The Influence of Technological Conditions of Co-Fermentation of Lignocellulosic and Starch Raw Materials on the Amount of Volatile By-Products Formed and the Quality of Obtained Bioethanol
Abstract
1. Introduction
- -
- the level of fermentable carbohydrates and amino acids for yeast through using 1G raw materials,
- -
- the level of glucose after lignocellulose decomposition and a significant reduction in feedstock costs through using 2G raw materials.
2. Materials and Methods
2.1. Raw Material
2.2. Pretreatment and Enzymatic Hydrolysis
2.2.1. Lignocellulosic Biomass
2.2.2. Corn Grain
2.3. Microorganisms
2.4. Fermentation Process
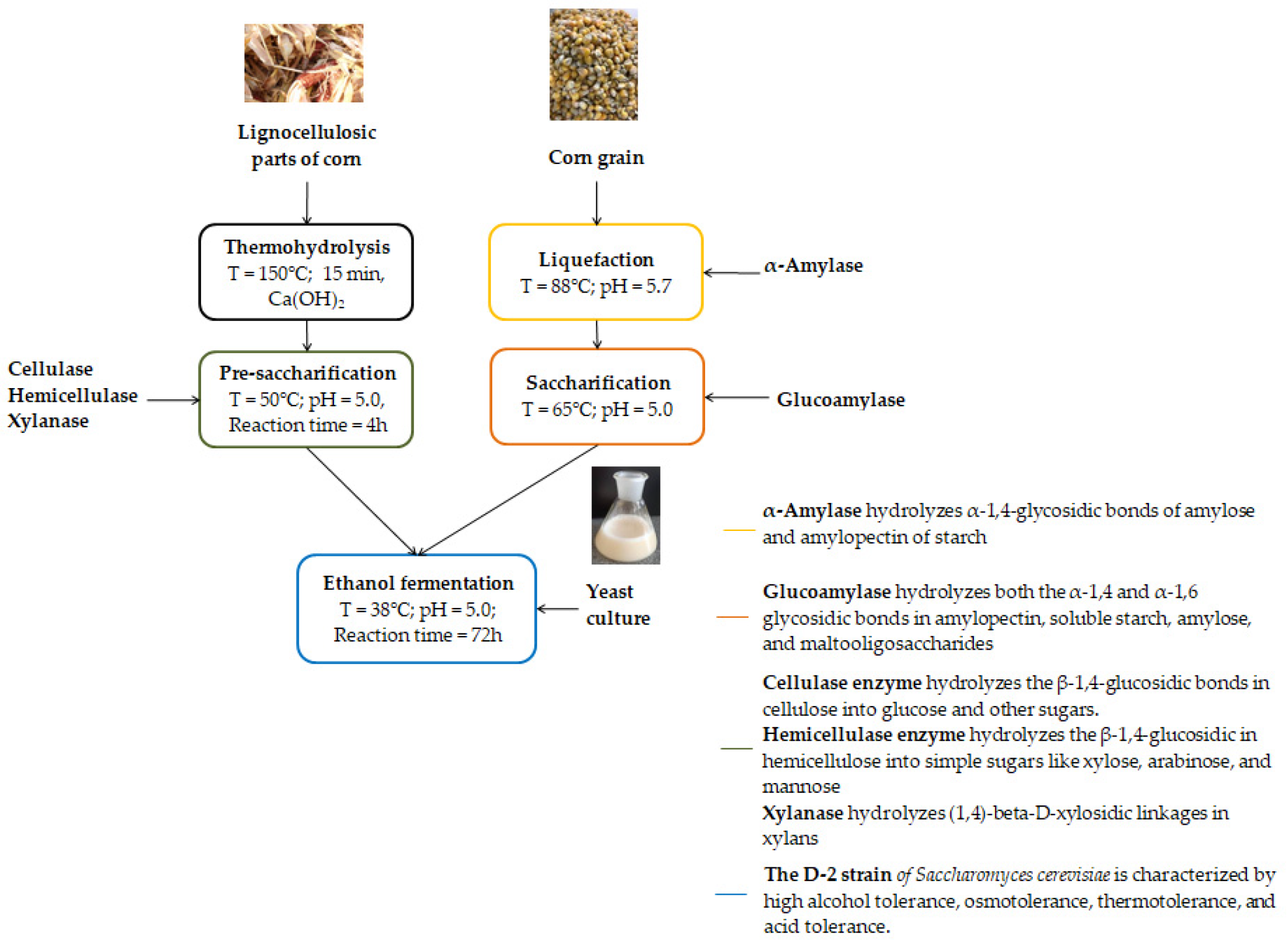
2.5. Process Variants
- Variant 1
- (1G + 2G, starch–lignocellulosic mash): starch and lignocellulosic biomasses were pretreated separately. The liquefied and saccharified corn suspension was combined with the pretreated lignocellulosic slurry, and the mixture was subjected to fermentation using the SHF/SSF method, as shown in Figure 1.
- Variant 2
- (1G, starch biomass): ground corn grain was liquefied with α-amylase and subsequently saccharified with glucoamylase. The resulting mash was then fermented to ethanol.
- Variant 3
- (2G, lignocellulosic biomass): the lignocellulosic feedstock was subjected to thermohydrolysis followed by pre-saccharification, after which the obtained slurry was fermented using the SHF/SSF method.
2.6. Sample Analysis
2.6.1. Dry Matter (%), Dry Organic Matter (% DM), pH
2.6.2. Cellulose, Hemicellulose and Lignin Content
2.6.3. Marking of the Volatile By-Products
2.6.4. Statistical Analysis
3. Results
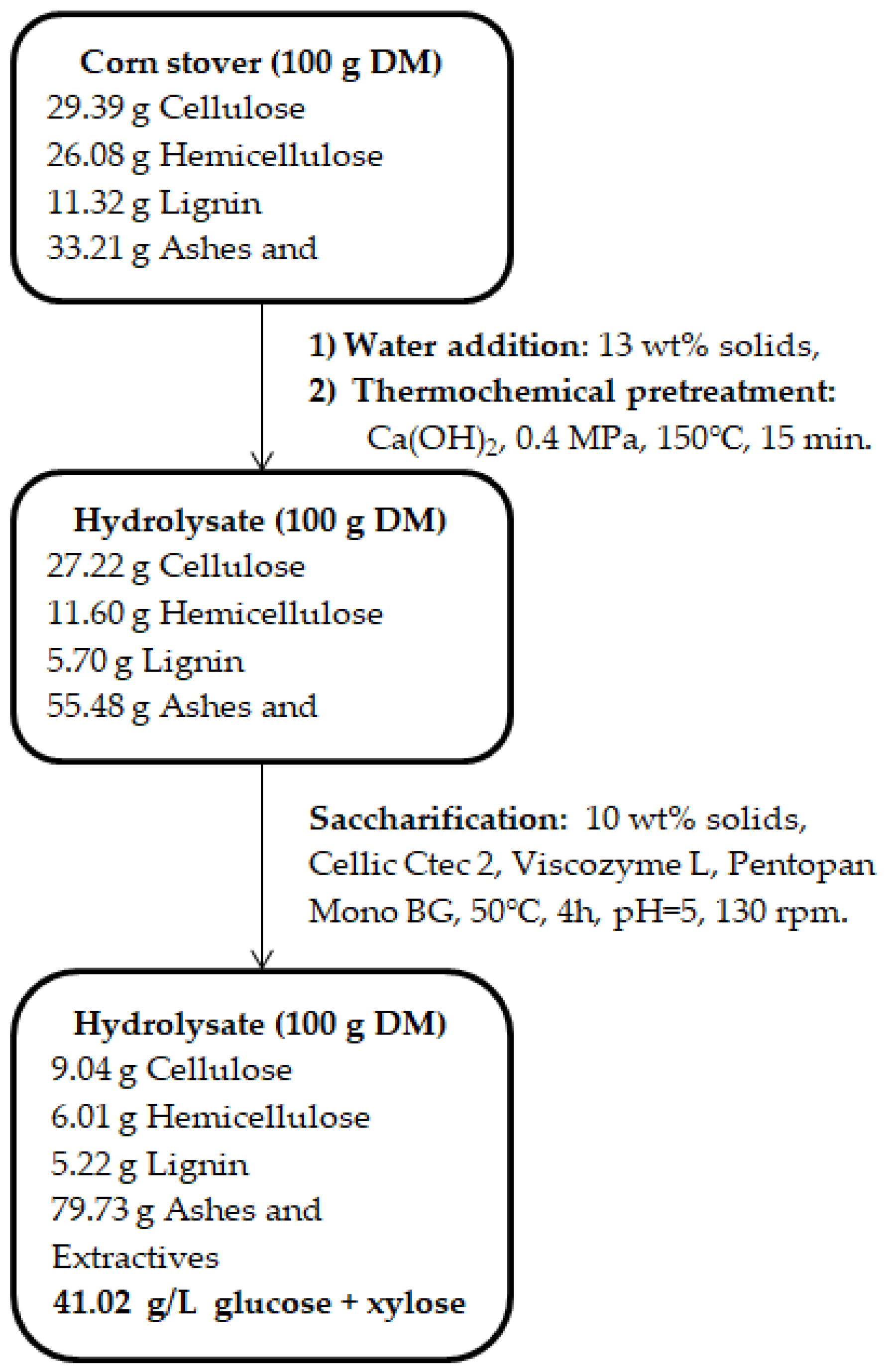
3.1. Decomposition of Lignocellulosic Biomass
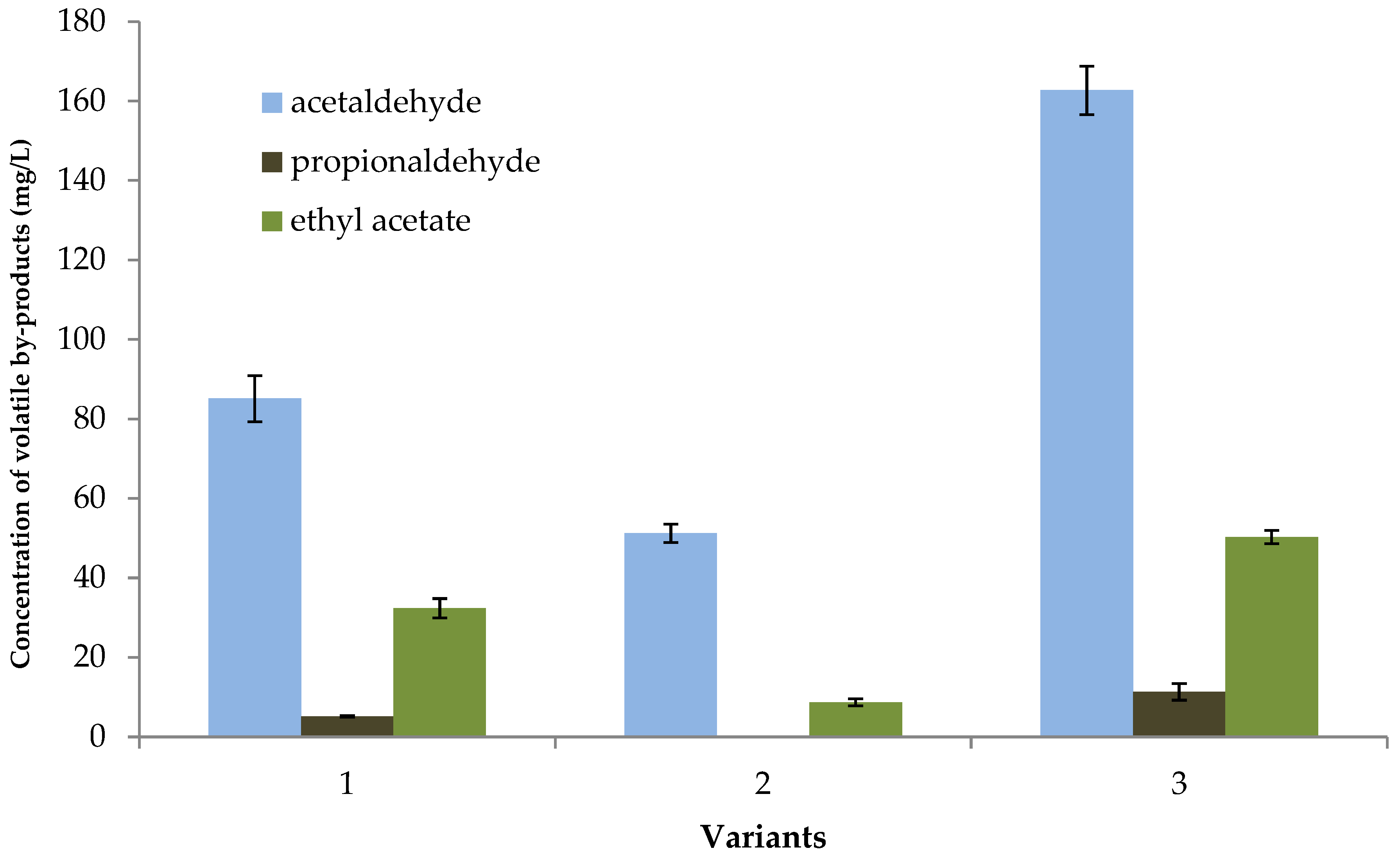
3.2. The Content of Volatile Chemical Compounds in Distillates Obtained from Starch-Lignocellulosic Mashes and Mono-Feedstock
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czyżyk, F.; Strzelczyk, M. Rational utilization of production residues generated in agri-food. Arch. Environ. Prot. 2015, 17, 99–106. [Google Scholar]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Erdei, B. Development of Integrated Cellulose- and Starch-Based Ethanol Production and Process Design for Improved Xylose Conversion. Ph.D. Thesis, Department of Chemical Engineering, Lund University, Lund, Sweden, 2013. [Google Scholar]
- Joelsson, E.; Erdei, B.; Galbe, M.; Wallberg, O. Techno-economic evaluation of integrated first- and second-generation ethanol production from grain and straw. Biotechnol. Biofuels 2016, 9, 1. [Google Scholar] [CrossRef]
- Li, Y.; Kesharwani, R.; Sun, Z.; Qin, R.; Dagli, C.; Zhang, M.; Wang, D. Economic viability and environmental impact investigation for the biofuel supply chain using co-fermentation technology. Appl. Energy 2020, 259, 114235. [Google Scholar] [CrossRef]
- Govil, T.; Wang, J.; Samanta, D.; David, A.; Tripathi, A.; Rauniyar, S.; Salem, D.R.; Sani, R.K. Lignocellulosic feedstock: A review of a sustainable platform for cleaner production of nature’s plastics. J. Clean. Prod. 2020, 270, 122521. [Google Scholar] [CrossRef]
- Erdei, B.; Barta, Z.; Sipos, B.; Réczey, K.; Galbe, M.; Zacchi, G. Ethanol production from mixtures of wheat straw and wheat meal. Biotechnol. Biofuels 2010, 3, 16. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Konda, N.M.; Shi, J.; Dutta, T.; Scown, C.D. Transforming biomass conversion with ionic liquids: Process intensification and the development of a high-gravity, one-pot process for the production of cellulosic ethanol. Energy Environ. Sci. 2016, 9, 1042. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D. Integrating starchy substrate into cellulosic ethanol production to boost ethanol titers and yields. Appl. Energy 2017, 195, 196–203. [Google Scholar] [CrossRef]
- Li, J.; Zhao, R.; Xu, Y.; Wu Bean, X.; Wang, D. Fuel ethanol production from starchy grain and other crops: An overview on feedstocks, affecting factors, and technical advances. Renew. Energy 2022, 188, 223–239. [Google Scholar] [CrossRef]
- Martínez-Jimenez, F.D.; Pereira, I.O.; Ribeiro, M.P.A.; Sargo, C.R.; dos Santos, A.A.; Zanella, E.; Stambuk, B.U.; Ienczak, J.L.; Morais, E.R.; Costa, A.C. Integration of first- and second-generation ethanol production: Evaluation of a mathematical model to describe sucrose and xylose cofermentation by recombinant Saccharomyces cerevisiae. Renew. Energy 2022, 192, 326–339. [Google Scholar] [CrossRef]
- Katanski, A.; Vučurović, V.; Vučurović, D.; Bajić, B.; Šaranović, Ž.; Šereš, Z.; Dodić, S. Bioethanol production from a-starch milk and b-starch milk as intermediates of industrial wet-milling wheat processing. Fermentation 2024, 10, 144. [Google Scholar] [CrossRef]
- Dias, M.O.S.; Junqueira, T.L.; Rossell, C.E.V.; Filho, R.M.; Bonomi, A. Evaluation of process configurations for second generation integrated with first generation bioethanol production from sugarcane. Fuel Process. Technol. 2012, 109, 84–89. [Google Scholar] [CrossRef]
- Cavalett, O.; Junqueira, T.L.; Dias, M.O.S.; Jesus, C.D.F.; Mantelatto, P.E.; Cunha, M.P.; Franco, H.C.J.; Cardoso, T.F.; Filho, R.M.; Rossell, C.E.V.; et al. Environmental and economic assessment of sugarcane first generation biorefineries in Brazil. Clean Technol. Environ. Policy 2012, 14, 399–410. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Cruz, A.J.G.; Costa, C.B.B. Improving second generation bioethanol production in sugarcane biorefineries through energy integration. Appl. Therm. Eng. 2016, 109, 819–827. [Google Scholar] [CrossRef]
- Erdei, B.; Frankó, B.; Galbe, M.; Zacchi, G. Separate hydrolysis and co-fermentation for improved xylose utilization in integrated ethanol production from wheat meal and wheat straw. Biotechnol. Biofuels 2012, 5, 12. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, D.; Cristhian, C.; Jiang, J. Simultaneous saccharification and cofermentation of lignocellulosic residues from commercial furfural production and corn kernels using different nutrient media. Biotechnol. Biofuels 2011, 4, 22. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, M.; Roozeboom, K.; Wang, D. Integrated bioethanol production to boost low-concentrated cellulosic ethanol without sacrificing ethanol field. Bioresour. Technol. 2018, 250, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Seguinot, P.; Ortiz-Julien, A.; Camarasa, C. Impact of nutrient availability on the fermentation and production of aroma compounds under sequential inoculation with M. pulcherrima and S. cerevisiae. Front. Microbiol. 2020, 11, 305. [Google Scholar]
- Mengesha, Y.; Tebeje, A.; Tilahun, B. A review on factors influencing the fermentation process of Teff (Eragrostis teff) and other cereal-based Ethiopian Injera. Int. J. Food Sci. 2022, 2022, 4419955. [Google Scholar] [CrossRef] [PubMed]
- Cheraiti, N.; Guezenec, S.; Salmon, J.-M. Very early acetaldehyde production by industrial Saccharomyces cerevisiae strains: A new intrinsic character. Appl. Microbiol. Biotechnol. 2010, 2, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.O.S.; Cavalett, O.; Filho, R.M.; Bonomi, A. Integrated first and second generation ethanol production from sugarcane. Chem. Eng. Trans. 2014, 37, 445–450. [Google Scholar]
- Moonsamy, T.; Mandegari, M.; Farzad, S.; Görgens, J. A new insight into integrated first and second-generation bioethanol production from sugarcane. Ind. Crops Prod. 2022, 188, 115675. [Google Scholar] [CrossRef]
- Iram, A.; Çekmecelioğlu, D.; Demirci, A. Integrating 1G with 2G bioethanol production by using distillers’ dried grains with solubles (DDGS) as the feedstock for lignocellulolytic enzyme production. Fermentation 2022, 8, 101559. [Google Scholar] [CrossRef]
- EN 15376:2014; Automotive Fuels—Ethanol as a Blending Component for Petrol—Requirements and Test Methods. European Committee for Standardization (CEN): Brussels, Belgium, 2014.
- Dziemianowicz, W.; Kotarska, K.; Świerczyńska, A. Increase butanol production from corn straw by mineral compounds supplementation. Energies 2022, 15, 6899. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- ISO 16472:2006; Animal Feeding Stuffs—Determination of Amylase-Treated Neutral Detergent Fibre Content (aNDF). ISO: Geneva, Switzerland, 2006.
- ISO 13906:2008; Animal Feeding Stuffs—Determination of Acid Detergent Fibre (ADF) and Acid Detergent Lignin (ADL) Contents. ISO: Geneva, Switzerland, 2008.
- Dziemianowicz, W.; Kotarska, K.; Świerczyńska, A. The effect of technological conditions on abe fermentation and butanol production of rye straw and the composition of volatile compounds. Molecules 2024, 29, 3398. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Su, J.; Hu, H.; Yang, M.; Huang, Z.; Chena, D.; Wu, J.; Feng, Z. Overcoming biomass recalcitrance by synergistic pretreatment of mechanical activation and metal salt for enhancing enzymatic conversion of lignocellulose. Biotechnol. Biofuels 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef]
- Kululo, W.W.; Habtu, N.G.; Abera, M.K.; Sendekle, Z.B.; Fanta, S.W.; Yemata, T.A. Advances in various pretreatment strategies of lignocellulosic substrates for the production of bioethanol: A comprehensive review. Discov. Appl. Sci. 2025, 7, 476. [Google Scholar] [CrossRef]
- Broda, M.; Yelle, D.J.; Serwańska, K.J.M. Bioethanol production from lignocellulosic biomass—Challenges and solutions. Molecules 2022, 27, 8717. [Google Scholar] [CrossRef]
- Fan, J.; Lu, Y.; An, N.; Zhu, W.; Li, M.; Gao, M.; Wang, X.; Wu, C.; Wang, Y. Pretreatment Technologies for Lignocellulosic Biomass: Research Progress, Mechanisms, and Prospects. BioResources 2025, 20, 4897–4924. [Google Scholar] [CrossRef]
- Grimaldi, M.P.; Marques, M.P.; Laluce, C.; Cilli, E.M.; Sponchiado, R.P. Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol. Biofuels 2015, 8, 205. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.-S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 25, 1115–1127. [Google Scholar] [CrossRef]
- Baksi, S.; Saha, D.; Saha, S.; Sarkar, U.; Basu, D.; Kuniyal, J.C. Pre-treatment of lignocellulosic biomass: Review of various physico-chemical and biological methods influencing the extent of biomass depolymerization. Int. J. Environ. Sci. Technol. 2023, 20, 13895–13922. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Lian, Y.; Xiong, Q.; Hayashi, J.I.; Huang, C.; Ragauskas, A.J.; Meng, X.; Zhou, Y. Insights into environmentally friendly solvent pretreatment of lignocellulosic biomass: Strategies, mechanisms, and future perspectives. Carbohydr. Polym. 2025, 367, 124027. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Ağbulut, Ü.; Len, C.; Nguyen, P.Q.P.; Chen, W.H. Steam explosion as sustainable biomass pretreatment technique for biofuel production: Characteristics and challenges. Bioresour. Technol. 2023, 385, 129398. [Google Scholar] [CrossRef]
- Sai Bharadwaj, A.V.S.L.; Dev, S.; Zhuang, J.; Wang, Y.; Yoo, C.G.; Jeon, B.H.; Aggarwal, S.; Park, S.H.; Kim, T.H. Review of chemical pretreatment of lignocellulosic biomass using low-liquid and low-chemical catalysts for effective bioconversion. Bioresour. Technol. 2023, 368, 128339. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cai, D.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Zhang, C.; Wang, Z.; Tan, T. Effect of acid pretreatment on different parts of corn stalk for second generation ethanol production. Bioresour. Technol. 2016, 206, 86–92. [Google Scholar] [CrossRef]
- Santos, C.C.; de Souza, W.; Sant’Anna, C.; Brienzo, M. Elephant grass leaves have lower recalcitrance to acid pretreatment than stems, with higher potential for ethanol production. Ind. Crops Prod. 2018, 111, 193–200. [Google Scholar] [CrossRef]
- Nguyen, T.Y.; Cai, C.M.; Kumar, R.; Wyman, C.E. Co-solvent pretreatment reduces costly enzyme requirements for high sugar and ethanol yields from lignocellulosic biomass. Chem. Sus. Chem. 2015, 8, 1716–1725. [Google Scholar]
- Meng, F.; Li, N.; Yang, H.; Shi, Z.; Zhao, P.; Yang, J. Investigation of hydrogen peroxide-acetic acid pretreatment to enhance the enzymatic digestibility of bamboo residues. Bioresour. Technol. 2022, 344, 126162. [Google Scholar] [CrossRef]
- Fatriasari, W.; Ulwan, W.; Aminingsih, T.; Sari, F.P.; Suryanegara, L.; Iswanto, A.H.; Ghozali, M.; Kholida, L.N.; Hussin, M.H.; Fudholi, A. Optimization of maleic acid pretreatment of oil palm empty fruit bunches (OPEFB) using response surface methodology to produce reducing sugars. Ind. Crops Prod. 2021, 171, 113971. [Google Scholar] [CrossRef]
- Camesasca, L.; de Mattos, J.A.; Vila, E.; Cebreiros, F.; Lareo, C. Lactic acid production by Carnobacterium sp. isolated from a maritime Antarctic lake using eucalyptus enzymatic hydrolysate. Biotechnol. Rep. 2021, 31, e00643. [Google Scholar] [CrossRef]
- Shimizu, F.; Monteiro, P.; Ghiraldi, P.; Melati, R.; Pagnocca, F.; de Souza, W.; Sant’Anna, C.; Brienzo, M. Acid, alkali and peroxide pretreatments increase the cellulose accessibility and glucose yield of banana pseudostem. Ind. Crops Prod. 2018, 115, 62–68. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.-H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, Q.; Hu, X.; Li, X.; Huang, Y.; Li, W.; Ma, L. Structural evolution during corn stalk acidic and alkaline hydrogen peroxide pretreatment. Ind. Crops Prod. 2022, 176, 114386. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Santo, M.E.; Rezende, C.A.; Bernardinelli, O.D.; Pereira, N., Jr.; Curvelo, A.A.; Deazevedo, E.R.; Guimarães, F.E.; Polikarpov, I. Structural and compositional changes in sugarcane bagasse subjected to hydrothermal and organosolv pretreatments and their impacts on enzymatic hydrolysis. Ind. Crops Prod. 2018, 113, 64–74. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lim, C.H.; Shuit, S.H.; Lee, K.M.; Chong, C.T. Effects of Organic Solvents on the Organosolv Pretreatment of Degraded Empty Fruit Bunch for Fractionation and Lignin Removal. Sustainability 2021, 13, 6757. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, H.; Shan, J.; Sun, B.; Chen, Y.; Ji, L.; Ji, X.; Wang, J.; Zhu, C.; Ying, H. Ammonia–Mechanical Pretreatment of Wheat Straw for the Production of Lactic Acid and High-Quality Lignin. Fermentation 2023, 9, 177. [Google Scholar] [CrossRef]
- Teymouri, F.; Laureano-Perez, L.; Alizadeh, H.; Dale, B.E. Optimization of the ammonia fiber explosion (AFEX) treatment parameters for enzymatic hydrolysis of corn stover. Bioresour. Technol. 2005, 96, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, S.; Zhai, R.; Yuan, X.; Yu, Y.; Shen, G.; Wang, Z.; Yu, J.; Jin, M. Lime pretreatment of pelleted corn stover boosts ethanol titers and yields without water washing or detoxifying pretreated biomass. Renew. Energy 2022, 192, 396–404. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unloking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Fírvida, I.; del Río, P.G.; Gullón, P.; Gullón, B.; Garrote, G.; Romaní, A. Alternative Lime Pretreatment of Corn Stover for Second-Generation Bioethanol Production. Agronomy 2021, 11, 155. [Google Scholar] [CrossRef]
- Chang, M.; Li, D.; Wang, W.; Chen, D.; Zhang, Y.; Hu, H.; Ye, X. Comparison of sodium hydroxide and calcium hydroxide pretreatments on the enzymatic hydrolysis and lignin recovery of sugarcane bagasse. Bioresour. Technol. 2017, 244, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Dar, R.A.; Baganz, F.; Smoliński, A.; Rasmey, A.-H.M.; Liu, R.; Zhang, L. Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation 2025, 11, 121. [Google Scholar] [CrossRef]
- Tian, L.; Qi, T.; Zhang, F.; Tran, V.G.; Yuan, J.; Wang, Y.; He, N.; Cao, M. Synthetic biology approaches to improve tolerance of inhibitors in lignocellulosic hydrolysates. Biotechnol. Adv. 2025, 78, 108477. [Google Scholar] [CrossRef]
- Cámara, E.; Olsson, L.; Zrimec, J.; Zelezniak, A.; Geijer, C.; Nygård, Y. Data mining of Saccharomyces cerevisiae mutants engineered for increased tolerance towards inhibitors in lignocellulosic hydrolysates. Biotechnol. Adv. 2022, 57, 107947. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Wiman, M.; Heer, D.; Brink, D.P.; Sauer, U.; Hahn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Physiological and molecular characterization of yeast cultures pre-adapted for fermentation of lignocellulosic hydrolysate. Fermentation 2023, 9, 72. [Google Scholar] [CrossRef]
- Kim, S.K.; Jin, Y.S.; Choi, I.G.; Park, Y.C.; Seo, J.H. Enhanced tolerance of Saccharomyces cerevisiae to multiple lignocellulose-derived inhibitors through modulation of spermidine contents. Metab. Eng. 2015, 29, 46–55. [Google Scholar] [CrossRef]
- Gorsich, S.W.; Dien, B.S.; Nichols, N.N.; Slininger, P.J.; Liu, Z.L.; Skory, C.D. Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2006, 71, 339–349. [Google Scholar] [CrossRef]
- Guaragnella, N.; Bettiga, M. Acetic acid stress in budding yeast: From molecular mechanisms to applications. Yeast 2021, 38, 391–400. [Google Scholar] [CrossRef]
- Hu, B.B.; Wang, J.L.; Wang, Y.T.; Zhu, M.J. Specify the individual and synergistic effects of lignocellulose-derived inhibitors on biohydrogen production and inhibitory mechanism research. Renew. Energy 2019, 140, 397–406. [Google Scholar] [CrossRef]
- Ko, J.K.; Um, Y.; Woo, H.M.; Kim, K.H.; Lee, S.M. Ethanol production from lignocellulosic hydrolysates using engineered Saccharomyces cerevisiae harboring xylose isomerase-based pathway. Bioresour. Technol. 2016, 209, 290–296. [Google Scholar] [CrossRef]
- Risco, A.; Plesu, V.; Heydenreich, J.A.; Bonet, J.; Bonet-Ruiz, A.E.; Calvet, A.; Iancu, P.; Llorens, J. Pressure selection for non-reactive and reactive pressure-swing distillation. Chem. Eng. Process-Process Intensif. 2019, 135, 9. [Google Scholar] [CrossRef]
- Hazelwood, L.; Daran, J.-M.; van Maris, A.; Pronk, J.; Dickinson, J. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.; Vendruscolo, F.; Carvalho, W.R.; Soares Júnior, M.S.; Pinheiro, M.V.M.; Caliari, M. Influence of the number of distillations on the composition of organic sugarcane spirit. J. Inst. Brew. 2013, 119, 133–138. [Google Scholar] [CrossRef]
- Geng, K.; Lin, Y.; Zheng, X.; Li, C.; Chen, S.; Ling, H.; Yang, J.; Zhu, X.; Liang, S. Enhanced Expression of Alcohol Dehydrogenase I in Pichia pastoris Reduces the Content of Acetaldehyde in Wines. Microorganisms 2023, 12, 38. [Google Scholar] [CrossRef]
- Jackowetz, J.N.; Dierschke, S.; Mira de Orduña, R. Multifactorial analysis of acetaldehyde kinetics during alcoholic fermentation by Saccharomyces cerevisiae. Food Res. Int. 2011, 44, 310–316. [Google Scholar] [CrossRef]
- Raj, S.B.; Ramaswamy, S.; Plapp, B.V. Yeast Alcohol Dehydrogenase Structure and Catalysis. Biochemistry 2014, 53, 5791–5803. [Google Scholar] [CrossRef] [PubMed]
- Pech-Canul, A.; Hammer, S.; Ziegler, S.; Richardson, I.; Sharma, B.; Maloney, M.; Bomble, Y.; Lynd, L.; Olson, D. The role of AdhE on ethanol tolerance and production in Clostridium thermocellum. J. Biol. Chem. 2024, 300, 107559. [Google Scholar] [CrossRef]
- Modig, T.; Lidén, G.; Taherzadeh, M. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Jilani, S.; Olson, D. Mechanism of furfural toxicity and metabolic strategies to engineer tolerance in microbial strains. Microb. Cell Factories 2023, 22, 37891678. [Google Scholar] [CrossRef]
- Auld, D.; Bergman, T. Medium- and short-chain dehydrogenase/reductase gene and protein families: The role of zinc for alcohol dehydrogenase structure and function. Cell. Mol. Life Sci. 2008, 65, 3961–3970. [Google Scholar] [CrossRef]
- de Smidt, O.; du Preez, J.C.; Albertyn, J. The alcohol dehydrogenases of Saccharomyces cerevisiae: A comprehensive review. FEMS Yeast Res. 2008, 8, 967–978. [Google Scholar] [CrossRef]
- Leskovac, V.; Trivić, S.; Pericin, D. The three zinc-containing alcohol dehydrogenases from baker’s yeast, Saccharomyces cerevisiae. FEMS Yeast Res. 2002, 2, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Stanzer, D.; Hanousek Čiča, K.; Blesić, M.; Smajić Murtić, M.; Mrvčić, J.; Spaho, N. Alcoholic Fermentation as a Source of Congeners in Fruit Spirits. Foods 2023, 12, 1951. [Google Scholar] [CrossRef]
- Malcorps, P.; Cheval, J.M.; Jamil, S.; Dufour, J.P. A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae during fermentation. J. Am. Soc. Brew. Chem. 1991, 49, 47–53. [Google Scholar] [CrossRef]
- Roca-Mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.-J. Nitrogen preferences during alcoholic fermentation of different non-Saccharomyces yeasts of oenological interest. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, S.; McKinnon, A.; Musarurwa, H.T.; Ferreira, A.C.; Bauer, F.F. The Impact of Single Amino Acids on Growth and Volatile Aroma Production by Saccharomyces cerevisiae Strains. Front. Microbiol. 2017, 8, 2554. [Google Scholar] [CrossRef]
- Swidah, R.; Ogunlabi, O.; Grant, C.M.; Ashe, M.P. n-Butanol production in S. cerevisiae: Co-ordinate use of endogenous and exogenous pathways. Appl. Microbiol. Biotechnol. 2018, 102, 9857–9866. [Google Scholar] [CrossRef]
- Macrelli, S.; Galbe, M.; Wallberg, O. Effects of production and market factors on ethanol profitability for an integrated first and second generation ethanol plant using the whole sugarcane as feedstock. Biotechnol. Biofuels 2014, 7, 26. [Google Scholar] [CrossRef]
- Rohowsky, B.; Häßler, T.; Gladis, A.; Remmele, E.; Schieder, D.; Faulstich, M. Feasibility of simultaneous saccharification and juice co-fermentation on hydrothermal pretreated sweet sorghum bagasse for ethanol production. Appl. Energy 2013, 102, 211–219. [Google Scholar] [CrossRef]
- Chandra, R.; Castillo-Zacarias, C.; Delgado, P.; Parra-Saldívar, R.A. Biorefinery approach for dairy wastewater treatment and product recovery towards establishing a biorefinery complexity index. J. Clean. Prod. 2018, 183, 1184–1196. [Google Scholar] [CrossRef]
- Ayodele, B.; Alsaffar, M.; Mustapa, S. An overview of integration opportunities for sustainable bioethanol production from first- and second-generation sugar-based feedstocks. J. Clean. Prod. 2020, 245, 118857. [Google Scholar] [CrossRef]
- Furlan, F.; Borba Costa, C.; de Castro Fonseca, G.; de Pelegrini Soares, R.; Secchi, A.; da Cruz, A.J.; de Campos Giordano, R. Assessing the production of first and second generation bioethanol from sugarcane through the integration of global optimization and process detailed modeling. Comput. Chem. Eng. 2012, 43, 1–9. [Google Scholar] [CrossRef]
- Semaan, G.; Öztürk, A.; Kumar, G. Comparative techno-economic assessment of multi-feedstock to multi-product integrated lignocellulosic biorefined. Biochem. Eng. J. 2025, 224, 109892. [Google Scholar] [CrossRef]
- Formenti, L.R.; Nørregaard, A.; Bolic, A.; Hernandez, D.Q.; Hagemann, T.; Heins, A.L.; Larsson, H.; Mears, L.; Mauricio-Iglesias, M.; Krühne, U.; et al. Challenges in industrial fermentation technology research. Biotechnol. J. 2014, 9, 727–738. [Google Scholar] [CrossRef]
| Lignocellulosic Biomass | |||
| Dry matter (%) | Dry organic matter (%DM) | Crude fiber (%) | |
| 90.65 ± 0.09 | 93.82 ± 0.11 | 28.43 ± 0.29 | |
| Corn grain | |||
| Dry matter (%) | Starch (%) | ||
| 89.95 ± 0.06 | 60.03 ± 0.43 | ||
| Chemical Pretreatment Method | Advantages | Disadvantages | Pretreatment Effect | Reference |
|---|---|---|---|---|
| Acid hydrolysis | (I) Low requirements of temperatures and pressure (II) High sugar release efficiency (III) Solubilizes hemicellulose | (I) Generates toxic by-products (e.g., furfural) (II) Corrosive (III) Requires neutralization (IV) Recovery acids for further use | Hemicellulose removal: 96% Glucose yield: 94.2% | [42] |
| Hemicellulose removal: 85% (leaf), 77% (stem), 75% (whole plant) Glucose yield: 89% (leaf), 43% (stem), 76% (whole plant) | [43] | |||
| Delignification: 76% hemicellulose removal: 69% glucose yield: > 80% | [44] | |||
| Cellulose yield: 65% Hemicellulose yield: 23% Lignin yield: 9% | [45] | |||
| Cellulose yield: 44.43% Hemicellulose yield: 19.11% | [46] | |||
| Alkaline hydrolysis | (I) Low inhibitor formation (II) Effective lignin removal (III) Ease to recover and reuse reagents | (I) High amount of alkali solution is required (II) Requires neutralization (III) Requires long residence time | Removal of 43% lignin | [47] |
| Cellulose/glucose recovery: 75.48% | [48] | |||
| Cellulose yield: 46.8% Saccharification field: 58% | [49] | |||
| Cellulose yield: 33.0% Hemicellulose yield: 16.5% Saccharification field: 24.7% | [50] | |||
| Organosolv pretreatment | (I) High yield of pentose sugar (II) Effective removal of lignin | (I) Requires expensive solvents | Delignification: 63% Cellulose conversion: 89.2% | [51] |
| Delignification: 62% Hemicellulose removal: 75% Glucose yield: 63% | [52] | |||
| Delignification: 75.1% Hemicellulose removal: 81.5% | [53] | |||
| Ammonia fiber explosion (AFEX) | (I) High yield of pentose sugar (II) Low formation of inhibitors | (I) Recycling of ammonia is needed (II) Less effective process with increasing lignin content (III) Highly corrosive ammonia | Delignification: 62.5% Total sugar yield: 89.4% | [54] |
| Fermentable sugars conversion: 90% | [55] |
| Component | After Thermohydrolysis | After Thermohydrolysis and Enzymatic Hydrolysis |
|---|---|---|
| Sugar conversion (%) | 24.29 ± 0.28 | 65.78 ± 0.42 |
| Total sugars (g/L) | 15.13 ± 0.12 | 41.02 ± 0.38 |
| Saccharification (%) | - | 66.57 ± 0.54 |
| Variants | Ethanol Concentration (g/L) | Ethanol Yield (L/100 kg of Raw Material) | ||||
|---|---|---|---|---|---|---|
| Fermentation Time (h) | Fermentation Time (h) | |||||
| 24 | 48 | 72 | 24 | 48 | 72 | |
| Variant 1 | 43.18 b ± 0.58 | 47.91 b ± 0.37 | 49.39 b ± 0.22 | 27.36 b ± 0.46 | 30.36 b ± 0.31 | 31.32 b ± 0.17 |
| Variant 2 | 56.30 c ± 0.84 | 60.08 c ± 0.28 | 61.86 c ± 0.45 | 35.68 c ± 0.53 | 38.08 c ± 0.18 | 39.20 c ± 0.28 |
| Variant 3 | 18.96 a ± 0.20 | 24.65 a ± 0.28 | 27.93 a ± 0.43 | 12.02 a ± 0.17 | 15.62 a ± 0.21 | 17.72 a ± 0.35 |
| Variants | 1-Propanol, (mg/L) | Isobutanol, (mg/L) | n-Butanol, (mg/L) | 2-Methyl-1-Butanol, (mg/L) |
|---|---|---|---|---|
| 1 | 3365.03 c ± 29.86 | 1690.27 b ± 41.75 | 233.43 b ± 12.20 | 336.57 a ± 8.16 |
| 2 | 365.10 a ± 7.10 | 1100.50 a ± 11.10 | 5.90 a ± 0,30 | 546.67 b ± 5.70 |
| 3 | 2945.50 b ± 50.24 | 1978.13 c ± 32.84 | 296.10 c ± 17.10 | 622.23 c ± 11.86 |
| Variants | Aldehydes (mg/L) | Higher Alcohols (mg/L) | Esters (mg/L) | Methanol (mg/L) |
|---|---|---|---|---|
| 1 | 90.27 b ± 0.95 | 5625.30 b ± 91.55 | 32.37 b ± 0.45 | 54.11 b ± 0.44 |
| 2 | 51.20 a ± 2.30 | 2018.17 a ± 12.20 | 8.70 a ± 0.20 | 1.33 a ± 0.03 |
| 3 | 246.40 c ± 3.20 | 5840.41 b ± 46.10 | 50.27 c ± 0.70 | 61.91 c ± 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotarska, K.; Dziemianowicz, W.; Świerczyńska, A. The Influence of Technological Conditions of Co-Fermentation of Lignocellulosic and Starch Raw Materials on the Amount of Volatile By-Products Formed and the Quality of Obtained Bioethanol. Appl. Sci. 2025, 15, 11315. https://doi.org/10.3390/app152111315
Kotarska K, Dziemianowicz W, Świerczyńska A. The Influence of Technological Conditions of Co-Fermentation of Lignocellulosic and Starch Raw Materials on the Amount of Volatile By-Products Formed and the Quality of Obtained Bioethanol. Applied Sciences. 2025; 15(21):11315. https://doi.org/10.3390/app152111315
Chicago/Turabian StyleKotarska, Katarzyna, Wojciech Dziemianowicz, and Anna Świerczyńska. 2025. "The Influence of Technological Conditions of Co-Fermentation of Lignocellulosic and Starch Raw Materials on the Amount of Volatile By-Products Formed and the Quality of Obtained Bioethanol" Applied Sciences 15, no. 21: 11315. https://doi.org/10.3390/app152111315
APA StyleKotarska, K., Dziemianowicz, W., & Świerczyńska, A. (2025). The Influence of Technological Conditions of Co-Fermentation of Lignocellulosic and Starch Raw Materials on the Amount of Volatile By-Products Formed and the Quality of Obtained Bioethanol. Applied Sciences, 15(21), 11315. https://doi.org/10.3390/app152111315





