Microalgae: A Comprehensive Review on Characterisation, Ultrastructure, and Enzymatic Disruption. Fermentation 2024, 10, 608. [Google Scholar] [CrossRef]
- Paswan, R.; Das, S. Meso-Structural Degradation and Mechanical Property Evolution in Cementitious Mortars Containing Microencapsulated Phase Change Materials under Extended Freeze-Thaw Cycles. Constr. Build. Mater. 2024, 457, 139405. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion. Int. J. Environ. Res. Public Healh 2023, 20, 4234. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Long-Term Pre-Treatment of Municipal Sewage Sludge with Solidified Carbon Dioxide (SCO2)—Effect on Anaerobic Digestion Efficiency. Appl. Sci. 2023, 13, 3075. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals; Springer US: Boston, MA, USA, 1975; pp. 29–60. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a L’etude D’une Cyanobacterie: Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthese de Spirulina maxima (Setchell et Gardner). Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Jamil, M.; Iqbal, A.; He, N.; Cheok, Q. Thermophysical Properties and Heat Transfer Performance of Novel Dry-Ice-Based Sustainable Hybrid Lubri-Coolant. Sustainability 2022, 14, 2430. [Google Scholar] [CrossRef]
- Available online: https://www.co2meter.com/blogs/news/dry-ice-dangers-uses-safety-best-practices (accessed on 18 September 2025).
- Usevičiūtė, L.; Januševičius, T.; Danila, V.; Mažeikienė, A.; Zagorskis, A.; Pranskevičius, M.; Marčiulaitienė, E. Performance and Kinetics of Anaerobic Digestion of Sewage Sludge Amended with Zero-Valent Iron Nanoparticles, Analyzed Using Sigmoidal Models. Energies 2025, 18, 1425. [Google Scholar] [CrossRef]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal Pretreatment of Microalgae for Biomethane Production: Experimental Studies, Kinetics and Energy Analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Statistica—StatSoft. Available online: https://www.statsoft.pl/programy/statistica/ (accessed on 18 October 2025).
- Hawrot-Paw, M.; Koniuszy, A.; Ratomski, P.; Sąsiadek, M.; Gawlik, A. Biogas Production from Arthrospira platensis Biomass. Energies 2023, 16, 3971. [Google Scholar] [CrossRef]
- Aljabri, H.; Cherif, M.; Siddiqui, S.A.; Bounnit, T.; Saadaoui, I. Evidence of the drying technique’s impact on the biomass quality of Tetraselmis subcordiformis (Chlorophyceae). Biotechnol. Biofuels Bioprod. 2023, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Dębowski, M.; Zieliński, M. Comparison of biogas production from anaerobic digestion of microalgae species belonged to various taxonomic groups. Arch. Environ. Prot. 2023, 46, 33–40. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Inoue, D.; Ike, M. Mitigating ammonia-inhibition in anaerobic digestion by bioaugmentation: A review. J. Water Process Eng. 2023, 52, 103506. [Google Scholar] [CrossRef]
- Spínola, M.P.; Mendes, A.R.; Prates, J.A.M. Chemical Composition, Bioactivities, and Applications of Spirulina (Limnospira platensis) in Food, Feed, and Medicine. Foods 2024, 13, 3656. [Google Scholar] [CrossRef]
- Arora Soni, R.; Sudhakar, K.; Rana, R.S. Biochemical and Thermal Analysis of Spirulina Biomass through FTIR, TGA, CHN. Energy Eng. 2021, 118, 1045–1056. [Google Scholar] [CrossRef]
- Ryndin, K.G.; Butyrin, A.V.; Grigorenko, A.V.; Chunzhuk, E.A.; Chernova, N.I.; Kiseleva, S.V.; Malaniy, S.Y.; Bakumenko, E.A.; Slavkina, O.V.; Ossipov, K.; et al. From the Cultivation of Arthrospira platensis at an Increased CO2 Concentration to the Bio-Oil Production by Hydrothermal Liquefaction. Appl. Sci. 2023, 13, 9950. [Google Scholar] [CrossRef]
- Saadaoui, I.; Cherif, M.; Siddiqui, S.A.; Esakkimuthu, S.; AbdulQuadir, M.; El Anbari, M.; Sayadi, S. Unveiling Tetraselmis Subcordiformis Lipidome Dynamics during Large-Scale Cultivation in Open Raceway Pond. J. Appl. Phycol. 2024, 36, 1125–1134. [Google Scholar] [CrossRef]
- Montoya Montoya, L.M.; Pérez, A.A.A.; Giraldo Calderón, N.D.; Garcés, L.A. Analysis of Cell Growth, Photosynthetic Behavior and the Fatty Acid Profile in Tetraselmis Subcordiformis under Different Lighting Scenarios. J. Appl. Phycol. 2024, 36, 1679–1695. [Google Scholar] [CrossRef]
- Astals, S.; José Chávez-Fuentes, J.; Capson-Tojo, G.; Hutňan, M.; Jensen, P.D. The interaction between lipids and ammoniacal nitrogen mitigates inhibition in mesophilic anaerobic digestion. Waste Manag. 2021, 136, 244–252. [Google Scholar] [CrossRef]
- Machnicka, A.; Grübel, K.; Wacławek, S.; Sikora, K. Waste-activated sludge disruption by dry ice: Bench scale study and evaluation of heat phase transformations. Environ. Sci. Pollut. Res. 2019, 26, 26488–26499. [Google Scholar] [CrossRef]
- Senousy, H.H.; El-Sheekh, M.M.; Saber, A.A.; Khairy, H.M.; Said, H.A.; Alhoqail, W.A.; Abu-Elsaoud, A.M. Biochemical Analyses of Ten Cyanobacterial and Microalgal Strains Isolated from Egyptian Habitats, and Screening for Their Potential against Some Selected Phytopathogenic Fungal Strains. Agronomy 2022, 12, 1340. [Google Scholar] [CrossRef]
- Cheong, B.E.; Yu, D.; Martinez-Seidel, F.; Ho, W.W.H.; Rupasinghe, T.W.T.; Dolferus, R.; Roessner, U. The Effect of Cold Stress on the Root-Specific Lipidome of Two Wheat Varieties with Contrasting Cold Tolerance. Plants 2022, 11, 1364. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of microalgae with high lipid content and their potential as sources of nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- Kavitha, S.; Schikaran, M.; Yukesh Kannah, R.; Gunasekaran, M.; Kumar, G.; Rajesh Banu, J. Nanoparticle induced biological disintegration: A new phase separated pretreatment strategy on microalgal biomass for profitable biomethane recovery. Bioresour. Technol. 2019, 289, 121624. [Google Scholar] [CrossRef]
- Marques, A.L.; Pinto, F.P.; Araujo, O.Q.D.F.; Cammarota, M.C. Assessment of Methods to Pretreat Microalgal Biomass for Enhanced Biogas Production. J. Sustain. Dev. Energy Water Environ. Syst. 2018, 6, 394–404. [Google Scholar] [CrossRef]
- Feki, F.; Cherif, M.; Masmoudi, M.A.; Chamkha, M.; Saadaoui, I.; Das, P.; Sayadi, S. Methane production enhancement from Tetraselmis biomass co-digestion using frying oil residue as co-substrate and ultrasonication as pretreatment. Environ. Technol. Innov. 2024, 33, 103478. [Google Scholar] [CrossRef]
- Inglesby, A.E.; Griffiths, M.J.; Harrison, S.T.L.; van Hille, R.P. Anaerobic digestion of Spirulina sp. and Scenedesmus sp.: A comparison and investigation of the impact of mechanical pre-treatment. J. Appl. Phycol. 2015, 27, 1891–1900. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Feng, Y.; Ge, J.; Show, P.L.; Song, C.; Wu, L.; Ma, Z.; Gao, G. Using high CO2 concentrations to culture microalgae for lipid and fatty acid production: Synthesis based on a meta-analysis. Aquaculture 2025, 594, 741386. [Google Scholar] [CrossRef]
- Dai, X.; Hu, C.; Zhang, D.; Dai, L.; Duan, N. Impact of a high ammonia-ammonium-pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion. Bioresour. Technol. 2017, 245, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Dingeo, C.; Rizzello, C.G.; Pontonio, E. Lactic Acid Bacteria Fermentation and Endopeptidase Treatment Improve the Functional and Nutritional Features of Arthrospira Platensis. Front. Microbiol. 2021, 12, 744437. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Lan, C.Q.; Liao, D. Effects of sodium bicarbonate on cell growth, lipid accumulation, and morphology of Chlorella vulgaris. Microb. Cell Factories 2018, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Fitria; Kumar, A.; Zhang, L.; Liu, J.; Fatriasari, W.; Yang, B. Understanding the Effects of Ash Content on Various Pretreatment Technologies for the Bioconversion of Corn Stover. BioEnergy Res. 2025, 18, 14. [Google Scholar] [CrossRef]
- Čabarkapa, I.; Rakita, S.; Popović, S.; Tomičić, Z.; Spasevski, N.; Vulić, J.; Đuragic, O. Characterization of Organic Spirulina Spp. and Chlorella vulgaris as One of the Most-Dense Food. J. Food Saf. Food Qual. 2022, 73, 78–85. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Yang, L.; Zhang, W.; Chen, H. Patterns of differential responses of the bacterial and methanogens to short-term nitrogen and phosphorus addition in swamp meadow of the Tibetan Plateau. J. Environ. Manag. 2025, 392, 126897. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.C.; Bassin, I.D.; Cammarota, M.C. Microalgae and Cyanobacteria Biomass Pretreatment Methods: A Comparative Analysis of Chemical and Thermochemical Pretreatment Methods Aimed at Methane Production. Fermentation 2022, 8, 497. [Google Scholar] [CrossRef]
- Szaja, A.; Bartkowska, I. Implementation of solidified carbon dioxide to anaerobic co-digestion of municipal sewage sludge and orange peel waste. Arch. Environ. Prot. 2024, 50, 72–79. [Google Scholar] [CrossRef]
- Nowicka, E.; Machnicka, A.; Grűbel, K. Improving of Anaerobic Digestion by Dry Ice Disintegration of Surplus Activated Sludge. Ecol. Chem. Eng. A 2014, 21, 211–219. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Innovative Method for Biomethane Production Based on a Closed Cycle of Biogas Upgrading and Organic Substrate Pretreatment—Technical, Economic, and Technological Fundamentals. Energies 2025, 18, 1033. [Google Scholar] [CrossRef]
- Zawieja, I. Effect of Dry Ice Modification of Excess Sludge on the Methane Fermentation Process. Annu. Set Environ. Prot. 2018, 20, 558–573. [Google Scholar]
- Zawieja, I.E. The Course of the Methane Fermentation Process of Dry Ice Modified Excess Sludge. Arch. Environ. Prot. 2019, 45, 50–58. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M. Characteristics of Solidified Carbon Dioxide and Perspectives for Its Sustainable Application in Sewage Sludge Management. Int. J. Mol. Sci. 2023, 24, 2324. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2020, 14, 150. [Google Scholar] [CrossRef]
- Machnicka, A.; Grübel, K. The Effect of Pre-Treatment and Anaerobic Digestion for Pathogens Reduction in Agricultural Utilization of Sewage Sludge. Environ. Sci Pollut Res. 2022, 30, 13801–13810. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Comparison of ultrasound and thermal pretreatment of Scenedesmus biomass on methane production. Bioresour. Technol. 2012, 110, 610–616. [Google Scholar] [CrossRef]
- Córdova, O.; Passos, F.; Chamy, R. Physical pretreatment methods for improving microalgae anaerobic biodegradability. Appl. Biochem. Biotechnol. 2018, 185, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Gutiérrez, R.; Brockmann, D.; Steyer, J.-P.; García, J.; Ferrer, I. Microalgae production in wastewater treatment systems, anaerobic digestion and modelling using ADM1. Algal. Res. 2015, 10, 55–63. [Google Scholar] [CrossRef]
- Passos, F.; Solé, M.; Garcia, J.; Ferrer, I. Biogas production from microalgae grown in wastewater: Effect of microwave pretreatment. Appl. Energy 2013, 108, 168–175. [Google Scholar] [CrossRef]
- Scarcelli, P.G.; Serejo, M.L.; Paulo, P.L.; Bóncz, M.A. Evaluation of biomethanization during co-digestion of thermally pretreated microalgae and waste activated sludge, and estimation of its kinetic parameters. Sci. Total Environ. 2020, 706, 135745. [Google Scholar] [CrossRef]
- Ometto, F.; Quiroga, G.; Pšenička, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pre-treatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- Çakmak, E.K.; Ugurlu, A. Enhanced biogas production of red microalgae via enzymatic pretreatment and preliminary economic assessment. Algal. Res. 2020, 50, 101979. [Google Scholar] [CrossRef]
- Giménez, J.B.; Aguado, D.; Bouzas, A.; Ferrer, J.; Seco, A. Use of rumen microorganisms to boost the anaerobic biodegradability of microalgae. Algal. Res. 2017, 24, 309–316. [Google Scholar] [CrossRef]
- Hom-Diaz, A.; Passos, F.; Ferrer, I.; Vicent, T.; Blánquez, P. Enzymatic pretreatment of microalgae using fungal broth from Trametes versicolor and commercial laccase for improved biogas production. Algal. Res. 2016, 19, 184–188. [Google Scholar] [CrossRef]
- Mahdy, A.; Mendez, L.; Ballesteros, M.; González-Fernández, C. Enhanced methane production of Chlorella vulgaris and Chlamydomonas reinhardtii by hydrolytic enzymes addition. Energy Convers. Manag. 2014, 85, 551–557. [Google Scholar] [CrossRef]
- Cheng, Q.; Deng, F.; Li, H.; Qin, Z.; Wang, M.; Li, J. Nutrients removal from the secondary effluents of municipal domestic wastewater by Oscillatoria tenuis and subsequent co-digestion with pig manure. Environ. Technol. 2018, 39, 3127–3134. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Lewis, D. Pre-treatment options for halophytic microalgae and associated methane production. Bioresour. Technol. 2015, 177, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Teuling, E.; Wierenga, P.A.; Schrama, J.W.; Gruppen, H. Comparison of Protein Extracts from Various Unicellular Green Sources. J. Agric. Food Chem. 2017, 65, 7989–8002. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Biohythane Production in Hydrogen-Oriented Dark Fermentation of Aerobic Granular Sludge (AGS) Pretreated with Solidified Carbon Dioxide (SCO2). Int. J. Mol. Sci. 2023, 24, 4442. [Google Scholar] [CrossRef]
- Şahan, M.; Fardinpoor, M.; Yılmaz, V.; Yılmaz, F.; Perendeci, N.A. Effects of High Temperature & Pressure Pretreatment Process on Methane Production from Cyanobacteria. Fermentation 2023, 9, 240. [Google Scholar] [CrossRef]
- Dar, R.A.; Phutela, U.G. Enzymatic and Hydrothermal Pretreatment of Newly Isolated Spirulina subsalsa BGLR6 Biomass for Enhanced Biogas Production. Waste Biomass Valorization 2020, 11, 3639–3651. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Świca, I.; Zieliński, M. Ultrasonic Disintegration to Improve Anaerobic Digestion of Microalgae with Hard Cell Walls—Scenedesmus sp. and Pinnularia sp. Plants 2022, 12, 53. [Google Scholar] [CrossRef]

| Parameter | Unit | Species-Series | |
|---|---|---|---|
| T. subcordiformis Series 1 | L. platensis Series 2 | ||
| Total solids (TS) | [%FM *] | 5.1 ± 0.3 | 5.2 ± 0.4 |
| Volatile solids (VS) | [%TS] | 84.5 ± 1.5 | 89.6 ± 1.2 |
| Mineral solids (MS) | [%TS] | 15.5 ± 1.5 | 10.4 ± 1.2 |
| Total carbon (TC) | [mg/gTS] | 475 ± 28 | 442 ± 35 |
| Total organic carbon (TOC) | [mg/gTS] | 437 ± 21 | 418 ± 26 |
| Total nitrogen (TN) | [mg/gTS] | 53.6 ± 2.7 | 63.4 ± 3.1 |
| C/N ratio | – | 8.9 ± 0.3 | 7.0 ± 0.2 |
| Total phosphorus (TP) | [mg/gTS] | 12.6 ± 1.1 | 19.8 ± 1.4 |
| pH | – | 7.85 ± 0.06 | 9.42 ± 0.08 |
| Proteins | [%TS] | 33.5 ± 1.9 | 46.2 ± 2.5 |
| Lipids | [%TS] | 18.4 ± 0.9 | 8.5 ± 0.7 |
| Saccharides | [%TS] | 26.1 ± 1.8 | 18.9 ± 1.5 |
| Seria | Variant | SCO2:Biomass | COD Dissolved [mg O2/L] | TOC Dissolved [mg/L] | Degree of Solubilisation COD [%] | Degree of Solubilisation TOC [%] |
|---|---|---|---|---|---|---|
| Tetraselmis subcordiformis —seria 1 | V0 | – | 384 ± 28 | 142 ± 17 | – | – |
| V1 | 1:10 | 2634 ± 186 | 1040 ± 83 | 5.4 ± 0.4 | 2.9 ± 0.5 | |
| V2 | 1:5 | 4817 ± 256 | 3273 ± 179 | 10.6 ± 0.6 | 13.8 ± 1.0 | |
| V3 | 1:3 | 5955 ± 447 | 3996 ± 241 | 13.2 ± 0.9 | 16.7 ± 0.7 | |
| V4 | 1:2.5 | 5998 ± 401 | 4327 ± 288 | 13.4 ± 0.7 | 18.3 ± 1.4 | |
| V5 | 1:2 | 6041 ± 379 | 4375 ± 312 | 13.5 ± 0.6 | 18.5 ± 1.2 | |
| Limnospira platensis—seria 2 | V0 | – | 392 ± 34 | 153 ± 19 | – | – |
| V1 | 1:10 | 2953 ± 197 | 1209 ± 98 | 6.0 ± 0.3 | 3.2 ± 0.6 | |
| V2 | 1:5 | 5412 ± 303 | 3904 ± 204 | 11.7 ± 0.8 | 16.3 ± 1.2 | |
| V3 | 1:3 | 6487 ± 549 | 4612 ± 277 | 14.2 ± 0.9 | 19.7 ± 0.8 | |
| V4 | 1:2.5 | 6504 ± 492 | 4980 ± 360 | 14.3 ± 0.8 | 21.5 ± 1.7 | |
| V5 | 1:2 | 6511 ± 438 | 5010 ± 392 | 14.3 ± 0.7 | 21.6 ± 1.6 |
| Parameter | Unit | T. subcordiformis Biomass—Series 1 | |||||
| V0 | V1 | V2 | V3 | V4 | V5 | ||
| CH4 | mL/gVS | 280 ± 11 | 298 ± 11 | 321 ± 12 | 337 ± 15 | 354 ± 16 | 377 ± 12 |
| k | 1/day | 0.16 ± 0.02 | 0.14 ± 0.01 | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.03 | 0.18 ± 0.01 |
| r | mL/day | 44.8 ± 2.1 | 41.7 ± 1.9 | 57.8 ± 1.3 | 60.7 ± 1.4 | 63.7 ± 1.4 | 67.9 ± 1.1 |
| Parameter | Unit | L. platensis Biomass—Series 2 | |||||
| V0 | V1 | V2 | V3 | V4 | V5 | ||
| CH4 | mL/gVS | 301 ± 10 | 322 ± 14 | 342 ± 14 | 362 ± 16 | 403 ± 18 | 420 ± 12 |
| k | 1/day | 0.19 ± 0.02 | 0.19 ± 0.02 | 0.22 ± 0.03 | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.22 ± 0.01 |
| r | mL/day | 57.2 ± 1.2 | 61.2 ± 1.4 | 75.2 ± 0.9 | 79.6 ± 1.0 | 88.7 ± 0.8 | 92.4 ± 0.9 |
| Species | Method | Pretreatment (PT) Conditions | Methane Yield (mL CH4/g VS) | Reference | |
|---|---|---|---|---|---|
| Without PT | With PT | ||||
| Scenedesmus sp., 4.48 g/L TS | Ultrasound | 80 W, 30 min, 128.9 MJ/kg TS | 81.80 | 153.5 | [65] |
| Thermal | 70 °C, 25 min 80 °C, 25 min | 89.3 128.7 | |||
| Chlorella sorokiniana, 13.8 g/L CODT | Ultrasound | 220 W, 30 min 400 W, 20 min 400 W, 30 min 400 W, 40 min | 317.66 | 458.43 414.12 424.68 421.87 | [66] |
| Thermal | 80 °C, 20 min | 374.81 | |||
| Mixed biomass (Nitzschia sp., Stigeoclonium sp., Navicula sp., Monoraphidium sp.), 31.49 g/L TS | Ultrasound Microwave | 70 W, 30 min, 20 kHz, 27 MJ/kg TS 900 W, 3 min, 34.3 MJ/kg TS | 105.6 | 113.7 127.7 | [67] |
| Microalgae mixed biomass from high-rate ponds, 16.7 g/L CODT | Microwave | 300 W, 9 min, 64,400 kJ/kg TS 600 W, 4.5 min, 64,400 kJ/kg TS 900 W, 3 min, 64,400 kJ/kg TS | 117.63 | 167.24 188.34 210.06 | [68] |
| Chlorella sp., 27.9 g/L COD | Thermal | 65 °C, 4 h | 211 | 297 | [69] |
| Scenedesmus obliquus, 20 g/L TS | Hydrothermal | 165 °C, 7 bar, 30 min | 159 | 383.6 | [70] |
| Porphyridium cruentum, 3.4 g CODT/L | Enzymatic | Protease 0.5 mL/g dry biomass, pH 8.0–8.5, 55 °C, 9 h | 130 | 230 | [71] |
| Scenedesmus sp., 60.9 g/L CODT | Biological | TSAD, rumen m-orgs as pretreatment in the 1st stage, fermentation reactor: 40 d, SRT = 7 d, HRT = 7 d. | na | 214 | [72] |
| Mixed culture of bacteria and microalgae, composed mainly by Oocystis sp., 31.3 g/L CODT | Biological | 100 U/L laccase-rich broth from Trametes versicolor, 100 rpm/20 min 100 U/L commercial laccase, 20 min, 100 rpm, 25 °C | 83 | 144 100 | [73] |
| Scenedesmus sp., 6 g/L CODT | Thermochemical | H2SO4 0.1% v/v, 150 °C, 1 h | 130.9 | 253.1 | [44] |
| Chlorella sp. | Thermochemical | NaOH 0.05%, 50 °C, 24 h NaOH 2.0%, 50 °C, 24 h NaOH 5.0%, 50 °C, 24 h NaOH 0.05%, 50 °C, 48 h NaOH 2.0%, 50 °C, 48 h NaOH 5.0%, 50 °C, 48 h | 137.17 | 110.00 125.00 155.00 130.00 160.00 135.00 | [74] |
| Oscillatoria tenuis | Chemical | H2SO4 4M, pH 2; room temp. | 191 | 210 | [75] |
| Tetraselmis subcordiformis Biomass—Series 1 | ||||||||||||||||
| Variant | SCO2/Cv | ρCv | VCv | MCv | ρSCO2 | VSCO2 | MSCO2 | PSCO2 | WSCO2 | Es | Y CH4 | Y CH4 | CV CH4 | Eout | Enout | Enet |
| kg/L | L | kg | kg/L | L | kg | W | kg/h | Wh | L/kgVS | L/kg FM | Wh/L | Wh | Wh | Wh | ||
| 0 | 0 | 1.03 | 1 | 1.04 | - | 280 | 12.1 | 9.17 | 110.7 | 0 | 0 | |||||
| 1 | 0.1 | 1.56 | 0.1 | 0.156 | 4500 | 1090 | 0.644 | 298 | 12.8 | 117.8 | 7.1 | 6.4 | ||||
| 2 | 0.2 | 0.2 | 0.312 | 1.288 | 321 | 13.8 | 126.9 | 16.2 | 14.9 | |||||||
| 3 | 0.3 | 0.3 | 0.468 | 1.932 | 337 | 14.5 | 133.2 | 22.5 | 20.5 | |||||||
| 4 | 0.4 | 0.4 | 0.624 | 2.576 | 354 | 15.3 | 139.9 | 29.2 | 26.6 | |||||||
| 5 | 0.5 | 0.5 | 0.78 | 3.22 | 377 | 16.2 | 149.0 | 38.3 | 35.1 | |||||||
| Limnospira platensis Biomass—Series 2 | ||||||||||||||||
| Variant | SCO2/Cv | ρCv | VCv | MCv | ρCO2 | VSCO2 | MSCO2 | PSCO2 | WSCO2 | Es | Y CH4 | Y CH4 | CV CH4 | Eout | Enout | Enet |
| kg/L | L | kg | kg/L | L | kg | W | kg/h | Wh | L/kgVS | L/kg FM | Wh/L | Wh | Wh | Wh | ||
| 0 | 0 | 1.03 | 1 | 1.04 | - | 301 | 14.0 | 9.17 | 128.6 | 0 | 0 | |||||
| 1 | 0.1 | 1.56 | 0.1 | 0.156 | 4500 | 1090 | 0.644 | 322 | 15.0 | 137.6 | 9.0 | 8.3 | ||||
| 2 | 0.2 | 0.2 | 0.312 | 1.288 | 342 | 15.9 | 146.1 | 17.5 | 16.2 | |||||||
| 3 | 0.3 | 0.3 | 0.468 | 1.932 | 362 | 16.9 | 154.7 | 26.1 | 24.1 | |||||||
| 4 | 0.4 | 0.4 | 0.624 | 2.576 | 403 | 18.8 | 172.2 | 43.6 | 41.0 | |||||||
| 5 | 0.5 | 0.5 | 0.78 | 3.22 | 420 | 19.6 | 179.4 | 50.8 | 47.6 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Świca, I.; Zieliński, M.; Kazimierowicz, J. The Effect of Pretreatment of Tetraselmis subcrodiformis (Wille) Butcher and Limnospira platensis (Gomont) Ciferri et Tiboni Biomass with Solidified Carbon Dioxide on the Efficiency of Anaerobic Digestion. Appl. Sci. 2025, 15, 11373. https://doi.org/10.3390/app152111373
Dębowski M, Świca I, Zieliński M, Kazimierowicz J. The Effect of Pretreatment of Tetraselmis subcrodiformis (Wille) Butcher and Limnospira platensis (Gomont) Ciferri et Tiboni Biomass with Solidified Carbon Dioxide on the Efficiency of Anaerobic Digestion. Applied Sciences. 2025; 15(21):11373. https://doi.org/10.3390/app152111373
Chicago/Turabian StyleDębowski, Marcin, Izabela Świca, Marcin Zieliński, and Joanna Kazimierowicz. 2025. "The Effect of Pretreatment of Tetraselmis subcrodiformis (Wille) Butcher and Limnospira platensis (Gomont) Ciferri et Tiboni Biomass with Solidified Carbon Dioxide on the Efficiency of Anaerobic Digestion" Applied Sciences 15, no. 21: 11373. https://doi.org/10.3390/app152111373
APA StyleDębowski, M., Świca, I., Zieliński, M., & Kazimierowicz, J. (2025). The Effect of Pretreatment of Tetraselmis subcrodiformis (Wille) Butcher and Limnospira platensis (Gomont) Ciferri et Tiboni Biomass with Solidified Carbon Dioxide on the Efficiency of Anaerobic Digestion. Applied Sciences, 15(21), 11373. https://doi.org/10.3390/app152111373










