Effects of Na and Na/CO2 Synergism on Gas/Tar Production During Rapid Coal Pyrolysis
Abstract
1. Introduction
2. Experimental Apparatus and Methods
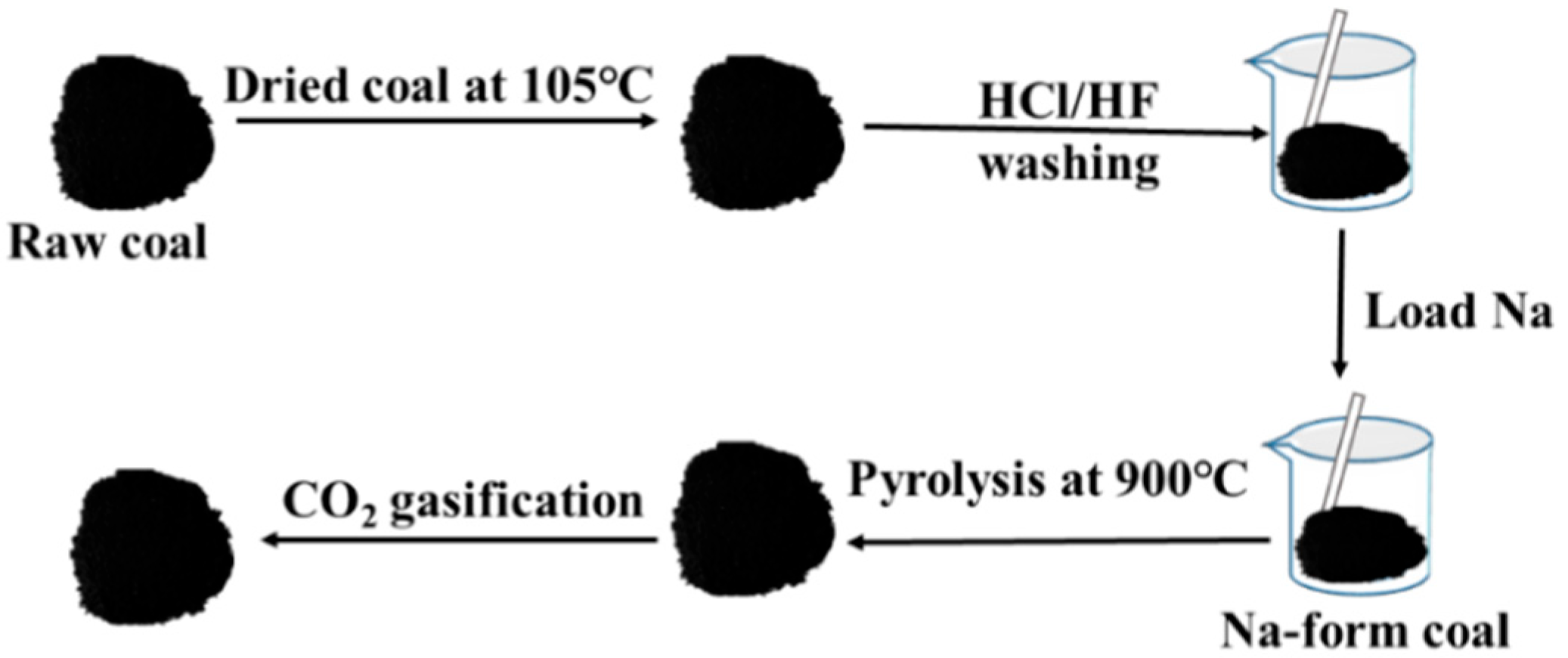
2.1. Experimental Raw Materials and Sample Preparation
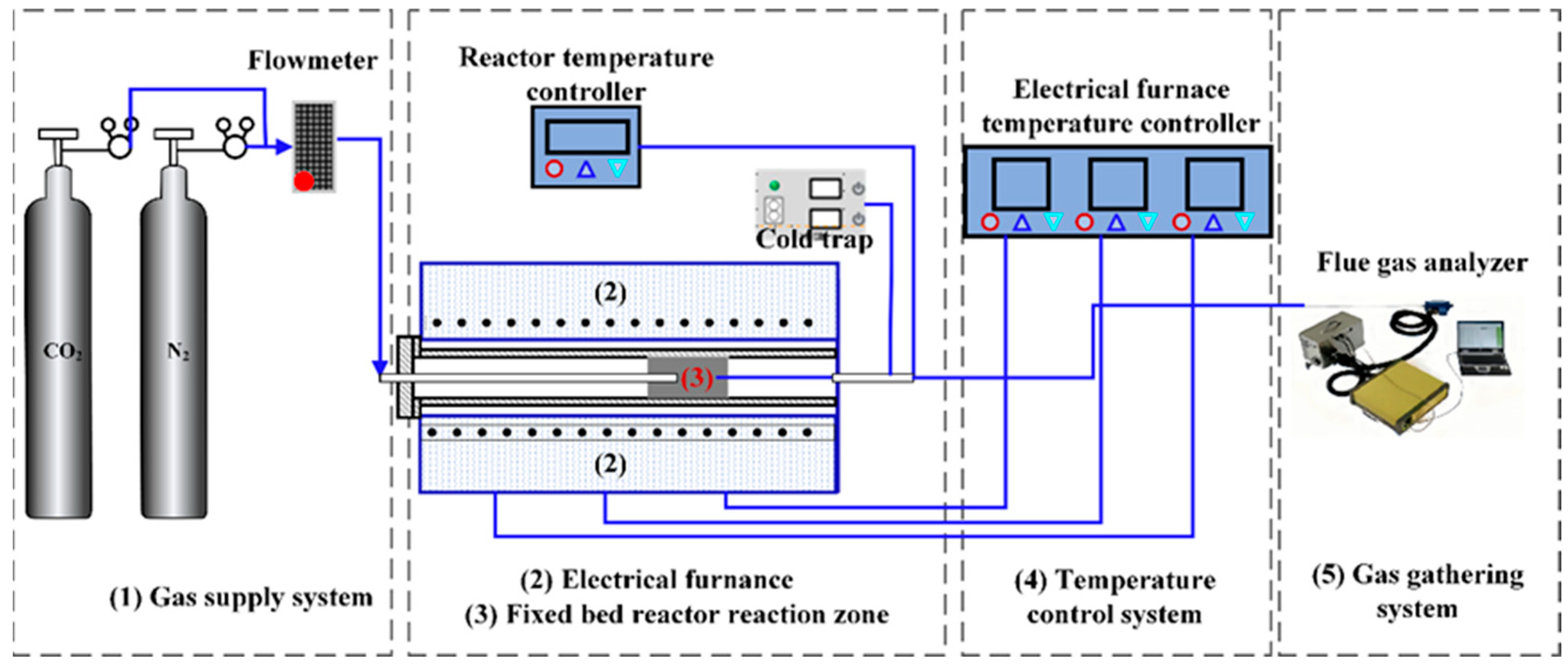
2.2. Preparation of Pyrolysis Tar and Online Analysis of Light Gases
2.3. Analysis of Homogeneous Conversion Characteristics of Pyrolysis Tar Under CO2
3. Results and Discussion
3.1. Influence of Alkali Metal Na on Pyrolysis Gas and Tar Generation Characteristics
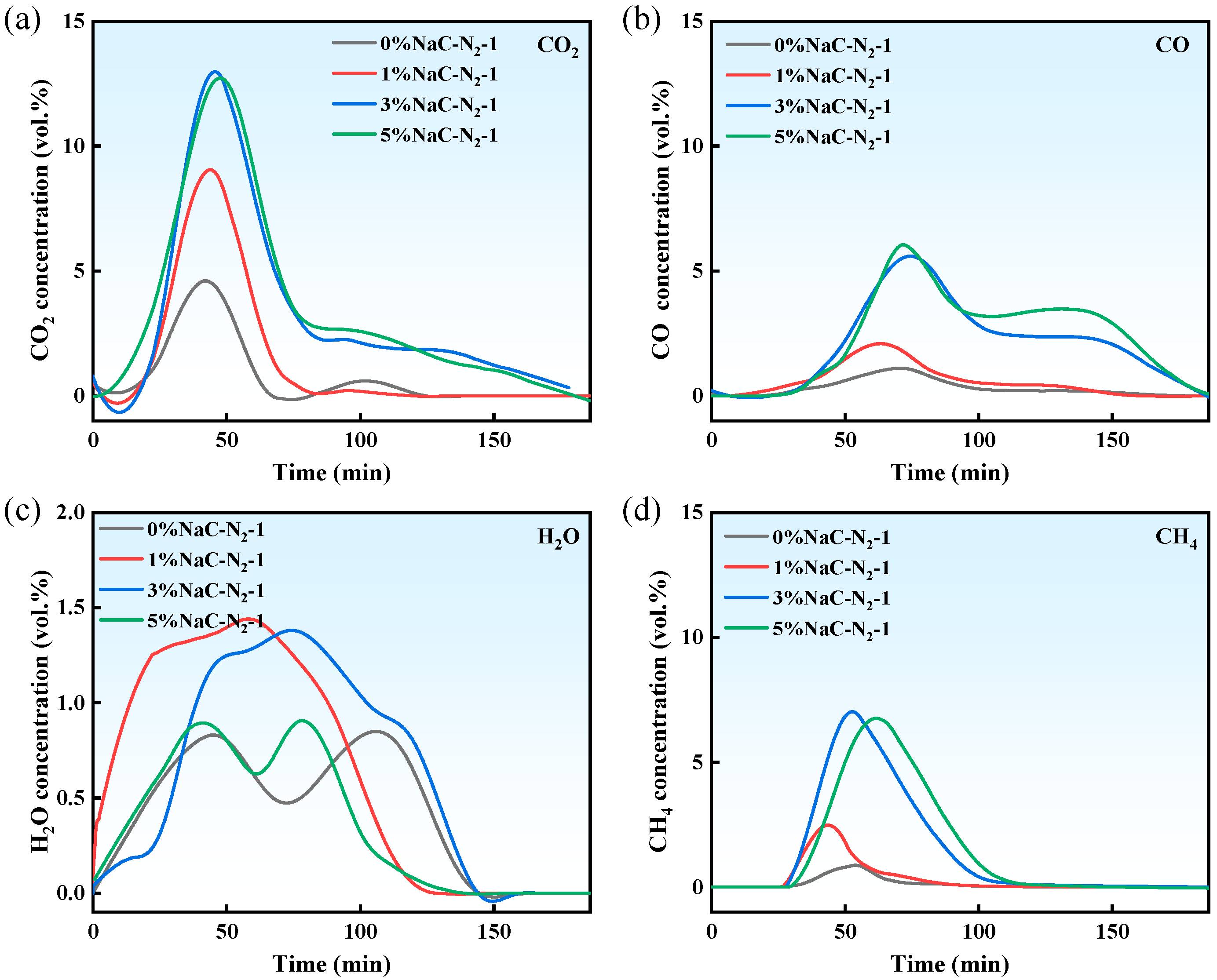
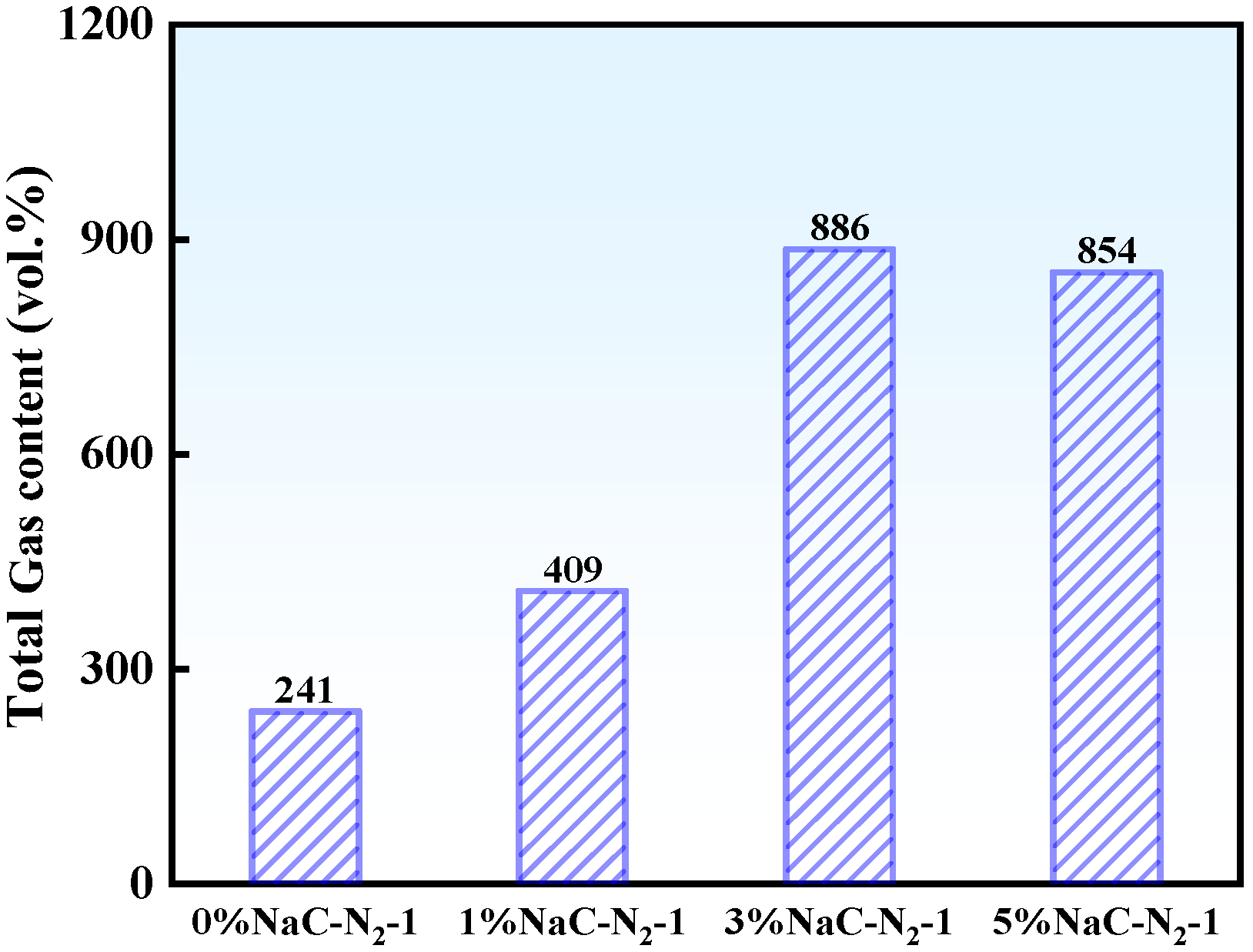
3.1.1. Effect of Na on Pyrolysis Gas Products
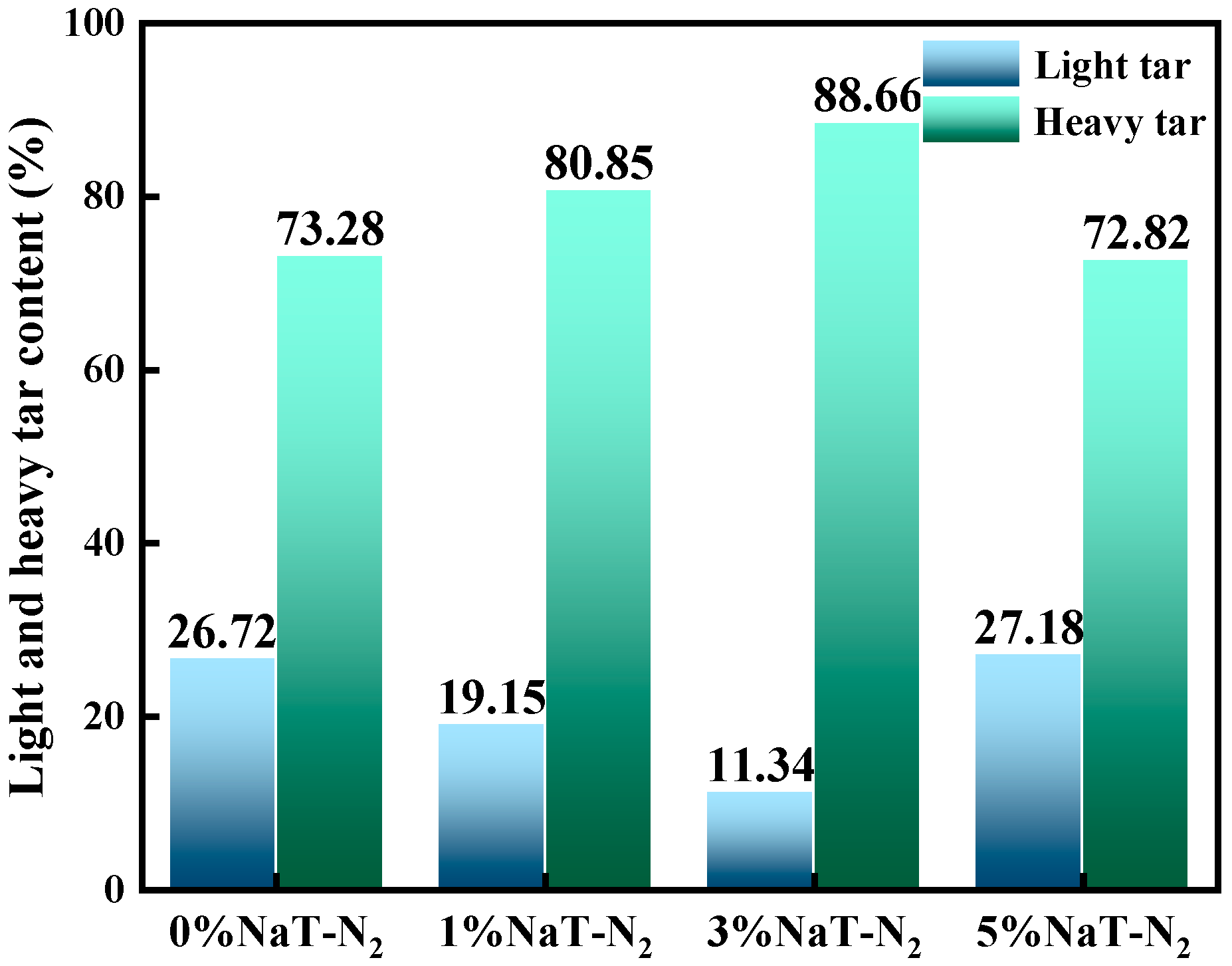
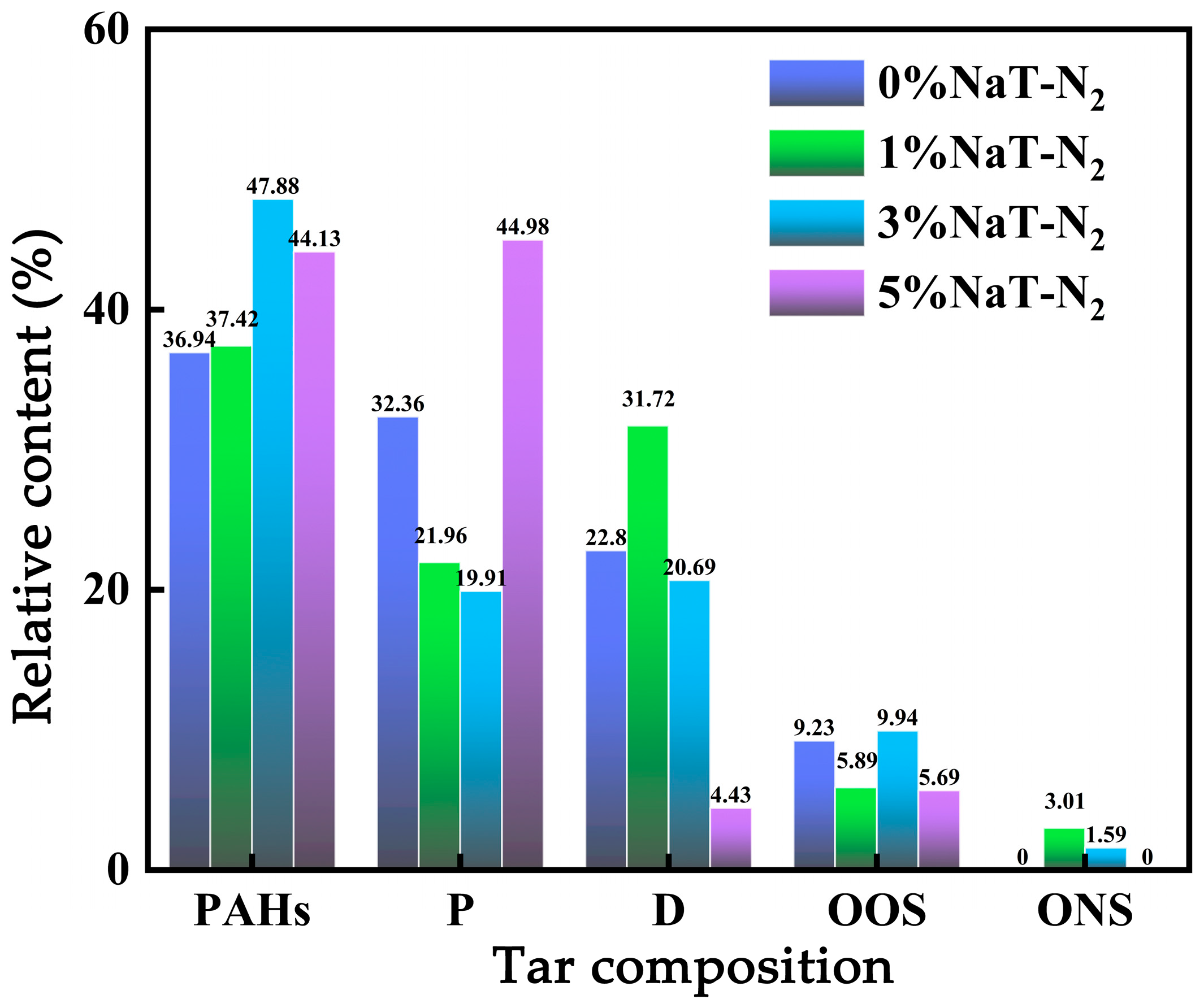
3.1.2. Effect of Na on Coal Tar Product Distribution
3.2. Homogeneous Transformation Characteristics of Coal Tar Under Na/CO2 Synergism
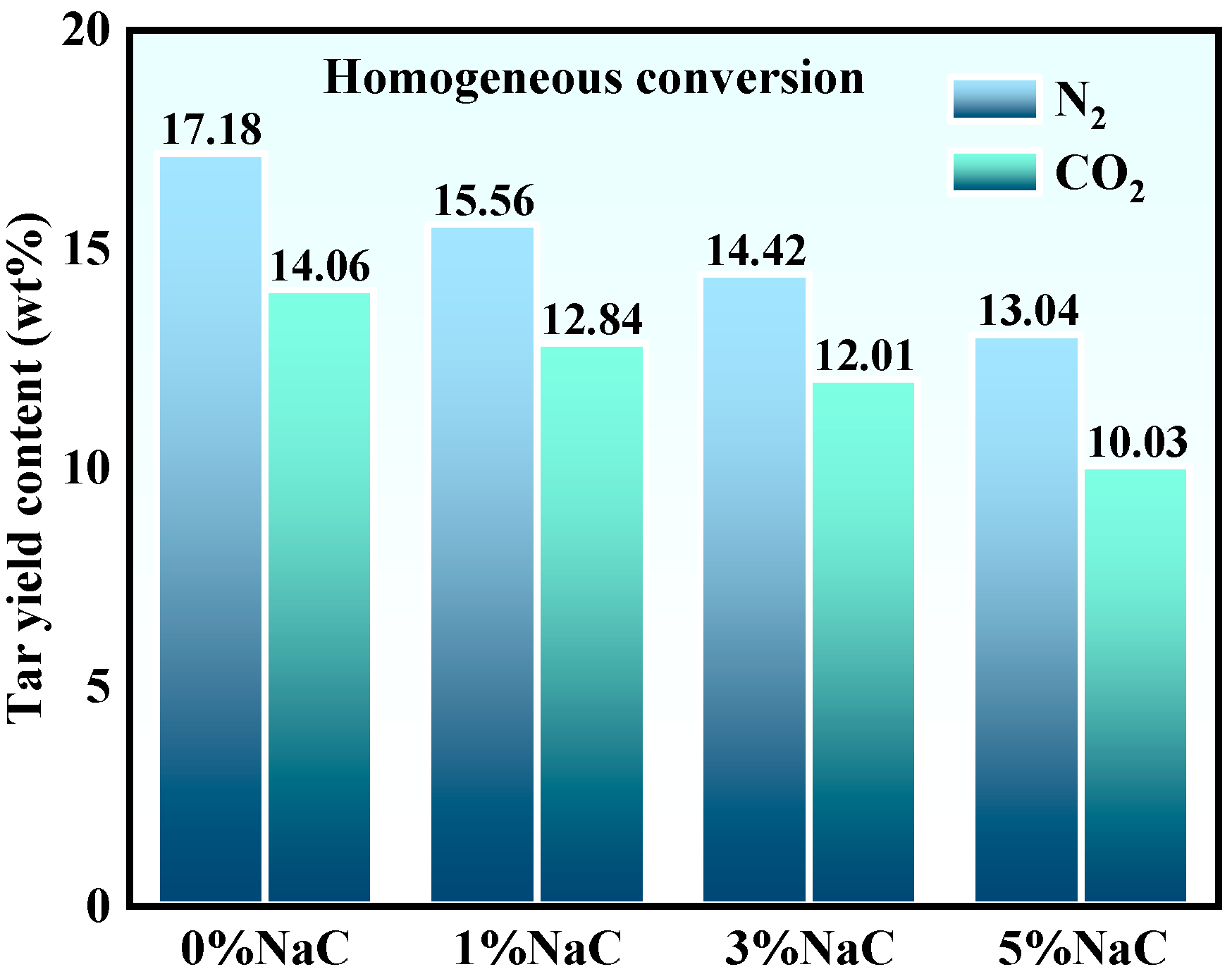
3.2.1. Coal Tar Yields
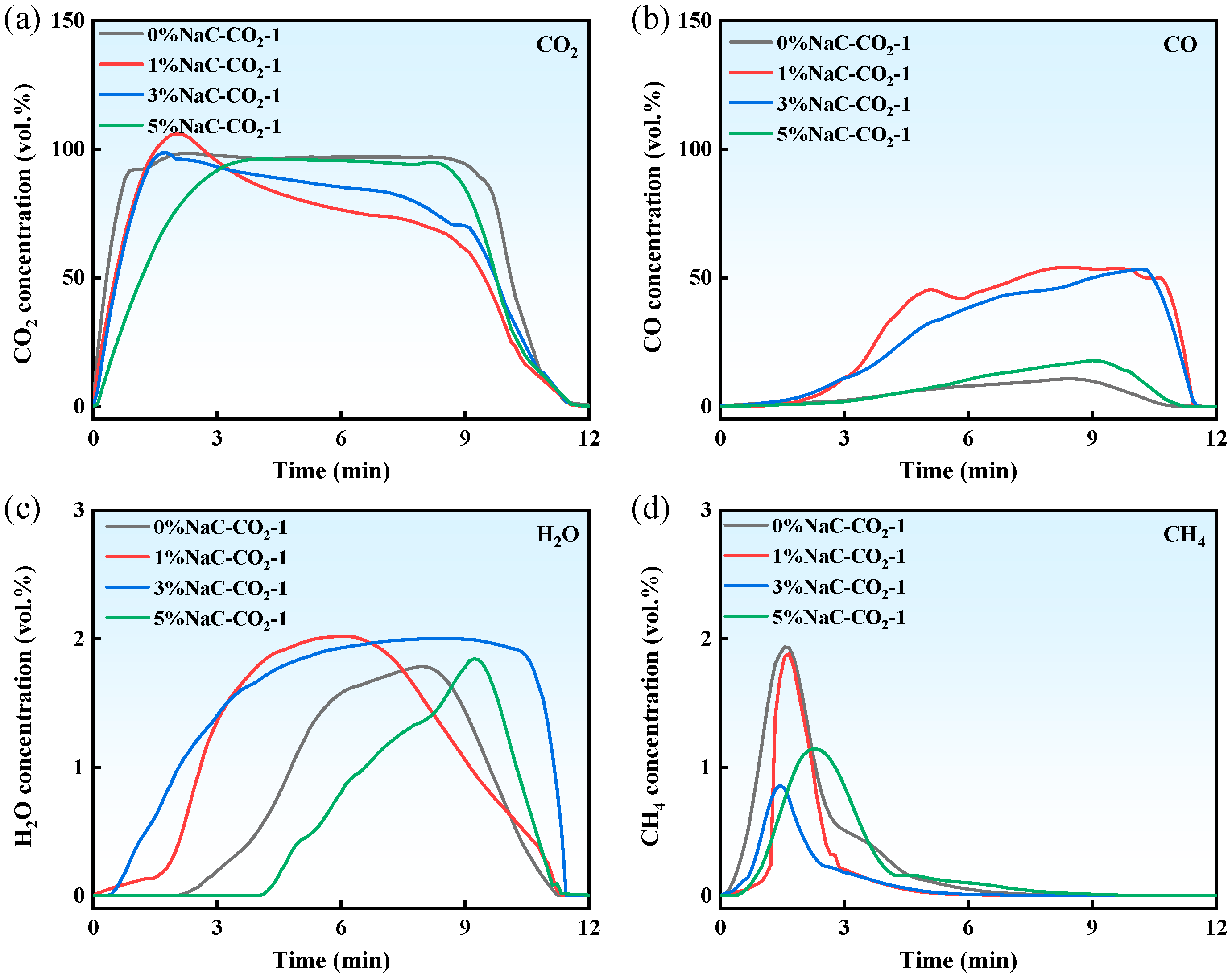
3.2.2. Effect of Na/CO2 Synergism on Gas Products
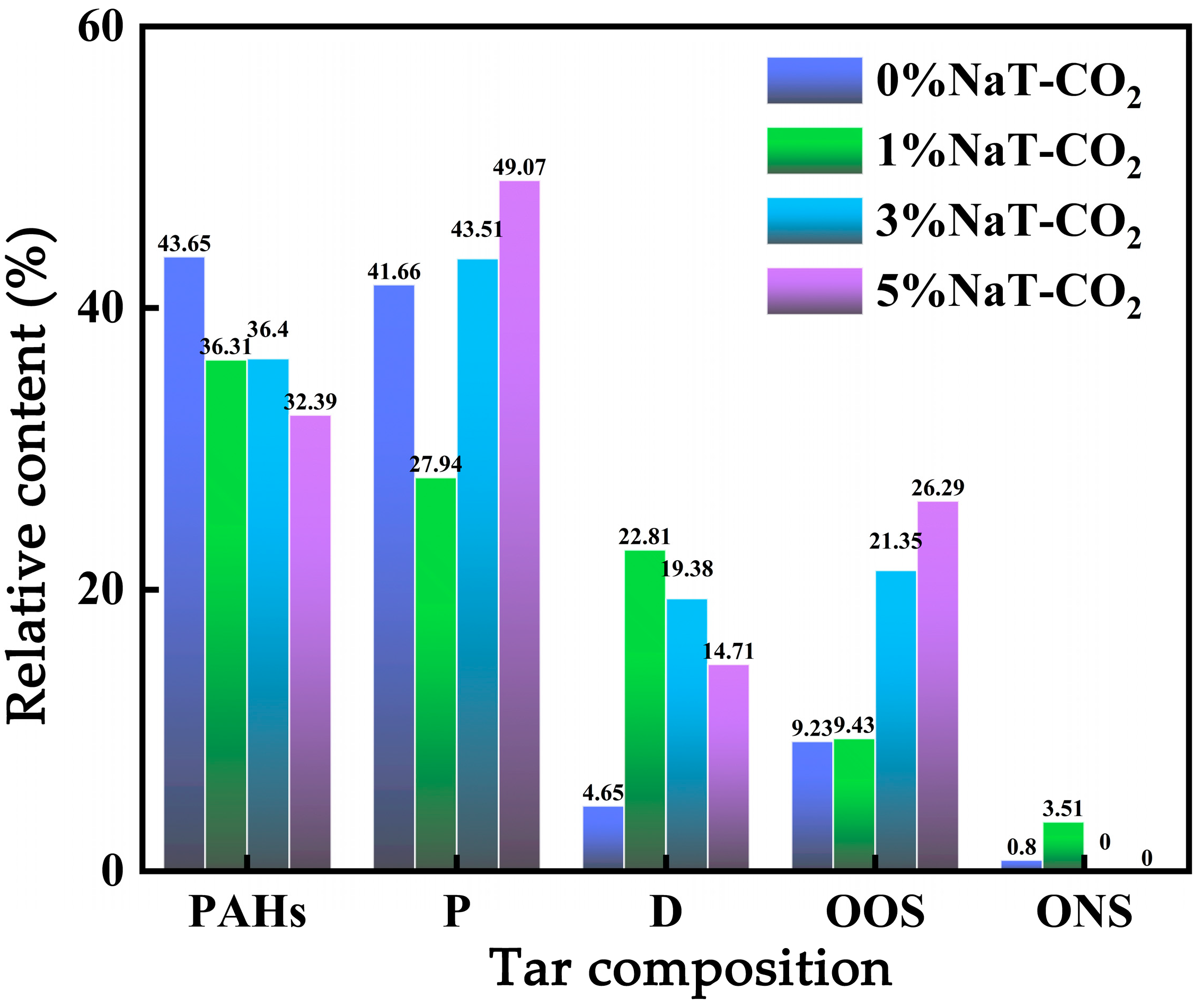
3.2.3. Effect of Na/CO2 Synergism on Homogeneous Transformation of Coal Tar
3.2.4. Homogeneous Transformation Mechanism of Coal Tar Under Na/CO2 Synergism
4. Conclusions
- (1)
- Na prolonged the gas release duration and increased the yields of H2O, CO, CO2, and CH4. At 5% Na loading, gas production increased sharply: CO2, CO, and CH4 yields increased by approximately 49 vol.%, 382 vol.%, and 251 vol.%, respectively. The increments for CO and CH4 were about 8 times and 3 times greater than those for CO2 and H2O.
- (2)
- The effect of Na on coal tar was closely dependent on its concentration. At low concentrations (1%, 3%), Na acted as a catalyst and interacted with cross-linking points during pyrolysis, promoting condensation reactions among light components. Conversely, at high concentration (5%), Na provided abundant active sites for tar cracking reactions. This facilitated the cleavage of large molecules in heavy oil (e.g., complex polycyclic aromatic hydrocarbons), decomposing them into more radical fragments and increasing light oil content. Furthermore, increasing Na content elevated the proportion of polycyclic aromatic hydrocarbons (PAHs) and phenolic compounds while reducing the proportions of cyclosiloxanes, oxygen-containing compounds, and nitrogen-containing compounds.
- (3)
- Light oil yields under the action of Na alone, CO2 alone, and Na/CO2 synergism were 27.18%, 27.93%, and 40.35%, respectively. This demonstrates the pronounced synergistic effect of Na/CO2 during tar homogeneous transformation. Na/CO2 synergism promoted tar cracking into radical fragments. Active radicals reacted with C-C bonds in aromatic rings, converting them into light tar molecules, thereby achieving homogeneous transformation of coal pyrolysis tar by CO2. It increased the content of phenolic compounds, cyclosiloxanes, and oxygen-containing compounds, while decreasing the content of PAHs.
- (4)
- The Na/CO2 synergism provides a scalable technical route for clean coal valorization—40.35% light oil yield supports coal-to-liquid process upgrading, and enhanced CO production enables precise syngas H2/CO ratio tuning for Fischer–Tropsch synthesis, aligning with carbon neutrality via CO2 reuse. In the future, focus on molecular simulations and quantum chemical calculations, which are still required to investigate the formation mechanism of multi-active-site coke and the influence mechanism of the Na/CO2 coupling on the homogeneous conversion of coal tar at the molecular structural level.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, X.; Liu, Z.; Fan, W.; Zhou, C.; Liu, S.; Chen, J.; Guo, H. Effect of metal elements in coal ash on NO release characteristics during oxy-fuel combustion at high temperature. J. Energy Inst. 2021, 94, 107–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, H.; Wang, X.; Zhang, L.; Rao, P.; Zhu, H. Influence Mechanism of Water-Soluble Sodium on Zhundong Coal Pyrolysis. ACS Omega 2022, 7, 11862–11870. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Xie, Q.; Wei, Z.; Wan, C.; Li, G.; Cao, J. Transformation of alkali and alkaline earth metals in Zhundong coal during pyrolysis in an entrained flow bed reactor. J. Anal. Appl. Pyrolysis 2019, 142, 104661. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.; Yao, Q. Dynamic behavior of sodium release from pulverized coal combustion by phase-selective laser-induced breakdown spectroscopy. Proc. Combust. Inst. 2015, 35, 2339–2346. [Google Scholar] [CrossRef]
- Liu, N.; Huang, H.; Feng, J.; Li, R.; Huang, X.; Wu, Y. Effects of alkali and alkaline earth metals in biomass on co-pyrolysis characteristics and product distribution of coal and biomass. Fuel 2025, 389, 134551. [Google Scholar] [CrossRef]
- Yan, L.-J.; Bai, Y.-H.; Kong, X.-J.; Li, F. Effects of alkali and alkaline earth metals on the formation of light aromatic hydrocarbons during coal pyrolysis. J. Anal. Appl. Pyrolysis 2016, 122, 169–174. [Google Scholar] [CrossRef]
- He, Y.; Wang, J.F.; Zhang, Y.J.; Song, X.D.; Su, W.G.; Bai, Y.H.; Lü, P.; Yu, G.S. Influence of Structural Properties of Al2O3; on Alkali Metal Adsorption and Catalytic Tar Cracking Performance during High-Temperature Co-Pyrolysis of Coal and Biomass. Coal Convers. 2025, 48, 70–79. [Google Scholar] [CrossRef]
- Kong, L.; Bai, J.; Li, W. Viscosity-temperature property of coal ash slag at the condition of entrained flow gasification: A review. Fuel Process. Technol. 2021, 215, 106751. [Google Scholar] [CrossRef]
- He, Q.; Xu, J.; Jiang, X.; Wang, C.; Jiang, M.; Wei, Y.; Jiang, L.; Xu, K.; Wang, Y.; Su, S.; et al. Effects of AAEMs on the char heterogeneous structure evolution during Zhundong coal pyrolysis: Insights from micro-Raman spectroscopy. Fuel 2023, 347, 128378. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, X.; Chen, X.; Guo, W.; Wang, Y.; Zhang, J.; Kawa, O. Investigation on the effects of different forms of sodium, chlorine and sulphur and various pretreatment methods on the deposition characteristics of Na species during pyrolysis of a Na-rich coal. Fuel 2018, 234, 872–885. [Google Scholar] [CrossRef]
- Lu, R.; Wang, J.; Liu, Q.; Wang, Y.; Te, G.; Ban, Y.; Li, N.; Zhang, X.; He, R.; Zhou, H.; et al. Catalytic effect of sodium components on the microstructure and steam gasification of demineralized Shengli lignite char. Int. J. Hydrogen Energy 2017, 42, 9679–9687. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, C.; Shao, C.; Chen, G.; Sun, S.; Chen, G.; Zhang, L.; Pei, J.; Qiu, P.; Guo, S. Impacts of intrinsic alkali and alkaline earth metals on chemical structure of low-rank coal char: Semi-quantitative results based on FT-IR structure parameters. Fuel 2020, 278, 118229. [Google Scholar] [CrossRef]
- Qiu, P.; Du, C.; Liu, L.; Chen, L. Hydrogen and syngas production from catalytic steam gasification of char derived from ion-exchangeable Na- and Ca-loaded coal. Int. J. Hydrogen Energy 2018, 43, 12034–12048. [Google Scholar] [CrossRef]
- Liu, S.; Tuo, K.; Wang, L.; Chen, G.; Ma, W.; Fang, M. Microwave-assisted metal-catalyzed pyrolysis of low-rank coal: Promising option towards obtaining high-quality products. J. Energy Inst. 2020, 93, 1602–1614. [Google Scholar] [CrossRef]
- Li, Y.N. Study on Na/Ca-Catalyzed Coal Pyrolysis Characteristics and Tar Product Regulation. Master’s Thesis, Shenyang Aerospace University, Shenyang, China, 2022. [Google Scholar] [CrossRef]
- Wang, P.; Jin, L.; Liu, J.; Zhu, S.; Hu, H. Analysis of coal tar derived from pyrolysis at different atmospheres. Fuel 2013, 104, 14–21. [Google Scholar] [CrossRef]
- Lee, S.-R.; Lee, J.; Lee, T.; Tsang, Y.F.; Jeong, K.-H.; Oh, J.-I.; Kwon, E.E. Strategic use of CO2 for co-pyrolysis of swine manure and coal for energy recovery and waste disposal. J. CO2 Util. 2017, 22, 110–116. [Google Scholar] [CrossRef]
- Oh, J.-I.; Lee, J.; Lee, T.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Strategic CO2 utilization for shifting carbon distribution from pyrolytic oil to syngas in pyrolysis of food waste. J. CO2 Util. 2017, 20, 150–155. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, C.; Zhu, S.; Bai, Y.; Li, F. Tar formation during coal pyrolysis under N2 and CO2 atmospheres at elevated pressures. J. Anal. Appl. Pyrolysis 2016, 118, 130–135. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, P.; Yan, L.; Liu, C.; Li, F.; Xie, K. Effects of CO2 on gas evolution and char structure formation during lump coal pyrolysis at elevated pressures. J. Anal. Appl. Pyrolysis 2013, 104, 202–209. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, D.; Yao, Q.; Liu, Y.; Su, X.; Jia, C.Q.; Hao, Q.; Ma, X. Separation and Composition Analysis of GC/MS Analyzable and Unanalyzable Parts from Coal Tar. Energy Fuels 2018, 32, 7404–7411. [Google Scholar] [CrossRef]
- GB/T212-2008; Proximate Analysis of Coal. China Standards Press: Beijing, China, 2008.
- Xiao, N.; Luo, H.; Wei, W.; Tang, Z.; Hu, B.; Kong, L.; Sun, Y. Microwave-assisted gasification of rice straw pyrolytic biochar promoted by alkali and alkaline earth metals. J. Anal. Appl. Pyrolysis 2015, 112, 173–179. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Wang, X.; Bai, J.; Kong, L.; Bai, Z.; Li, J.; Li, H.; Xing, L.; Li, W. Insights into the effect of particle size on coal char particle gasification by thermogravimetric analyzer and high temperature stage microscope. Fuel 2022, 313, 123010. [Google Scholar] [CrossRef]
- Dong, L.; Han, S.; Yu, W.; Lei, Z.; Kang, S.; Zhang, K.; Yan, J.; Li, Z.; Shui, H.; Wang, Z.; et al. Effect of volatile reactions on the yield and quality of tar from pyrolysis of Shenhua bituminous coal. J. Anal. Appl. Pyrolysis 2019, 140, 321–330. [Google Scholar] [CrossRef]
- Xu, K.; Hu, S.; Wang, Y.; Zhang, L.; Su, S.; Jiang, L.; Xu, J.; Ouyang, Z.; Xiang, J. Relation between char structures and formation of volatiles during the pyrolysis of Shenfu coal: Further understanding on the effects of mobile phase and fixed phase. Fuel Process. Technol. 2018, 178, 379–385. [Google Scholar] [CrossRef]
- Wu, D.; Dong, H.; Luan, J.; Du, Q.; Gao, J.; Feng, D.; Zhang, Y.; Zhao, Z.; Li, D. Reaction Molecular Dynamics Study on the Mechanism of Alkali Metal Sodium at the Initial Stage of Naphthalene Pyrolysis Evolution. Energies 2023, 16, 6186. [Google Scholar] [CrossRef]
- Li, R.; Chen, Q.; Zhang, H. Detailed Investigation on Sodium (Na) Species Release and Transformation Mechanism during Pyrolysis and Char Gasification of High-Na Zhundong Coal. Energy Fuels 2017, 31, 5902–5912. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Du, Q.; Li, D.; Feng, D.; Gao, J.; Wu, S.; Luan, J. Roles of Ion-Exchangeable Sodium in the Conversion Process of Tar to Soot during Rapid Pyrolysis of Two Brown Coals in a Drop-Tube Reactor. ACS Omega 2020, 5, 9078–9092. [Google Scholar] [CrossRef]
- Lu, Q.; Guo, R.; Zhang, H.; Wang, J.-P.; Lu, T.; Li, G.-Y.; Liang, Y.-H. To stimulate, and to inhibit: A theoretical understanding of the sodium-catalytic mechanism of coke gasification. Chem. Eng. J. 2022, 435, 135091. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, J.; Du, Q.; Li, D.; Gao, J.; Dong, H.; Zhang, Y.; Wu, D.; Yang, X. The synergistic influence mechanism of the alkali metal sodium and CO2 on coal rapid pyrolysis soot: Experiments and DFT calculations. Fuel 2025, 382, 133294. [Google Scholar] [CrossRef]
- Pyshyev, S.; Korchak, B.; Miroshnichenko, D.; Lebedev, V.; Yasinska, A.; Lypko, Y. Obtaining New Materials from Liquid Pyrolysis Products of Used Tires for Waste Valorization. Sustainability 2025, 17, 3919. [Google Scholar] [CrossRef]
- Seiji, S.; Katsutoshi, I. Effect of Coal Interaction on Coke Strength. Tetsu Hagane-J. Iron Steel Inst. Jpn. 2001, 87, 238–244. [Google Scholar]
- Zhao, Z.; Gao, J.; Du, Q.; Li, D.; Dong, H.; Zhang, Y.; Li, H. Effect of Na on the condensation reaction of naphthalene molecules during coal pyrolysis. J. Energy Inst. 2021, 98, 313–321. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Fu, H.; Shi, Y.; Ling, L. Harmonizing the cyano-group and Na to enhance selective photocatalytic O2 activation on carbon nitride for refractory pollutant degradation. Proc. Natl. Acad. Sci. USA 2024, 121, e2318787121. [Google Scholar]
- Yao, R.; Wei, J.; Ge, Q.; Xu, J.; Han, Y.; Ma, Q.; Xu, H.; Sun, J. Monometallic iron catalysts with synergistic Na and S for higher alcohols synthesis via CO2 hydrogenation. Appl. Catal. B 2021, 298, 120556. [Google Scholar] [CrossRef]


| Sample | Industrial Analysis | Elemental Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | Cad | Had | Oad | Nad | Sad | |
| ZD | 25.92 | 4.22 | 19.75 | 50.10 | 67.66 | 3.58 | 27.4 | 0.74 | 0.62 |
| HZD | 24.25 | 1.54 | 16.44 | 57.77 | 73.45 | 3.48 | 21.18 | 1.24 | 0.65 |
| Sample | Na2O | K2O | CaO | MgO | SiO2 | SO3 | Al2O3 | Fe2O3 | P2O5 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| ZD | 5.57 | 0.70 | 32.29 | 10.44 | 1.02 | 39.19 | 3.00 | 8.70 | 0.21 | 0.10 |
| Study | Tar Yield Reduction | Gas Composition (Key Gases) | Methodology/Conditions | Main Findings and Notes |
|---|---|---|---|---|
| Current Study (Na/CO2 Synergism) | 7.15% decrease | CO, CH4 | Fixed-bed reactor, GC-MS | Na/CO2 synergism increased light oil yield and gas release. |
| Zhao et al. [31] (2025) | ~10% decrease | CO, CH4 | Fixed-bed reactor, NaNO3 with CO2 | NaNO3 with CO2 improved light oil yield and promoted tar cracking. |
| Luo et al. [19] (2016) | Increased with pressure | CO2, CH4 | Fixed-bed reactor, CO2 atmosphere | CO2 increased the gas yield, and Na/CO2 decreased the tar yield. |
| Dong et al. [29] (2020) | Significant decrease | CO, CH4 | Fixed-bed reactor, Na-loaded coal | Ion-exchange Na reduced tar and soot yield. |
| No. | RT (min) | M.W. | Composition Structure | Relative Content (%) | |||
|---|---|---|---|---|---|---|---|
| 0%NaT-CO2 | 1%NaT-CO2 | 3%NaT-CO2 | 5%NaT-CO2 | ||||
| 1 | 9.25 | 94 |  | 12.19 | 16.04 | 18.61 | 24.45 |
| 2 | 10.87 | 108 | 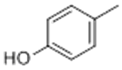 | 12.76 | 11.23 | 11.57 | 13.48 |
| 3 | 19.80 | 152 |  | 6.43 | 2.39 | 1.74 | 2.18 |
| 4 | 18.04 | 166 | 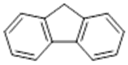 | 6.42 | 6.75 | 7.58 | 8.10 |
| 5 | 17.23 | 168 | 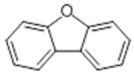 | 6.54 | 7.22 | 7.05 | 6.68 |
| 6 | 20.29 | 178 | 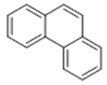 | 5.74 | 6.90 | 7.88 | 6.95 |
| 7 | 23.99 | 266 | 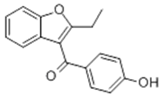 | 6.43 | 0.00 | 0.00 | 3.41 |
| 8 | 16.38 | 384 | 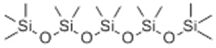 | 0.00 | 9.67 | 8.30 | 11.54 |
| 9 | 14.19 | 444 | 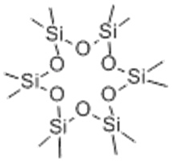 | 3.77 | 5.80 | 4.72 | 7.82 |
| 10 | 16.38 | 518 | 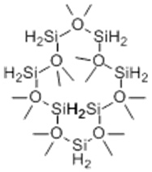 | 0.00 | 9.67 | 8.30 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Ma, R.; Wei, B.; Li, S.; Guo, L.; Lin, Q. Effects of Na and Na/CO2 Synergism on Gas/Tar Production During Rapid Coal Pyrolysis. Appl. Sci. 2025, 15, 11331. https://doi.org/10.3390/app152111331
Wang F, Ma R, Wei B, Li S, Guo L, Lin Q. Effects of Na and Na/CO2 Synergism on Gas/Tar Production During Rapid Coal Pyrolysis. Applied Sciences. 2025; 15(21):11331. https://doi.org/10.3390/app152111331
Chicago/Turabian StyleWang, Feng, Rui Ma, Bo Wei, Shuanglong Li, Liqing Guo, and Qianjin Lin. 2025. "Effects of Na and Na/CO2 Synergism on Gas/Tar Production During Rapid Coal Pyrolysis" Applied Sciences 15, no. 21: 11331. https://doi.org/10.3390/app152111331
APA StyleWang, F., Ma, R., Wei, B., Li, S., Guo, L., & Lin, Q. (2025). Effects of Na and Na/CO2 Synergism on Gas/Tar Production During Rapid Coal Pyrolysis. Applied Sciences, 15(21), 11331. https://doi.org/10.3390/app152111331






