1. Introduction
Spent ion exchange resins (SIERs), primarily generated during water purification and decontamination processes at nuclear facilities, constitute a major fraction of low and intermediate level radioactive waste. Although inorganic ion exchange resins are valued for their high selectivity toward certain species such as Cs
+ and Sr
2+, they cannot fully replace organic ion exchange resins, which remain dominant due to their reliability in maintaining the radiochemical control of nuclear reactor water systems [
1]. Effective management of SIERs requires both substantial volume reduction and the long-term immobilization of radionuclides as well as other hazardous contaminants [
2,
3].
Thermal treatments, such as plasma incineration, fluidized bed steam reforming, or pyrolysis, are widely preferred for processing SIERs, as they achieve high destruction of organics and reduce waste volume by up to 85–95%, while simultaneously producing an ash that is more compatible with conditioning matrices. However, the resulting ash is highly radioactive and chemically complex, often containing elevated concentrations of heavy metals, which poses challenges for handling, conditioning, and final disposal, especially due to uncertainties in the leaching behaviour of both radionuclides and toxic metals.
Traditionally, ash immobilization is carried out using cementitious matrices such as ordinary Portland cement (OPC), which are valued for their mechanical strength, chemical stability and cost-effectiveness [
4,
5], also providing excellent stability against radiation [
6]. Nevertheless, contaminants and residual organics present in SIERs can interfere with cement hydration and compromise waste form durability. For this reason, other novel cement systems, such as calcium aluminate and sulfoaluminate cements, magnesium phosphate cements, and alkali-activated cements such as geopolymer-type matrices, are being explored for the immobilization of LILW [
7,
8,
9,
10]. In any case, each cement formulation must be tested to ensure a relatively high waste loading capacity, homogeneous waste distribution within the matrix, and high leaching resistance for both radionuclides and the matrix itself.
Geopolymers, or alkali-activated binders, have recently emerged as promising alternatives to Portland cement for SIER encapsulation [
11,
12,
13]. They offer superior mechanical strength, enhanced thermal and radiation stability, lower energy requirements during production, a lower carbon footprint compared to conventional Portland cement-based materials, and the ability to accommodate a wide range of waste streams [
13,
14,
15,
16]. However, the main present challenge is understanding their long-term durability [
13,
17].
Combining thermal treatments with the immobilization of thermally treated waste in advanced matrices such as geopolymers can efficiently overcome the disposal challenges presented by conventional cementation. This increased compatibility helps to avoid operational issues such as corrosion of containment materials and gas generation in the disposal environment, both of which are critical for maintaining waste form stability and long-term durability and, thus, repository safety. Ultimately, this integrated approach produces a safer, more reliable waste form suited for long-term disposal.
This study uses a one-part, ready-to-use geopolymer binder, enabling a direct, quantitative comparison with standard CEM I/42.5 SR and CEM III/B 32.5 N/SR matrices for SIER immobilization. One-part geopolymers are dry formulations in which a solid alkali activator (for example, solid sodium silicate) and aluminosilicate precursors, such as metakaolin and blast furnace slag, are pre-mixed into a single powder. This composition is designed to be activated simply by the addition of water at the point of use, resembling the handling and application procedures of ordinary Portland cement [
18,
19,
20,
21].
In contrast, conventional two-part geopolymers require the preparation and precise dosing of a concentrated alkaline solution (such as sodium hydroxide or liquid sodium silicate), which must be mixed with the dry precursors immediately before application [
22]. This process presents significant operational challenges, particularly in controlled zones, due to the corrosive and hazardous nature of liquid alkalis. The storage, handling, and use of these reactive solutions increases the risk of chemical exposure, spills, and contamination within regulated working areas.
The one-part system offers a notable practical advantage by avoiding on-site handling of hazardous alkaline solutions, thereby enhancing both safety and operational simplicity. The adoption of a one-part “just-add-water” geopolymer formulation enables industrial-scale dry blending, reduces operational risks, and simplifies regulatory compliance.
This work serves as a comparative baseline for these materials under representative disposal conditions, providing evidence relevant to waste form durability, radionuclide retention, and regulatory assessment. The results give new insights into short-term waste form–EBS interactions and also provide a foundation for subsequent long-term assessments to be addressed in future work, as foreseen in the LOPERA project.
2. Materials and Methods
The surrogate mixed-bed ion exchange resins used in this study consisted of a 50 wt.% mix of commercial cationic and anionic resins, Amberlite IRN-77 and IRN-78, doped with B and traces of Cs, Sr, and activation products (Co, Cr, Zn, Fe, and Ag). Surrogate SIERs were thermally treated at 450 °C. Precise temperature control during heat treatment was ensured by employing a controlled heating ramp from 20 °C to 450 °C at a rate of 5 °C/min. This procedure enabled reproducible thermal decomposition of the resin surrogates and minimized variability between samples. The temperature was continuously monitored throughout the process to maintain uniformity and to achieve the target endpoint without exceeding the desired temperature. This relatively low-temperature thermal treatment was chosen to prevent the loss of volatile radionuclides, such as cesium. Thus, the resulting residue retains most of the doping elements, while also achieving a significant reduction in organics and water content, from an initial 70 wt.% to 3 wt.% (
Figure 1). Fourier Transform Infrared (FTIR) analysis confirmed the oxidative thermal decomposition of sulfonic (cationic) and amine (anionic) groups. The resulting product, namely reconditioned waste (RW), exhibited an acidic behaviour in aqueous media.
The CHNS elemental analysis revealed a substantial loss of N during the thermal treatment, leading to a carbonaceous solid with a variable content in S. Average chemical composition of the RW determined by elemental analysis is shown in
Table 1. Boron content in the ashes was significant, 16 wt.%. Cs
+ and Sr
2+ concentrations measured in the thermally treated surrogates were 23 and 121 mg·kg
−1, respectively.
Three cement matrices were prepared, a CEM I/42.5 SR/MSR (provided by Cementos Portland Valderribas, Pamplona, Spain), a CEM III/B 32.5 N/SR (provided by Cementos Tudela-Veguín, Oviedo, Spain) including blast furnace slag (BFS) with content in the range of 66–80 wt.% according to the norm EN 197-1.2011, and a one-part geopolymer (GPO) consisting of a mixture of 42.5 wt.% metakaolin and 42.5 wt.% BFS, used as precursors, with 15 wt.% Na
2SiO
3 used as a solid activator. The chemical composition of major elements found in the cement matrices, determined by X-ray Fluorescence (XRF), is presented in
Table 2.
Prismatic monoliths of 1 1 6 cm3 (pastes) were prepared, with and without (unreacted reference) homogenizing 20 wt.% of RW in the formulation and cured for 56 days at 21 °C and 99% relative humidity conditions.
Although the incorporation of the RW into the matrices did not have a significant effect on the mineralogy of the cement pastes, when studied after 28 days, the acid character reduced the pH of the pore solution. This, together with the high B content in the RW affected the kinetic of hydration, delaying the setting and hardening of the cement pastes. The total porosity of the three matrices was significantly affected by the incorporation of the RW to the matrix, decreasing their mechanical strength, but in all cases waste acceptance criterion (WAC) was met (compressive strength > 10 MPa); data not included in present paper.
The monoliths that, due to their dimensions, exhibited external surface areas of 26 cm
2, were immersed in 260 mL solutions placed in translucent high-density polyethylene (HDPE) containers with inner-fitted caps and outer polypropylene (PP) screw caps. These conditions allowed the experiments to be performed with a geometric external surface area of the monolith to liquid ratio of 1:10, in agreement with the standard test method for accelerated leach testing for measuring contaminant releases from solidified waste (ASTM C1308-21 [
23]). The solutions contained either (a) deionized water (DW), (b) disposal site water from the El Cabril disposal facility (DSW), or (c) synthetic cementitious water (SCW). To maximize the interaction between the external surface of the monoliths and leaching solutions, monoliths were hung from their inner caps with a thin nylon thread.
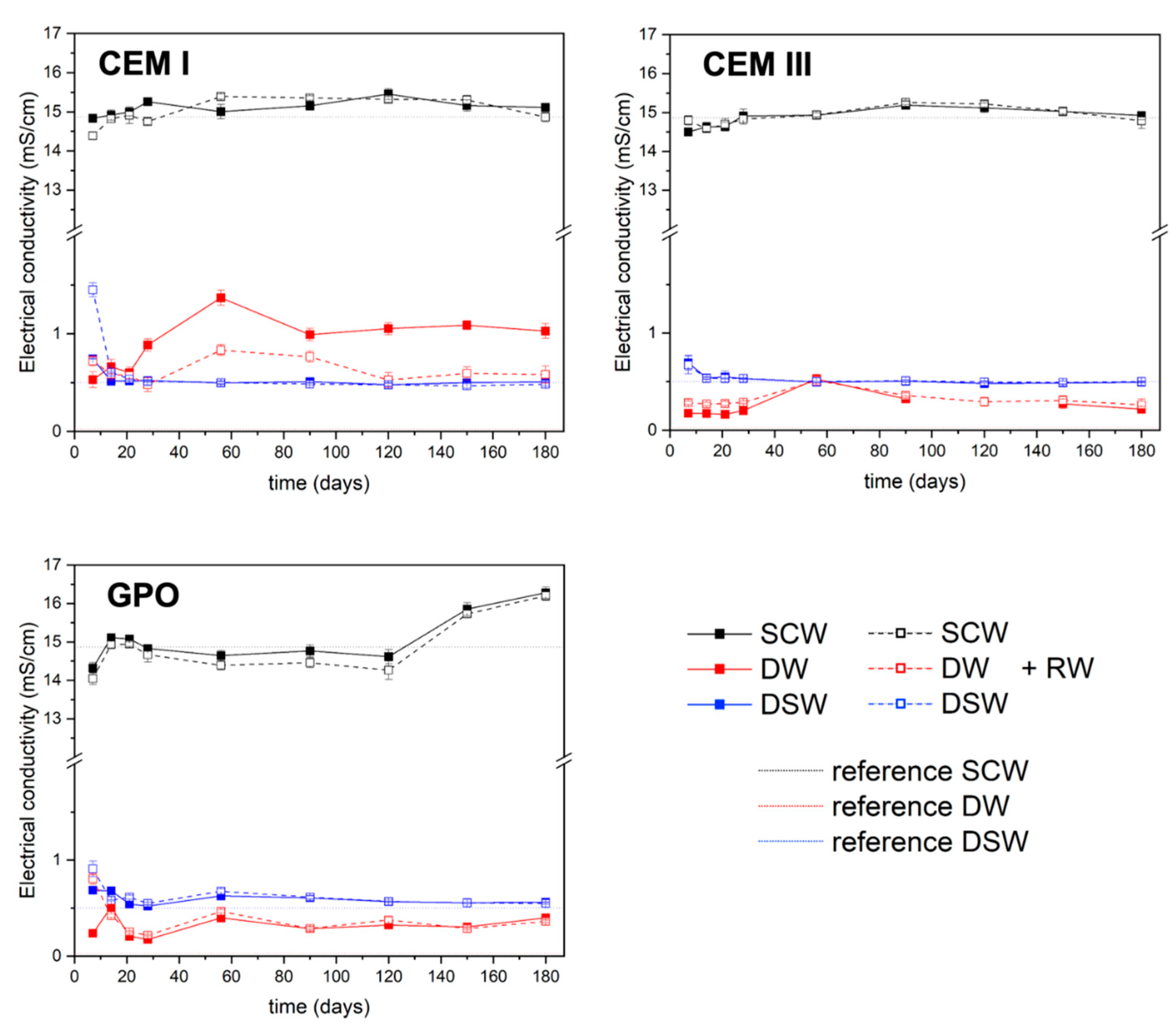
DW has an electrical conductivity (EC) below 0.05 S·cm−1 and was used, for general purposes, with the inductively coupled plasma mass spectrometry (ICP-MS) instrument to dilute samples and calibrate the equipment. Before use, the water was stirred and heated to degas potential absorbed atmospheric gases.
DSW is a sulphate-dominant neutral pH solution sampled in the vicinity of disposal platforms in the facility of El Cabril (Cordoba, Spain), whereas SCW is a synthetic solution representative of a CEM I porewater. The chemical compositions of DW and SCW are shown in
Table 3. The detailed composition of DSW is not provided due to confidentiality issues.
Solutions were renewed after 7 days for the first month and once a month thereafter, up to 6 months. The leaching experiments were performed by three different institutions, although the samples were produced in one single batch for each type of matrix to avoid handling effects and then distributed to the three organizations. Leaching experiments with DW were performed at the Autonomous University of Madrid (UAM), experiments with DSW were performed at CIEMAT, and experiments with SCW were performed at IET-cc (CSIC), which provided all the specimens. Each institution had different facilities and instrumentation to renew solutions, store, and analyze the samples; however, all procedures were agreed upon beforehand, and a common methodology was utilized according to the ISO 6961 standard [
24].
UAM performed a renovation of the solution under a hermetic chamber full of N2(g). Replacement of the solutions was only performed when the oxygen concentration was below the target level of 2%, in order to prevent CO2(g) reabsorption. Quantitative aqueous concentrations of Ca, Si, Al, and K and semiquantitative concentrations of B, Cs, and Sr were determined by ICP-MS with a NexION 300XX Perkin Elmer spectrometer (Shelton, CT, USA). SO42− and Cl− were determined by ionic chromatography (IC) with a Compact IC plus 882 device from Metrohm (Herisau, Switzerland). Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analyses were performed on solid samples immersed in DW using a Hitachi S–3000N microscope (Tokio, Japan) that was coupled with an EDX XFlash® 6130 Bruker detector (Billerica, MA, USA). The SEM operated under high vacuum conditions with an accelerating voltage of 20 keV, a lifetime of 40 s, a working distance of 15.0 to 18.5 mm, and a beam current of 300 mA.
CSIC performed a renovation of leachates in an Atmosbag (under N2 atmosphere). Quantitative aqueous concentrations of Ca, Si, Al, and K Sr were determined by an ICP- Optical Emission Spectrometer (OES) with a Varian 725-ES ICP-OES (Palo Alto, CA, USA). SO42− were determined by IC with a Metrosep ASupp7-250/4.0 and semiquantitative concentrations of B, Cs, and Sr by a NexION 300XX Perkin Elmer spectrometer.
CIEMAT performed leaching tests in a Pure Lab glove box under an inert N2 atmosphere. For the determination of Si and B, an ICP-OES Thermo ICAP Pro 6000 series was used (Waltham, MA, USA). Na, Ca, K, Mg, NH4, Cl−, and SO42− were quantified by means of IC (Advanced Compact IC 861 from Metrohm, Herisau, Switzerland). Al and trace elements (Sr, Cs, and metallic doping elements) were analyzed in an HR-ICP-MS Thermo Scientific Elements 2 (Waltham, MA, USA).
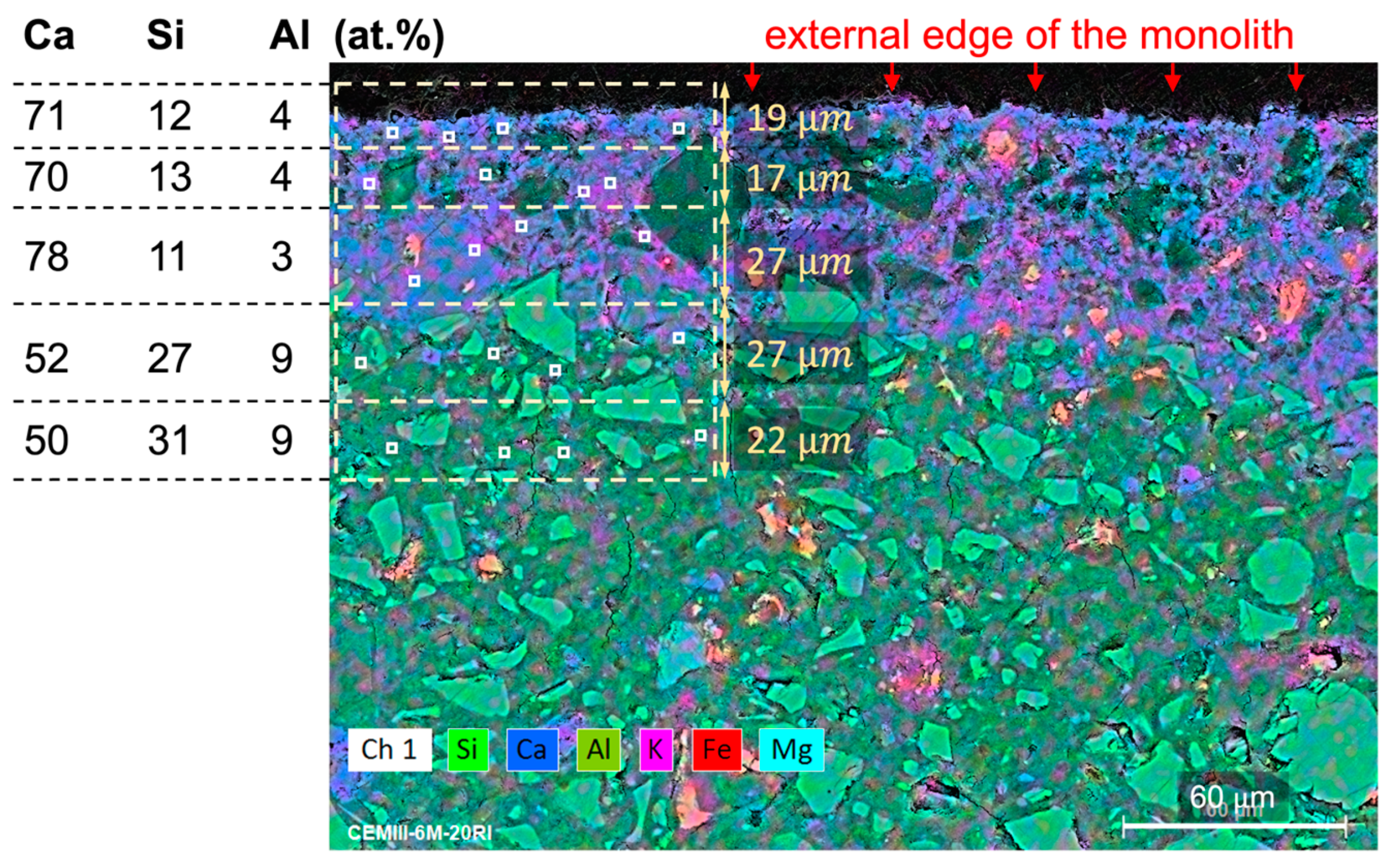
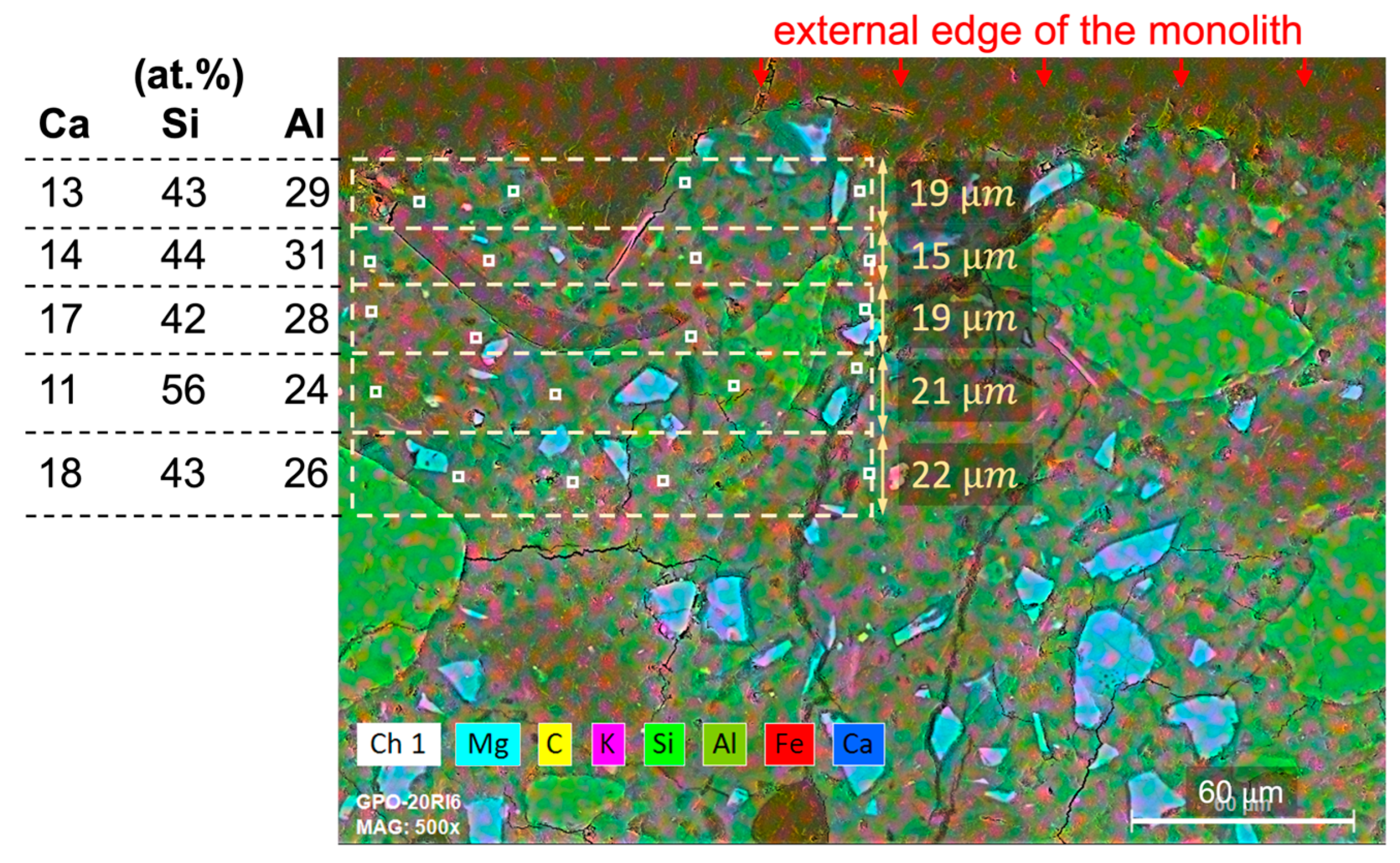
Monoliths for SEM-EDX analyses were cut and polished to study the variations in chemical compositions in a longitudinal profile of around 100 m, measured from the external surface facing the DW towards the sample’s core. Five sections were studied, with thicknesses ranging from 12 to 28 m. Four representative analyses taken on the cement matrices were considered to establish a mean value per section. The data were processed by removing C and O from the concentrations and recalculating the rest of the elements to achieve 100 wt.%.
Four replicates were used per sample in order to analyze the solutions and solids. Additional samples are presently running with the aim to study their leaching behaviour in the long-term. However, the present work is limited to studying the results obtained during the first 6 months, focusing on the aqueous phase evolution.
As already presented by Ferrand et al. [
25], the normalized loss of an element
i (
, in g·m
−2) was calculated according to Equation (1):
where
is the concentration of element
i in the leachate, subtracted from its concentration in the blank (g·m
3),
is the total volume of solution (m
3),
is the mass fraction of element
i in the sample (g·g
−1), and
is the total geometric surface area of the monolith (m
2).
The cumulative fraction leached (CFL) is defined as the sum of the fractions leached during all previous leaching intervals, including the fraction leached during the last interval using the initial amount of the element contained in the monolith [
26]. The CFL was calculated according to Equation (2):
where
is the quantity of species
i measured in the leachate recovered during the
nth test interval and
is the quantity of species
i in the leaching specimen at the beginning of the test.
The effective diffusion coefficient (
, in cm
2·s
−1) and the leachability index (
, dimensionless), both intrinsic mass transfer properties, can be calculated to predict the long-term leaching behaviour of elements of interest (B, Cs, Sr) contained in the RW.
was calculated as described in Equation (3):
where
is the slope of the linear regression fit of the CFL of element
i vs. time. The
is calculated from
, as expressed in Equation (4):
where
is a constant equal to 1 cm
2·s
−1.
4. Discussion
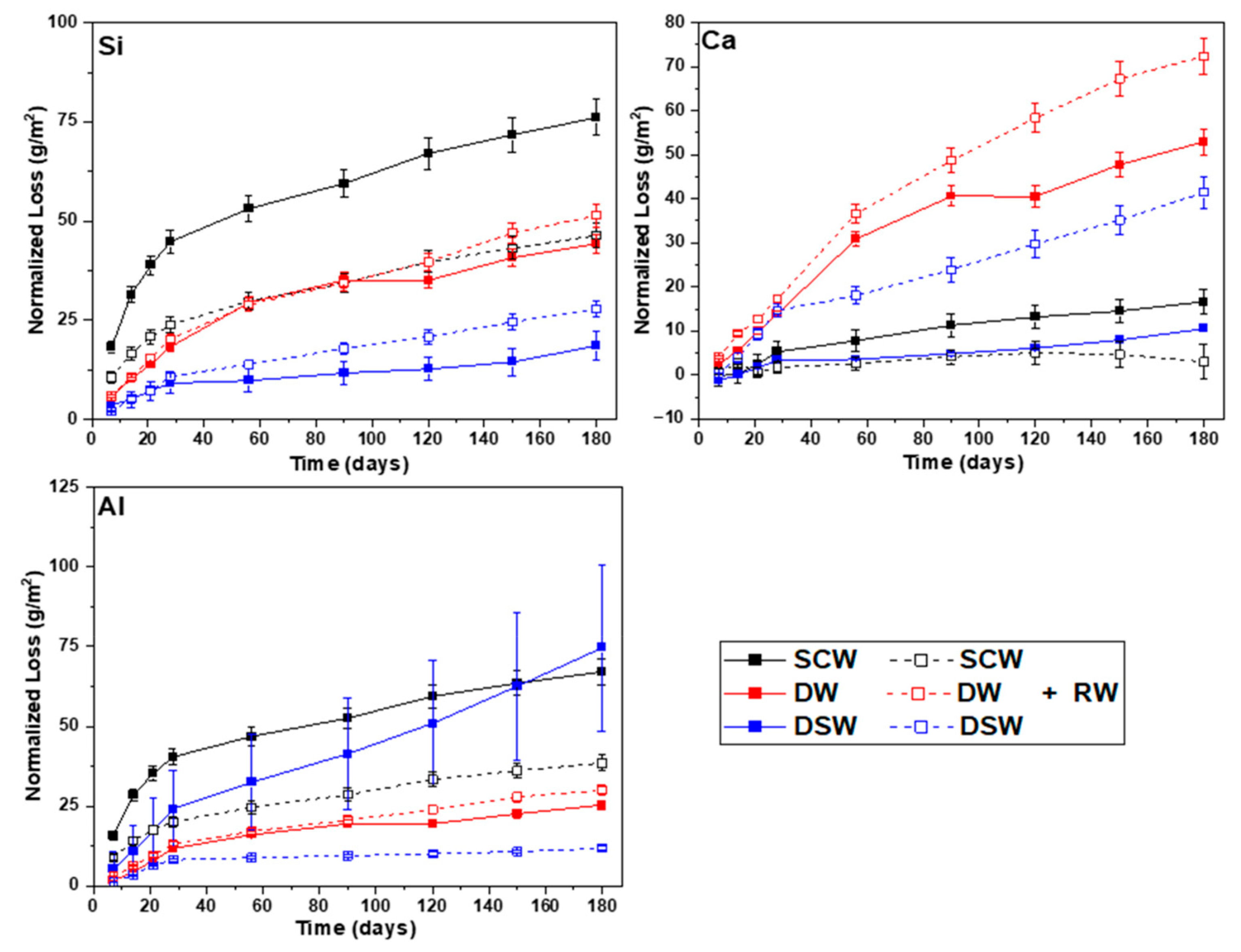
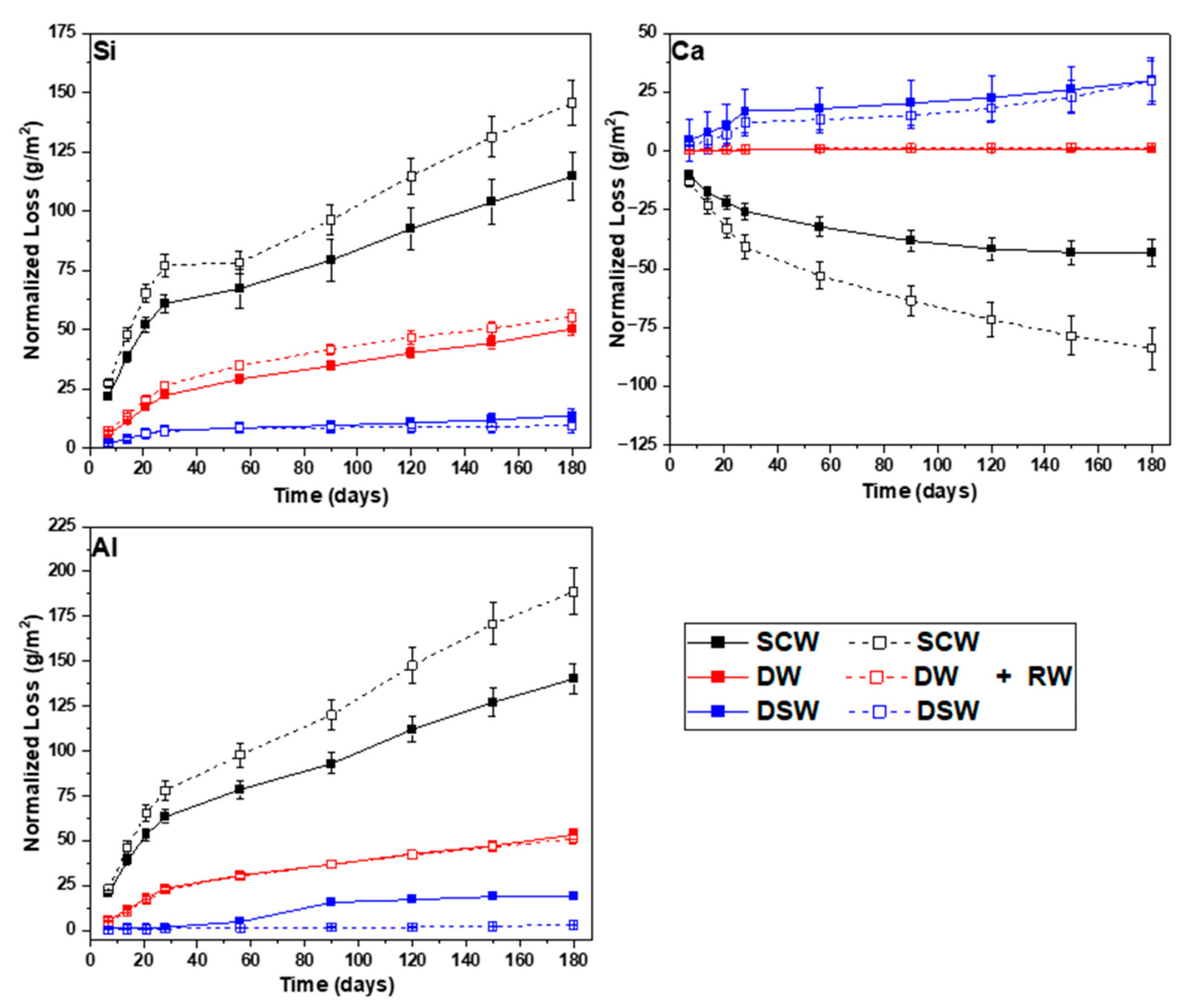
A net mass balance calculation was performed for Si, Ca, and Al considering the three cement matrices containing RW and the three leaching solutions (
Table 5). The balance was based on the accumulated net mass loss of each element from the monoliths, calculated at each time interval and expressed in weight percentage. Ca uptake (negative value) was observed for the three cement matrices immersed in DSW and the GPO immersed in SCW. Since the mass balance calculation did not consider associated errors, that might be influenced by the concentration errors determined at each interval, the initial element content in the monoliths, the initial mass of the monoliths, and the initial element concentration in solution. Errors in the range of
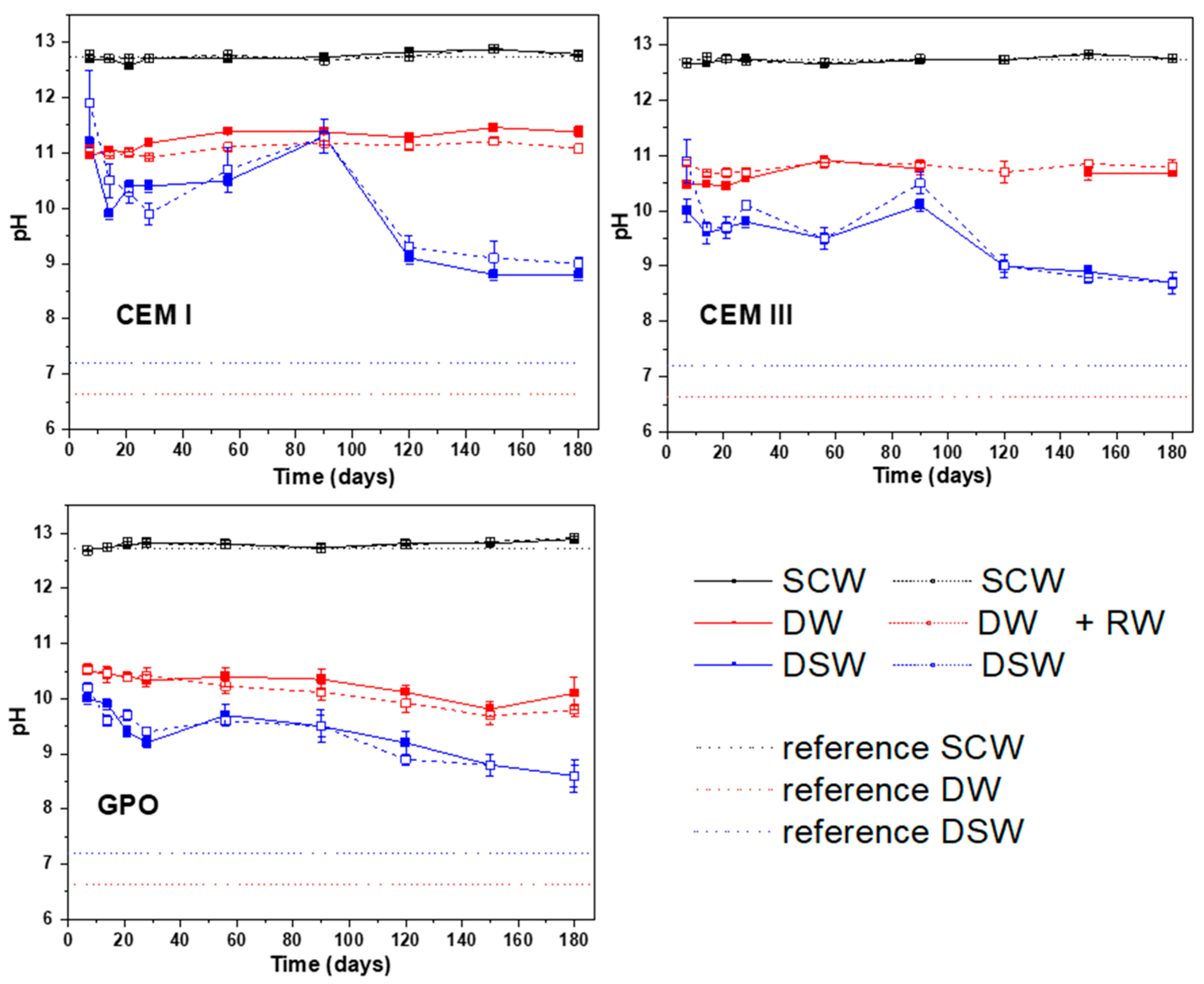
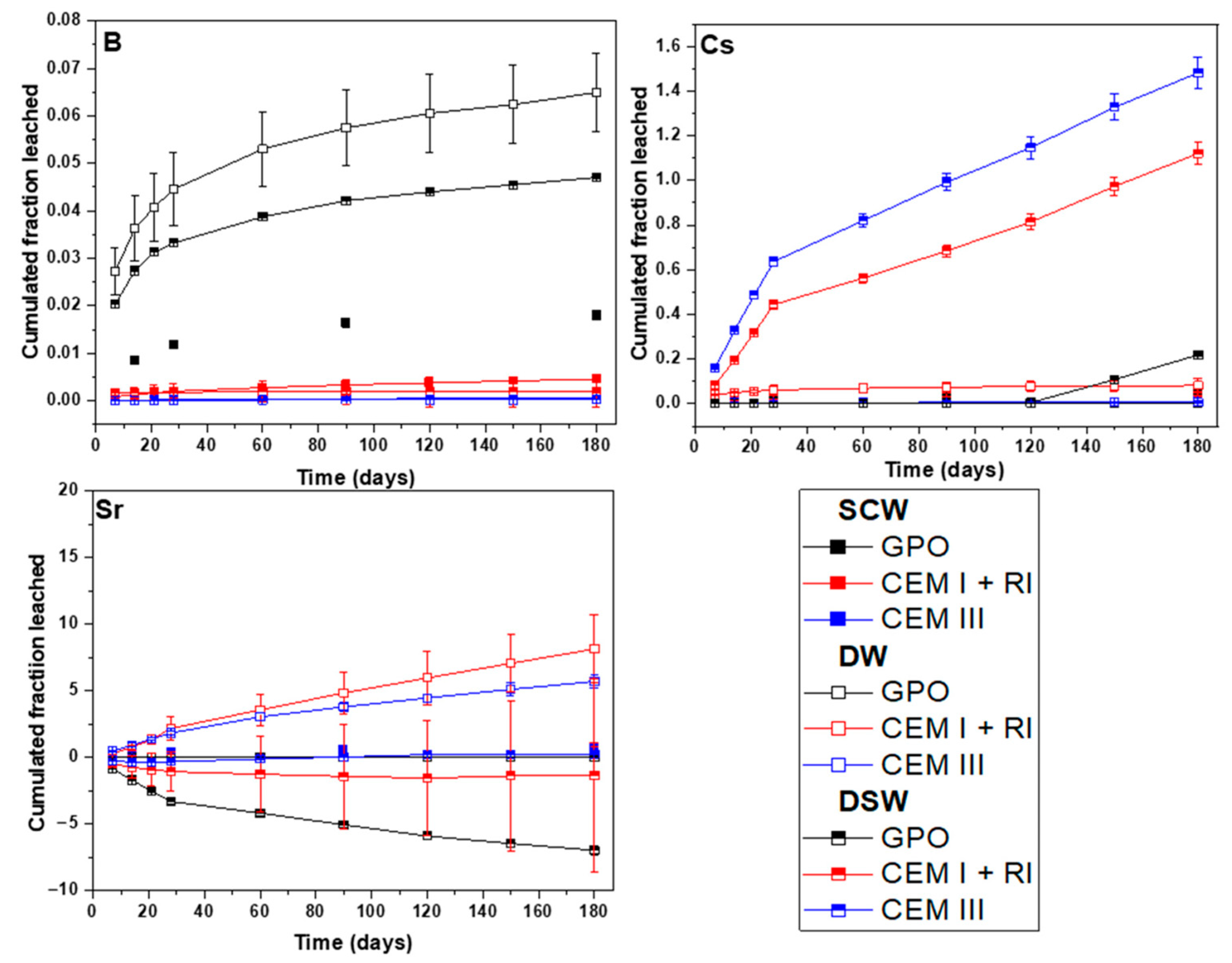
1 should be considered negligible. In general, mass losses lower than 5 wt.% were determined considering all experimental conditions. DW was revealed as the most aggressive leaching media for CEM I and CEM III, whereas SCW was shown to be the most aggressive leaching solution for GPO.
With the aim of understanding the Ca uptake observed in the experiments with GPO, a second Ca mass-balance calculation was performed, knowing the Ca concentrations in SCW and DSW, the volume of the solutions, the Ca wt.% within GPO and GPO+RW, and the mass of the monoliths. Ca content in the GPO+RW was initially 142 times higher than dissolved in DSW, and 324 times higher in the GPO than dissolved in SCW. Although only the external surfaces of the monoliths were exposed to the solution and, therefore, not all of the Ca contained in the monoliths could be considered to have quickly established an equilibrium with the solutions, the Ca uptake in these samples was unexpected. Due to the high pH determined in DSW (>9) and SCW (>12.5), one possible explanation could be attributed to the precipitation of carbonates from the interaction between soluble Ca and atmospheric CO2 prior to the filtration of aqueous samples and analysis by ICP-MS in the case of SCW. However, DSW had a HCO3− concentration of 85 ± 7 ppm.
Haga et al. [
29] observed a portlandite dissolution front in PC leached in DW, generated by the diffusion of Ca from the inner to the outer parts of the cement probe and then transferred to the DW through the contact surface. The portlandite dissolution front increased with increasing reaction time and increasing porosity (lower density). These authors did not observe an accumulation of Ca at the edges of the cement probes, as identified in the present study. Due to the large amount of carbon contained in the RW, a carbonation process might be plausible at the external surface, favoured by the high pH in CEM I and CEM III, delaying the release of Ca to the solution. This will be further investigated in the currently running longer-term experiments.
The leaching rate of B from the GPO matrix is consistently higher (one order of magnitude) than that from CEM I and CEM III, regardless of the leaching media. This difference is mainly attributed to the contrasting chemical composition and microstructure between GPO and the CEM matrices. The OPC-based matrices (CEM I and CEM III) contain significantly more Ca, which can bind B more effectively, reducing its mobility and leaching [
30]. In OPC-based matrices, Ca released during hydration can precipitate B-containing phases, or B can substitute into calcium silicates hydrates, limiting its release and leading to lower leachability [
31]. Ca accumulation near the external surface of the monoliths could be related to B immobilization, although this will need further analysis for confirmation.
The GPO matrix, in contrast, has lower Ca content and higher Si and Al content, as is inherent to the MK and BFS precursors. This composition produces a more porous geopolymeric microstructure, with fewer Ca-containing phases for B immobilization, hence allowing higher leaching. The SCW showed lower B leaching than DW and DSW, suggesting that high pH and ionic strength may partially limit B release due to the formation of secondary phases (through Ca-borates interaction) or matrix stability effects.
The leaching mechanisms of Cs and Sr were not studied in the present study, but in the case of geopolymer matrices, the primary mechanisms responsible for radionuclide immobilization involve a combination of physical encapsulation, chemical bonding, and ion-exchange interactions within the aluminosilicate network. The highly cross-linked geopolymer structure creates a dense matrix that significantly limits radionuclide mobility through physical containment. For specific radionuclides such as strontium, retention appears enhanced through incorporation into zeolite-like structures within the geopolymer framework and through ion-exchange processes at available cationic sites [
32,
33,
34,
35,
36,
37,
38]. In geopolymeric matrices, Cs and Sr have also been observed to partially displace sodium and potassium ions from charge-balancing sites in the aluminosilicate network [
39,
40], with their bonding capacity being influenced by both the matrix composition and the chemistry of the pore solution.
Lee et al. [
41] investigated the short-term leaching behaviour of Cs and Sr in deionized water from wet spent ion exchange resins (SIERs) encapsulated within a ground granulated blast furnace slag-based geopolymer matrix. After five days, the CFL for Cs and Sr from geopolymer matrices containing 20 wt.% wet SIER was 0.1 and 6 × 10
−4, respectively. Although the leaching period in the present study is longer, both works demonstrate that Si- and Al-rich geopolymers provide a promising matrix for Sr immobilization, as evidenced by their very low CFL values.
He et al. [
32] examined the leaching behaviour of Cs and Sr encapsulated in metakaolin-based geopolymers and Portland cement. Contrary to the results obtained here, they observed higher Cs leaching rates compared to Sr in deionized water for the geopolymer system. They attributed this to the smaller ionic radius of Sr, which facilitates its integration into zeolite-like structures within the geopolymer framework. According to their diffusion model analysis, Sr leaching occurred primarily via its dissolution in PC and surface wash-off in geopolymers, supporting the improved immobilization performance of the geopolymer matrix. These authors also noted a significant increase in Cs and Sr leaching when the medium changed from deionized water to a salt solution, which they attributed to the “corrosion” of the matrix and subsequent release of encapsulated elements.
SEM-EDX analyses further support these findings, revealing that microstructural integrity in geopolymer matrices is greater compared to conventional cement-based binders, which contributes to reduced radionuclide release potential.
The current results should be interpreted as representative of early-stage waste form performance only. While the observed cumulative fraction of leached values and leachability indexes meets current regulatory standards, these findings highlight the need for extended experimental studies to fully characterize matrix durability. Long-term testing remains essential to assess repository safety across relevant timescales.
5. Implications for Practical Application and Regulatory Compliance
Laboratory leaching kinetics provide critical input parameters for predictive models used in safety cases. However, because these experiments typically demonstrate short-term, non-equilibrium behaviour, strategies are needed to extrapolate such findings to operational timescales. By establishing baseline release rates from accelerated tests and identifying the key controlling mechanisms, such as diffusion or surface wash-off, laboratory data can be integrated into repository performance models that forecast long-term release trends under evolving environmental conditions.
For this work, three leaching solutions, deionized water (DW), synthetic cementitious water (SCW), and disposal site water (DSW), were selected to represent conditions relevant to the operational and long-term safety assessment of a Spanish disposal facility. DW simulates regulatory compliance testing and meteoric water ingress during normal operation. SCW reflects the alkaline porewater present in engineered cementitious barriers, characterizing near-field repository chemistry. DSW reproduces sulphate-rich, neutral pH groundwater conditions occurring after barrier failure, water table rise, or bath tubing events. This approach addresses a range of expected and accidental water pathway exposures, ensuring that matrix performance is assessed under all relevant scenarios as outlined in the facility safety case.
The results obtained from these leaching tests clarify how the performance of each waste form matrix varies across different leaching scenarios, each representing a different regulatory and environmental context. When subjected to DW, the geopolymer matrix consistently met the WAC for Cs and Sr according to NRC leachability indices, demonstrating a cumulative fraction leached below 0.5% for Cs and below 2% for Sr.
In scenarios using SCW, the results show a persistently high pH (12.5–13) and stable ionic strength across all matrices. However, the geopolymer exhibits enhanced performance by limiting element loss and exhibiting the lowest Ca and Al dissolution, thereby contributing to greater waste form stability under repository-relevant, high-pH conditions. The possibility of a hydration reaction in the GPO due to its interaction with the SCW cannot be discarded, as this changes the NASH and CASH balance of the matrix, which is needed for longer exposure times. This chemical stability suggests that geopolymer is more durable compared to conventional cement matrices and still exhibits a good compatibility with the cement-based Engineered Barrier System typical of LILW disposal facilities.
In the DSW scenario, the matrices are exposed to neutral pH, sulphate-dominated conditions that could occur after long-term repository evolution or accidental water infiltration. Here, the geopolymer again demonstrates superior retention, minimizing Cs and Sr leaching and even leading to Sr uptake, likely due to precipitation from the Sr present in groundwater. This indicates that the geopolymer performs well, not only under normal operation conditions, but also under potential failure or intrusion scenarios.
Overall, these results show that using a one-part geopolymer matrix offers safer long-term conditioning and meets regulatory standards for Sr and Cs retention. The one-part geopolymer performs well as a conditioning material, but longer-term studies are still needed, as short-term leaching results have not yet stabilized and continued testing is necessary to fully understand its long-term performance.
6. Conclusions
An integrated approach that combines thermal treatment with immobilization in one-part geopolymer, CEM I, and CEM III matrices was systematically evaluated for conditioning spent ion exchange resins under short-term leaching conditions. Geopolymer matrices showed significantly better retention of Cs and Sr compared to conventional cementitious binders, achieving leachability indices above regulatory thresholds. While geopolymers were chemically durable across all three leaching media, boron leaching was higher than in cement-based matrices. This emphasizes the role of matrix composition and structure in multi-element retention.
SEM-EDX analyses supported the calculated normalized losses of Ca, Si, and Al for all matrices, both with and without thermally treated waste. SEM results revealed microstructural and chemical changes after short-term leaching. Cement-based matrices often showed calcium accumulation and magnesium loss near the surface, indicating hydration and leaching effects. Geopolymer specimens showed degradation processes occurring to a more limited extent than in conventional OPC matrices when exposed to deionised water. Under synthetic cementitious water conditions, SEM observations revealed chemical interactions between the blast furnace slag and the alkaline leaching solution.
Although pH and conductivity data suggested a robust matrix stability, chemical equilibrium was not reached within six months, presenting the need for longer studies. These short-term results provide a solid foundation for predictive models and support using advanced waste forms. However, additional long-term testing and post-mortem analysis are important for confirming durability and environmental safety.
Overall, this study confirms that one-part geopolymers are a promising option for immobilizing Cs- and Sr-containing wastes. However, further research is needed to evaluate their long-term performance under disposal conditions.
Further research is necessary to clarify diffusion-controlled release mechanisms, microstructural evolution over time, and the long-term leaching resistance of these matrices under repository conditions. Ongoing long-term experiments are essential to validate the promising initial performance observed in these geopolymer matrices and to provide confidence for their industrial deployment.