Evaluation of the Effects of Carvacrol on Gram-Negative Bacilli Isolated from Wound Infections
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Carbapenemases Detection
2.3. DNA Extraction
2.4. DNA Amplification
2.5. Determination of Minimum Inhibitory Concentration
2.6. Antibiofilm Activity
2.7. Reactive Oxygen Species Measurements
3. Results
3.1. Detection of Carbapenemases
3.2. Detection of Virulence Genes
3.3. Carvacrol Susceptibility
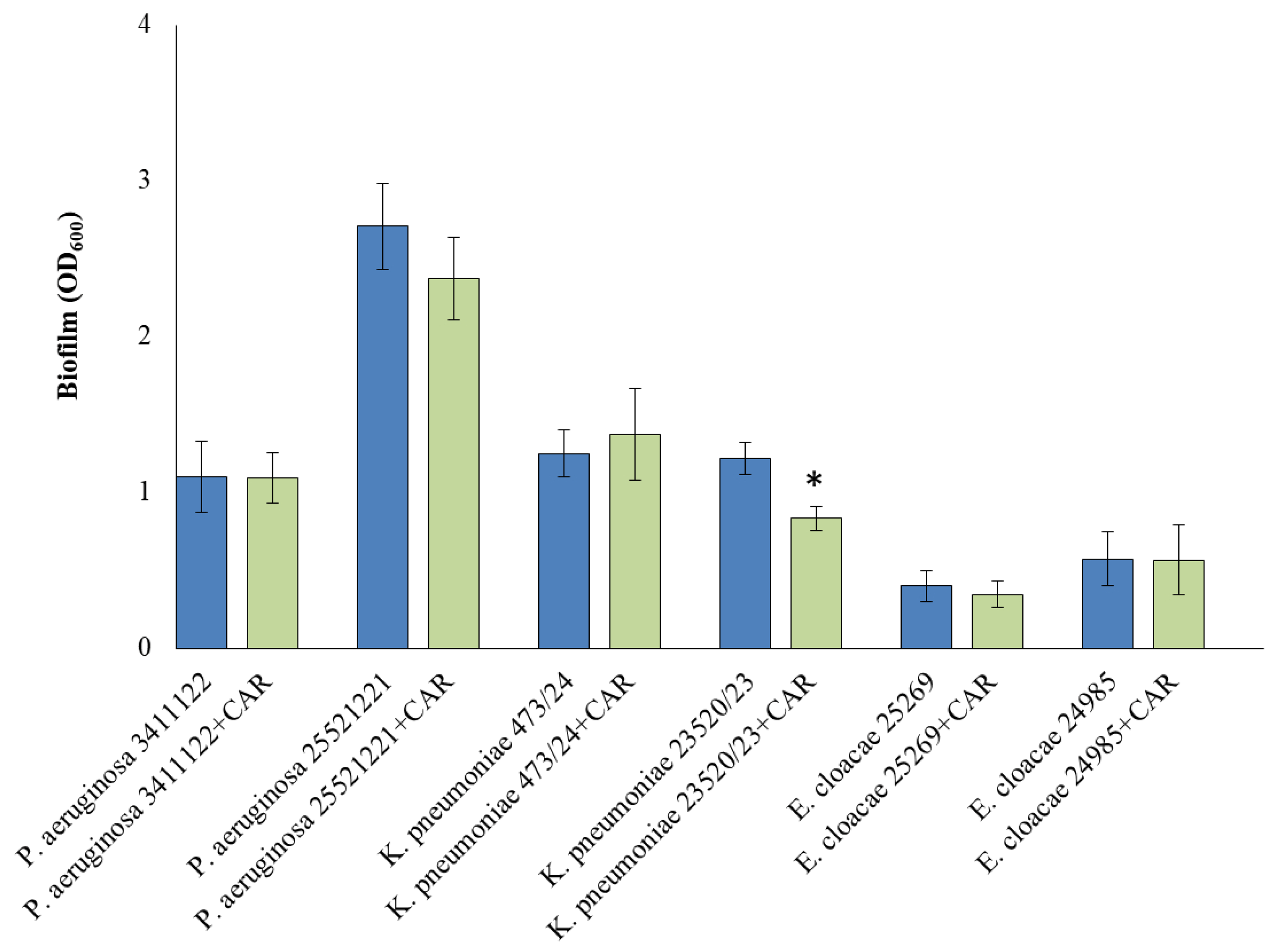
3.4. Biofilm Formation
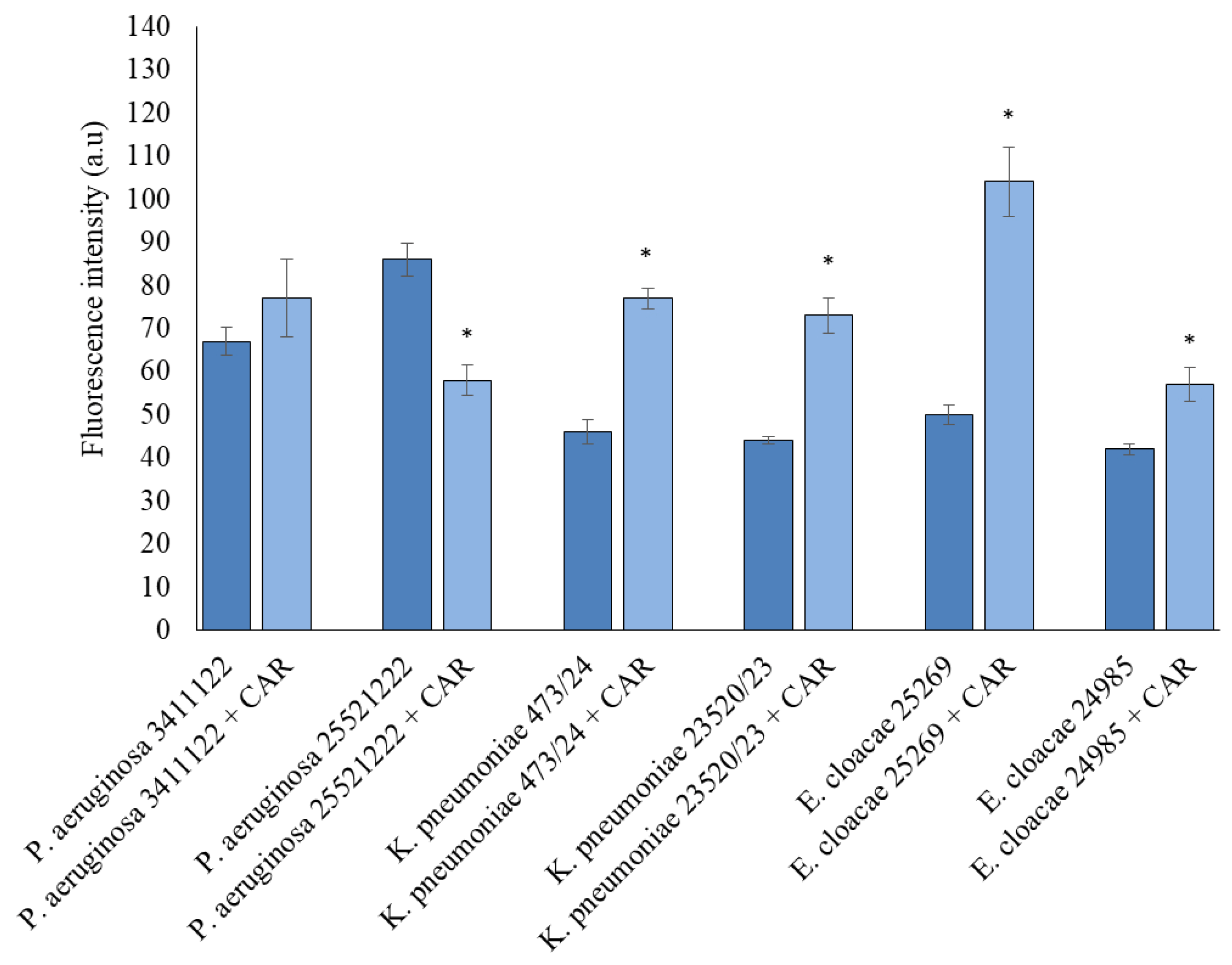
3.5. Detection of ROS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CAR | Carvacrol |
| CLSI | Clinical and Laboratory Standards Institute |
| DCF | 2′,7′-dichlorofluorescein |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| DNA | Deoxyribonucleic acid |
| ESBL | Extended-spectrum beta-lactamases |
| H2DCFDA | 2′,7′-dichlorodihydrofluorescein diacetate |
| KPC | Klebsiella pneumoniae carbapenemase |
| LPS | Lipopolysaccharide |
| MBC | Minimum bactericidal concentration |
| MBL | Metallo-beta-lactamases |
| MIC | Minimum inhibitory concentration |
| NDM | New Delhi metallo-beta-lactamases |
| PCR | Polymerase chain reaction |
| ROS | Reactive oxygen species |
| TTSS | The type III secretion system |
| VIM | Verona integron-encoded metallo-beta-lactamase |
References
- Malanovic, N.; Ön, A.; Pabst, G.; Zellner, A.; Lohner, K. Octenidine: Novel insights into the detailed killing mechanism of Gram-negative bacteria at a cellular and molecular level. Int. J. Antimicrob. Agents 2020, 56, 106146. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Rashid, R.B. Phylogenetic Analysis of Antibiotic Resistance Genes and Virulence Genes of Klebsiella species in silico. Dhaka Univ. J. Pharm. Sci. 2017, 16, 119–127. [Google Scholar] [CrossRef][Green Version]
- Bujňáková, D.; Puvača, N.; Ćirković, I. Virulence Factors and Antibiotic Resistance of Enterobacterales. Microorganisms 2022, 10, 1588. [Google Scholar] [CrossRef] [PubMed]
- Lazar, D.S.; Nica, M.; Dascalu, A.; Oprisan, C.; Albu, O.; Codreanu, D.R.; Kosa, A.G.; Popescu, C.P.; Florescu, S.A. Carbapenem-Resistant NDM and OXA-48-like Producing K. pneumoniae: From Menacing Superbug to a Mundane Bacteria; A Retrospective Study in a Romanian Tertiary Hospital. Antibiotics 2024, 13, 435. [Google Scholar] [CrossRef]
- Xiang, T.; Chen, C.; Wen, J.; Liu, Y.; Zhang, Q.; Cheng, N.; Wu, X.; Zhang, W. Resistance of Klebsiella pneumoniae Strains Carrying blaNDM–1 Gene and the Genetic Environment of blaNDM–1. Front. Microbiol. 2020, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, G. Verona Integron-Encoded Metallo-β-Lactamase (VIM)-Producing Pseudomonas aeruginosa Pyelonephritis in a Young Adult: A Case Report. Cureus 2025, 17, e78932. [Google Scholar] [CrossRef]
- da Silva, A.R.P.; do Socorro Costa, M.; Araújo, N.J.S.; de Freitas, T.S.; dos Santos, A.T.L.; Gonçalves, S.A.; da Silva, V.B.; Andrade-Pinheiro, J.C.; Tahim, C.M.; Lucetti, E.C.P.; et al. Antibacterial activity and antibiotic-modifying action of carvacrol against multidrug-resistant bacteria. Adv. Sample Prep. 2023, 7, 100072. [Google Scholar] [CrossRef]
- Cacciatore, F.A.; Maders, C.; Alexandre, B.; Barreto Pinilla, C.M.; Brandelli, A.; da Silva Malheiros, P. Carvacrol encapsulation into nanoparticles produced from chia and flaxseed mucilage: Characterization, stability and antimicrobial activity against Salmonella and Listeria monocytogenes. Food Microbiol. 2022, 108, 104116. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of Carvacrol and Thymol in Reducing Biofilm Formation on Technical Surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Ghorbani, G.; Rahimi, E.; Shakerian, A. Antibiotic resistance’s Genotypic and Phenotypic Characteristics and the Frequency of Virulence Factors in P. aeruginosa Isolates Isolated from Water Samples in Iran. Biomed. Res. Int. 2022, 2022, 7076433. [Google Scholar] [CrossRef]
- Wahl, A.; Fischer, M.A.; Klaper, K.; Müller, A.; Borgmann, S.; Friesen, J.; Hunfeld, K.P.; Ilmberger, A.; Kolbe-Busch, S.; Kresken, M.; et al. Presence of hypervirulence-associated determinants in Klebsiella pneumoniae from hospitalised patients in Germany. Int. J. Med. Microbiol. 2024, 314, 151601. [Google Scholar] [CrossRef]
- Shi, S.-F.; Jia, J.; Guo, X.; Zhao, Y.; Chen, D.; Guo, Y.; Zhang, X. Reduced Staphylococcus aureus biofilm formation in the presence of chitosan-coated iron oxide nanoparticles. Int. J. Nanomed. 2016, 11, 6499–6506. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6, 1–25. [Google Scholar]
- Coles, V.E.; Puri, L.; Bhandari, M.; Wood, T.J.; Burrows, L.L. The effects of chlorhexidine, povidone-iodine and vancomycin on growth and biofilms of pathogens that cause prosthetic joint infections: An in-vitro model. J. Hosp. Infect. 2024, 151, 99–108. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Periodontal ligament stem cell-derived exosome-loaded Emodin mediated antimicrobial photodynamic therapy against cariogenic bacteria. BMC Oral Health 2024, 24, 311. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, J. Expression and characterization of the new antimicrobial peptide AP138L-arg26 anti Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2024, 108, 111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Haqmal, M.A.; Liang, Y.; Muhammad, I.; Zhao, X.; Elken, E.M.; Gao, Y.; Jia, Y.; He, C.; Wang, Y.; et al. Antibacterial activity and cytotoxicity of a novel bacteriocin isolated from Pseudomonas sp. strain 166. Microb. Biotechnol. 2022, 15, 2337–2350. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Zhao, R.; Wang, J.; Cheng, Y.; Liu, Q.; Wang, Y.; Yang, S. Synergistic Interaction Between Paired Combinations of Natural Antimicrobials Against Poultry-Borne Pathogens. Front. Microbiol. 2022, 13, 811784. [Google Scholar] [CrossRef]
- Vasireddy, L.; Bingle, L.E.H.; Davies, M.S. Antimicrobial activity of essential oils against multidrug-resistant clinical isolates of the Burkholderia cepacia complex. PLoS ONE 2018, 13, e0201835. [Google Scholar] [CrossRef]
- Faleiro, M.L.; Miguel, M.G. Chapter 6-Use of Essential Oils and Their Components against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components, 1st ed.; Kumar Rai, M., Volodymyrivna Kon, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 65–94. [Google Scholar]
- Reichling, J. Anti-biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, M.X. Visualization and elimination of polymicrobial biofilms by a combination of ALA-carvacrol-blue light. J. Photochem. Photobiol. B Biol. 2022, 234, 112525. [Google Scholar] [CrossRef]
- Hasanvand, T.; Mohammadi, M.; Abdollahpour, F.; Kamarehie, B.; Jafari, A.; Ghaderpoori, A.; Karami, M.A. A comparative study on antibacterial activity of carvacrol and glutaraldehyde on Pseudomonas aeruginosa and Staphylococcus aureus isolates: An in vitro study. J. Environ. Health Sci. Eng. 2021, 19, 475–482. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Sun, Z.; Du, L.; Wang, D.; Zhao, T.; Doyle, M.P. Carvacrol oil inhibits biofilm formation and exopolysaccharide production of Enterobacter cloacae. Food Control 2021, 119, 107473. [Google Scholar] [CrossRef]
- Farhadi, K.; Rajabi, E.; Varpaei, H.A.; Iranzadasl, M.; Khodaparast, S.; Salehi, M. Thymol andcarvacrol against Klebsiella: Anti-bacterial, antibiofilm, and synergistic activities—Asystematic review. Front. Pharmacol. 2024, 15, 1487083. [Google Scholar] [CrossRef] [PubMed]
- Khakimova, M.; Ahlgren, H.G.; Harrison, J.J.; English, A.M.; Nguyen, D. The stringent response controls catalases in Pseudomonas aeruginosa and is required for hydrogen peroxide and antibiotic tolerance. J. Bacteriol. 2013, 195, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bahuguna, A.; Shukla, S.; Aziz, F.; Chauhan, A.K.; Ansari, M.B.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Antimicrobial potential of the food-grade additive carvacrol against uropathogenic E. coli based on membrane depolarization, reactive oxygen species generation, and molecular docking analysis. Microb. Pathog. 2020, 142, 104046. [Google Scholar] [CrossRef]
- da Cruz Nizer, W.S.; Inkovskiy, V.; Versey, Z.; Strempel, N.; Cassol, E.; Overhage, J. Oxidative Stress Response in Pseudomonas aeruginosa. Pathogens 2021, 10, 1187. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Pruss, A.; Grygorcewicz, B.; Wojciuk, B.; Dołęgowska, B.; Giedrys-Kalemba, S.; Kochan, E.; Sienkiewicz, M. Preliminary Study on the Antibacterial Activity of Essential Oils Alone and in Combination with Gentamicin Against Extended-Spectrum β-Lactamase-Producing and New Delhi Metallo-β-Lactamase-1-Producing Klebsiella pneumoniae Isolates. Microb. Drug Resist. 2018, 24, 1368–1375. [Google Scholar] [CrossRef]
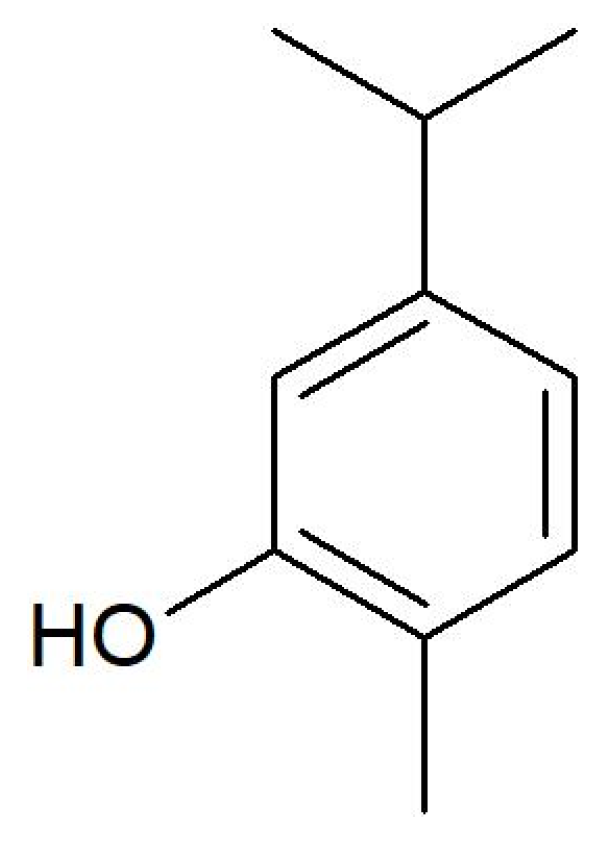
| Virulence Genes | Sequence (5′–3′) | Size of Product (bp) |
|---|---|---|
| phzM | F: CGTCGTGTTCAAGCAGATGGTGCTG R: CCGAACCGCTTCACCAGGC | 875 |
| phzS | F: CAATCATCTCAGCAGAACCC R: TGTCGTAGAGGATCTCCTG | 1752 |
| exoT | F: CATCGTCTACGCCATGAG R: AGCAGCACCTCGGAATAG | 1159 |
| exoY | F: GGAATGAACGAAGCGTTCTCCGAC R: TGGCGTCGACGAACACCTCG | 1035 |
| exoS | F: GTGTGCTTTATGCCATGAG R: GGTTTCCTTTTCCAGGTC | 444 |
| exoU | F: CTGCGCGGGTCTATGTGCC R: GATGCTGGACGGGTCGAG | 3308 |
| pilA | F: ATGGAGAGCGGGATCGACAG R: ATGCGGGTTTCCATCGGCAG | 1675 |
| pilB | F: TCGCCATGACCGATACGCTC R: ACAACCTGAGCCAGCCTTCC | 408 |
| apr | F: GCACGTGGTCATCCTGATGC R: TCCGTAGGCGTCGACGTAC | 1017 |
| plcH | F: CACACGGAAGGTTAATTCTGA R: CGGTTARACGGCTGAACCTG | 608 |
| Virulence Genes | Sequence (5′–3′) | Size of Product (bp) |
|---|---|---|
| uge | F: GAT CAT CCG GTC TCC CTG TA R: TCT TCA CGC CTT CCT TCA CT | 534 |
| kfu | F: GAA GTG ACG CTG TTT CTG GC R: TTT CGT GTG GCC AGT GAC TC | 797 |
| wabG | F: CGG ACT GGC AGA TCC ATA TC R: ACC ATC GGC CAT TTG ATA GA | 683 |
| fimH | F: ATG AAC GCC TGG TCC TTT GC R: GCT GAA CGC CTA TCC CCT GC | 688 |
| iroN | F: AAG TCA AAG CAG GGG TTG CCC G R: GAC GCC GAC ATT AAG ACG CAG | 665 |
| hlyA | F: AAC AAG GAT AAG CAC TGT TCT GGC T R: ACC ATA TAA GCG GTC ATT CCC GTC A | 1177 |
| rmpA | F: ACT GGG CTA CCT CTG CTT CA R: CTT GCA TGA GCC ATC TTT CA | 535 |
| magA | F: GGT GCT CTT TAC ATC ATT GC R: GCA ATG GCC ATT TGC GTT AG | 1280 |
| Presence | Absence | |
|---|---|---|
| P. aeruginosa 3411122 | exoT, aprA, phzS, plcH | phzM, exoY, exoS, exoU, pilA, pilB, aprA |
| P. aeruginosa 25521221 | exoT, phzS, plcH | phzM, exoY, exoS, exoU, pilA, pilB, aprA |
| K. pneumoniae 473/24 | fimH, wabG, uge, iroN | kfu, rmpA, magA, hlyA |
| K. pneumoniae 23520/23 | fimH, wabG, uge, kfu, rmpA | magA, hlyA |
| E. cloacae 25269 | fimH, kfu, iroN | hlyA |
| E. cloacae 24985 | fimH, kfu, iroN | hlyA |
| P. aeruginosa 3411122 | P. aeruginosa 25521221 | K. pneumoniae 473/24 | K. pneumoniae 23520/23 | E. cloacae 25269 | E. cloacae 24985 | |
|---|---|---|---|---|---|---|
| MIC [μg/mL] | 500 | 1000 | 250 | 250 | 250 | 125 |
| MBC [μg/mL] | 500 | 2000 | 250 | 250 | 250 | 250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruss, A.; Lichota, A.; Masiuk, H.; Kwiatkowski, P.; Słaba, M.; Sienkiewicz, M.; Dołęgowska, B. Evaluation of the Effects of Carvacrol on Gram-Negative Bacilli Isolated from Wound Infections. Appl. Sci. 2025, 15, 11309. https://doi.org/10.3390/app152111309
Pruss A, Lichota A, Masiuk H, Kwiatkowski P, Słaba M, Sienkiewicz M, Dołęgowska B. Evaluation of the Effects of Carvacrol on Gram-Negative Bacilli Isolated from Wound Infections. Applied Sciences. 2025; 15(21):11309. https://doi.org/10.3390/app152111309
Chicago/Turabian StylePruss, Agata, Anna Lichota, Helena Masiuk, Paweł Kwiatkowski, Mirosława Słaba, Monika Sienkiewicz, and Barbara Dołęgowska. 2025. "Evaluation of the Effects of Carvacrol on Gram-Negative Bacilli Isolated from Wound Infections" Applied Sciences 15, no. 21: 11309. https://doi.org/10.3390/app152111309
APA StylePruss, A., Lichota, A., Masiuk, H., Kwiatkowski, P., Słaba, M., Sienkiewicz, M., & Dołęgowska, B. (2025). Evaluation of the Effects of Carvacrol on Gram-Negative Bacilli Isolated from Wound Infections. Applied Sciences, 15(21), 11309. https://doi.org/10.3390/app152111309








