Enhancement of Visual Feedback Ownership in Hand Mirror Therapy Using Automated Control of Electrical Muscle Stimulation Based on Healthy Hand Movement
Abstract
1. Introduction
2. Related Research
2.1. Neuroplasticity in Rehabilitation
2.2. Mirror Therapy and Electrical Muscle Stimulation
2.3. Assessment of Perceived Body Representation Ownership Through Visual Feedback in Mirror Therapy
3. Methods
3.1. Participants
- Age under 18 years old.
- History of heart disease.
- Blood hypertension status.
- History of spinal cord/nerve injury.
- History of forearm/hand muscular injury.
- Having a metal implant, especially in the arm or hand.
- Pregnancy for the woman participant.
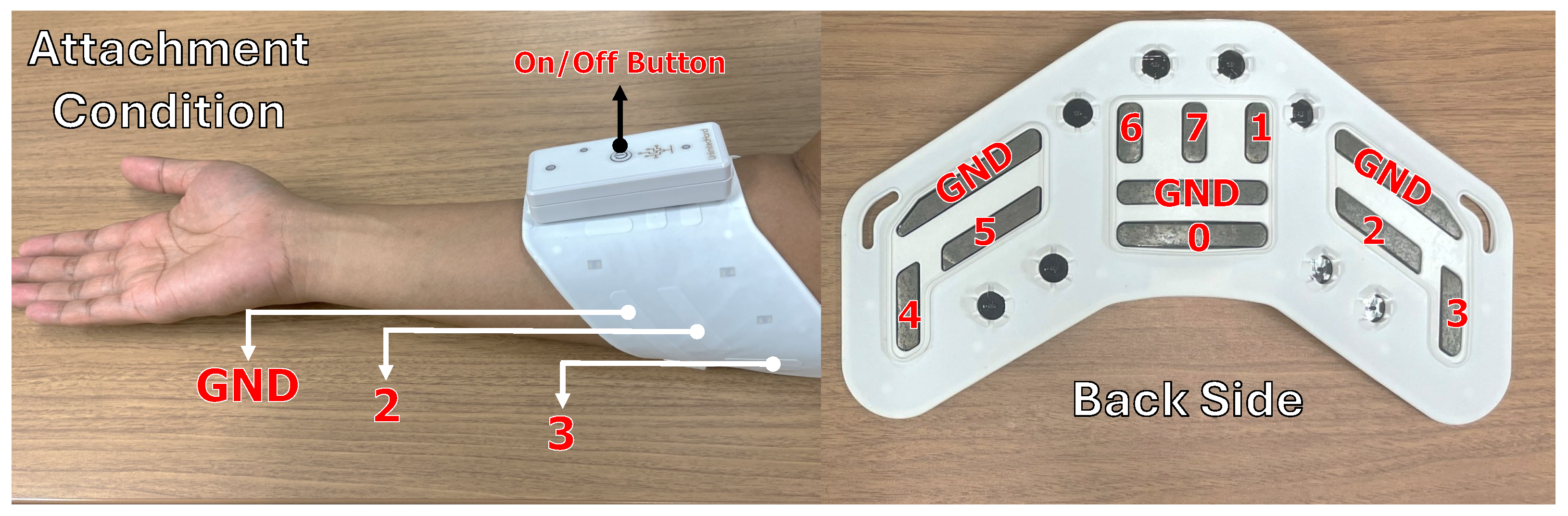
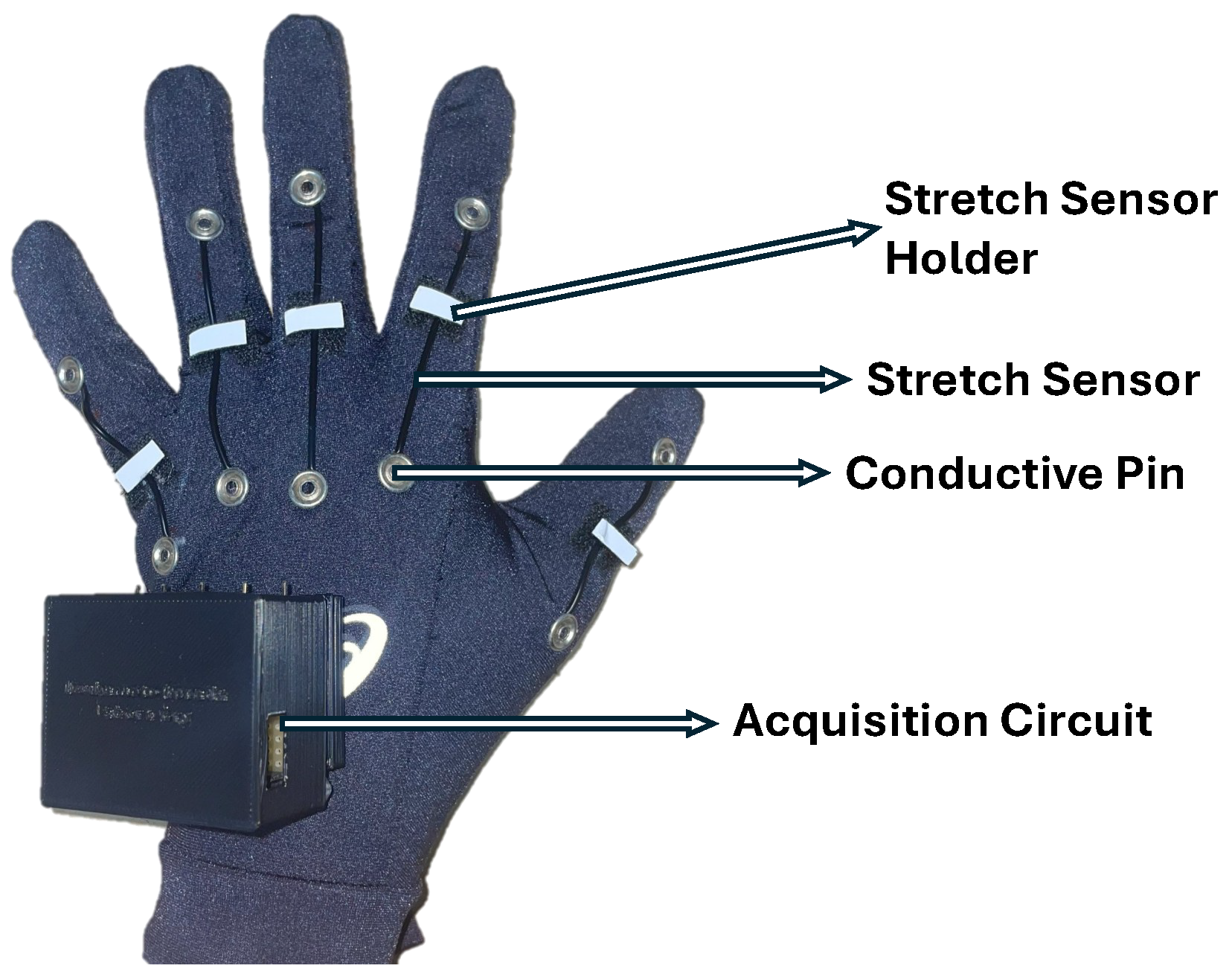
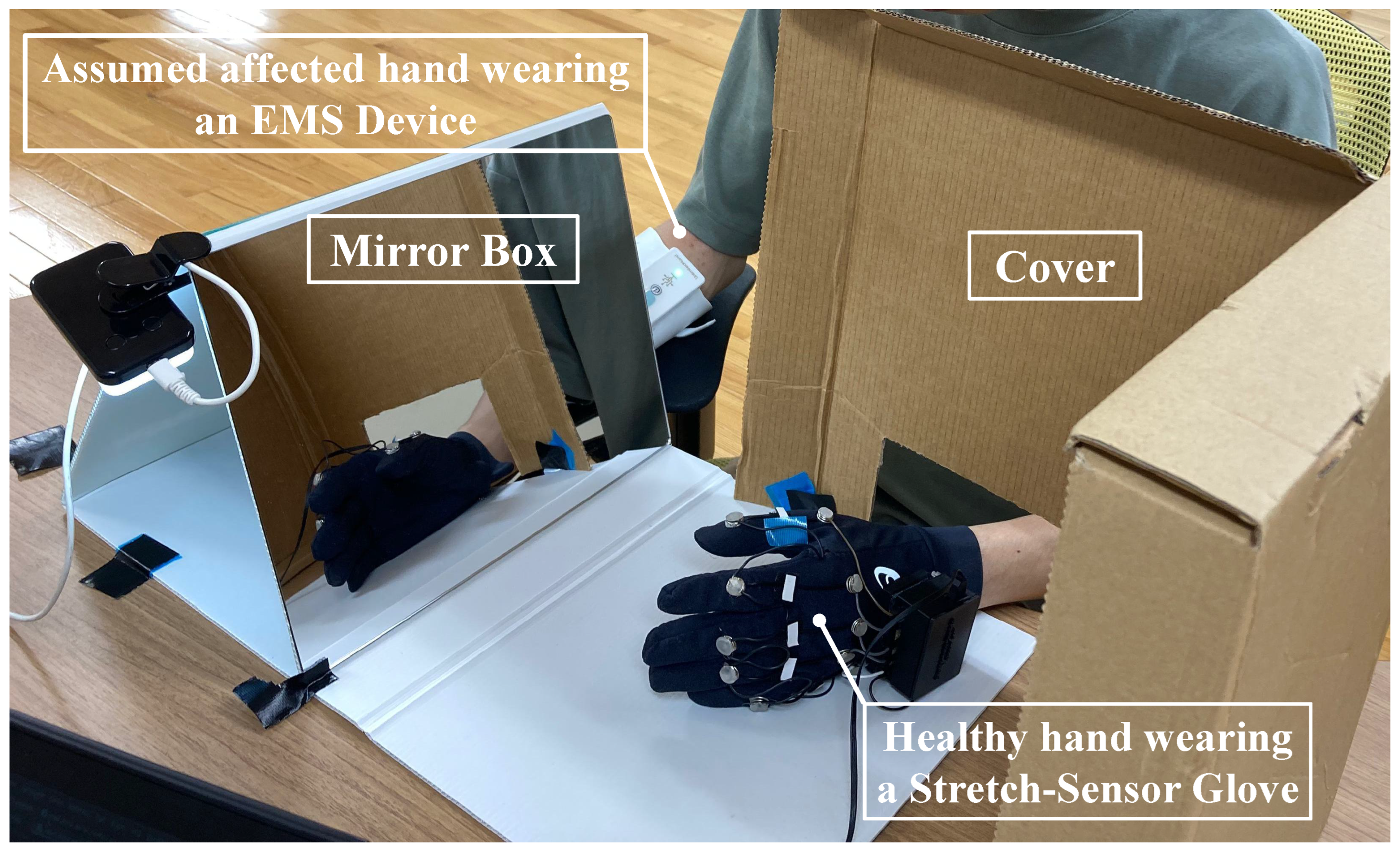
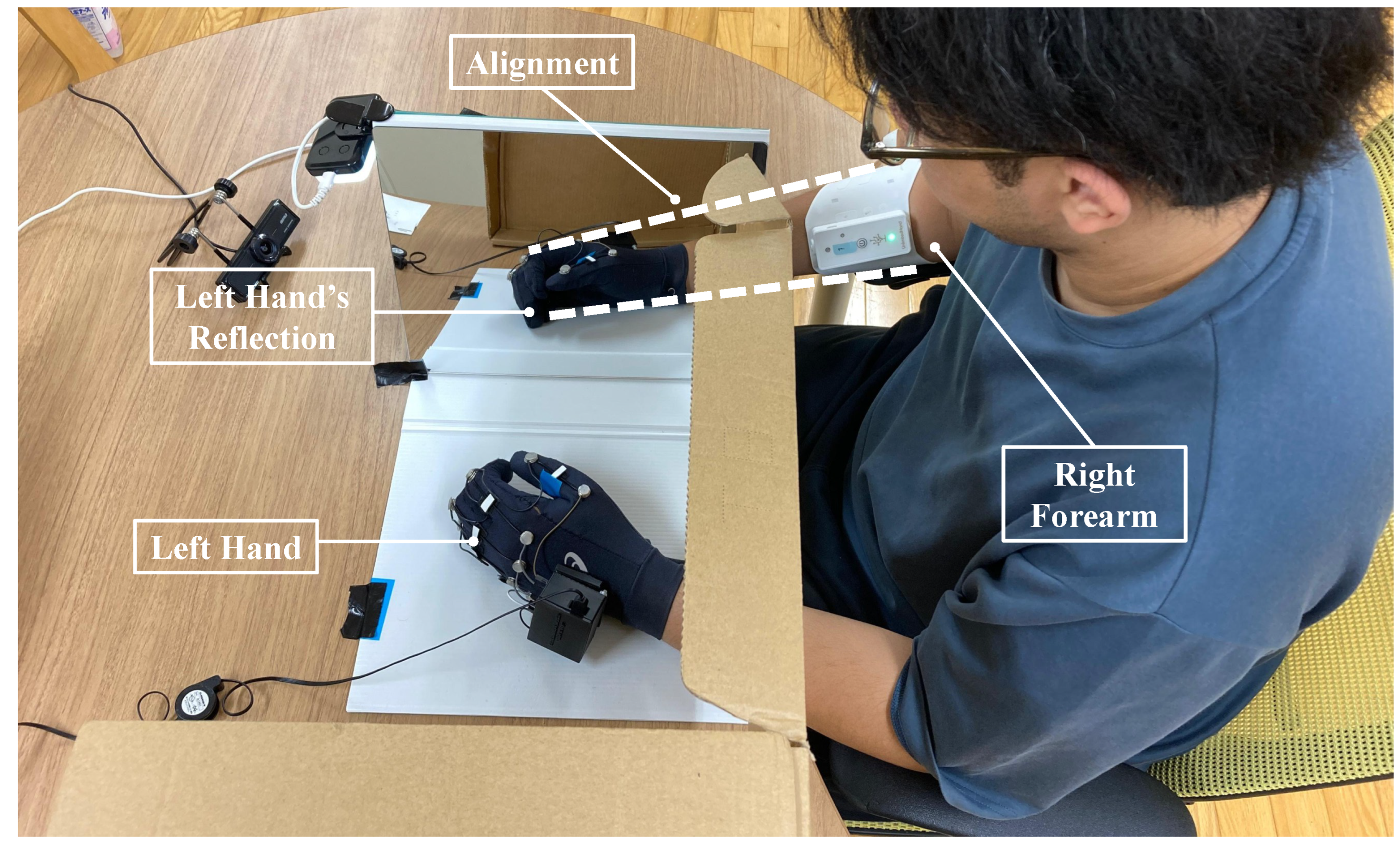
3.2. Instrumentation
3.3. Data Processing
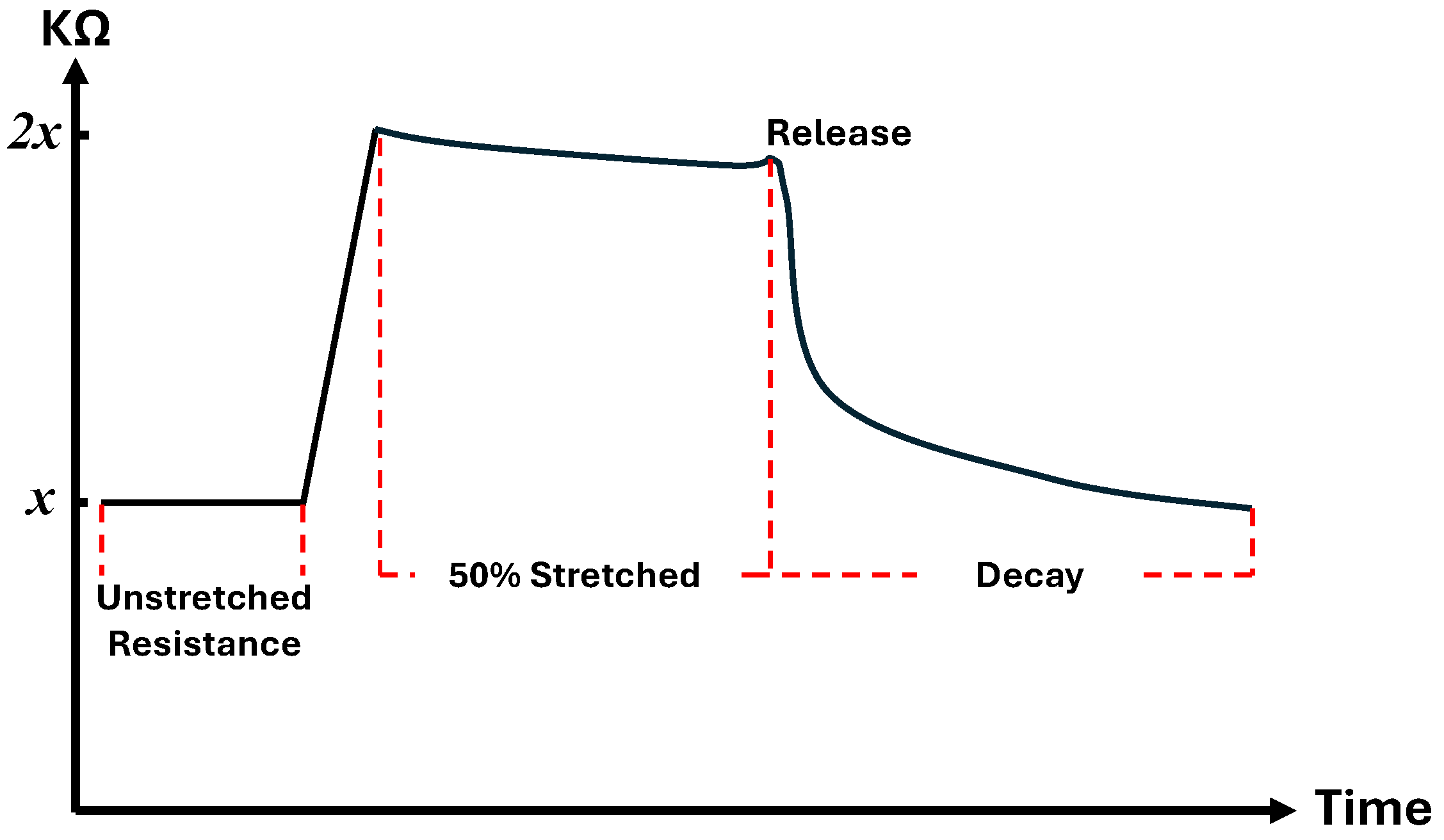
3.3.1. Stretch-Sensor Glove Data
3.3.2. Decay Modeling of Stretch-Sensor Glove Data
3.3.3. EMS Data
3.4. Evaluation
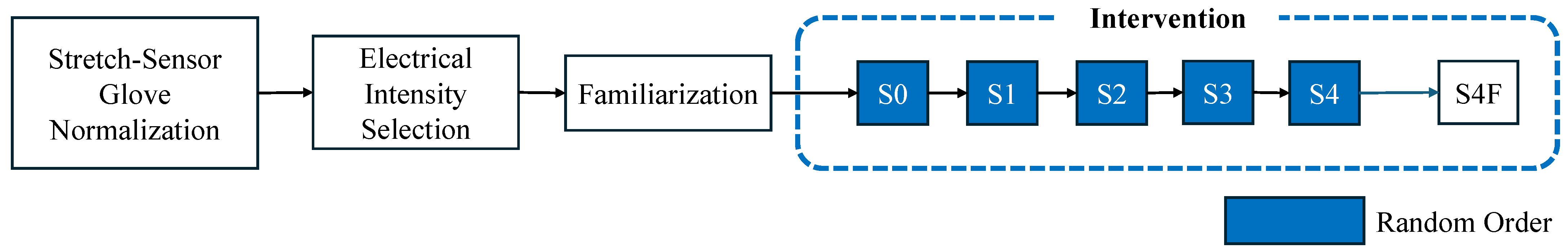
3.5. Experiment
3.5.1. Stretch-Sensor Glove Normalization
3.5.2. Electrical Intensity Selection
- Stimulation type 0 (S0) was the electrical intensity below the sensory level threshold, in which the stimulation was imperceptible (i.e., similar to MT only).
- Stimulation type 1 (S1) was the electrical intensity that induced sensory sensation (i.e., above sensory threshold and below motor threshold).
- Stimulation type 2 (S2) induced the motor threshold reaction.
- Stimulation type 3 (S3) stimulated functional finger movement with the intensity level slightly above S2.
- Stimulation type 4 (S4) applied a higher intensity level to induce fast and strong finger movement.
3.5.3. Familiarization
3.5.4. Intervention
3.5.5. Intervention Safety Measure
3.6. Statistical Analysis
4. Results
4.1. Correlation of Physical Characteristics and Personalized Electrical Intensity
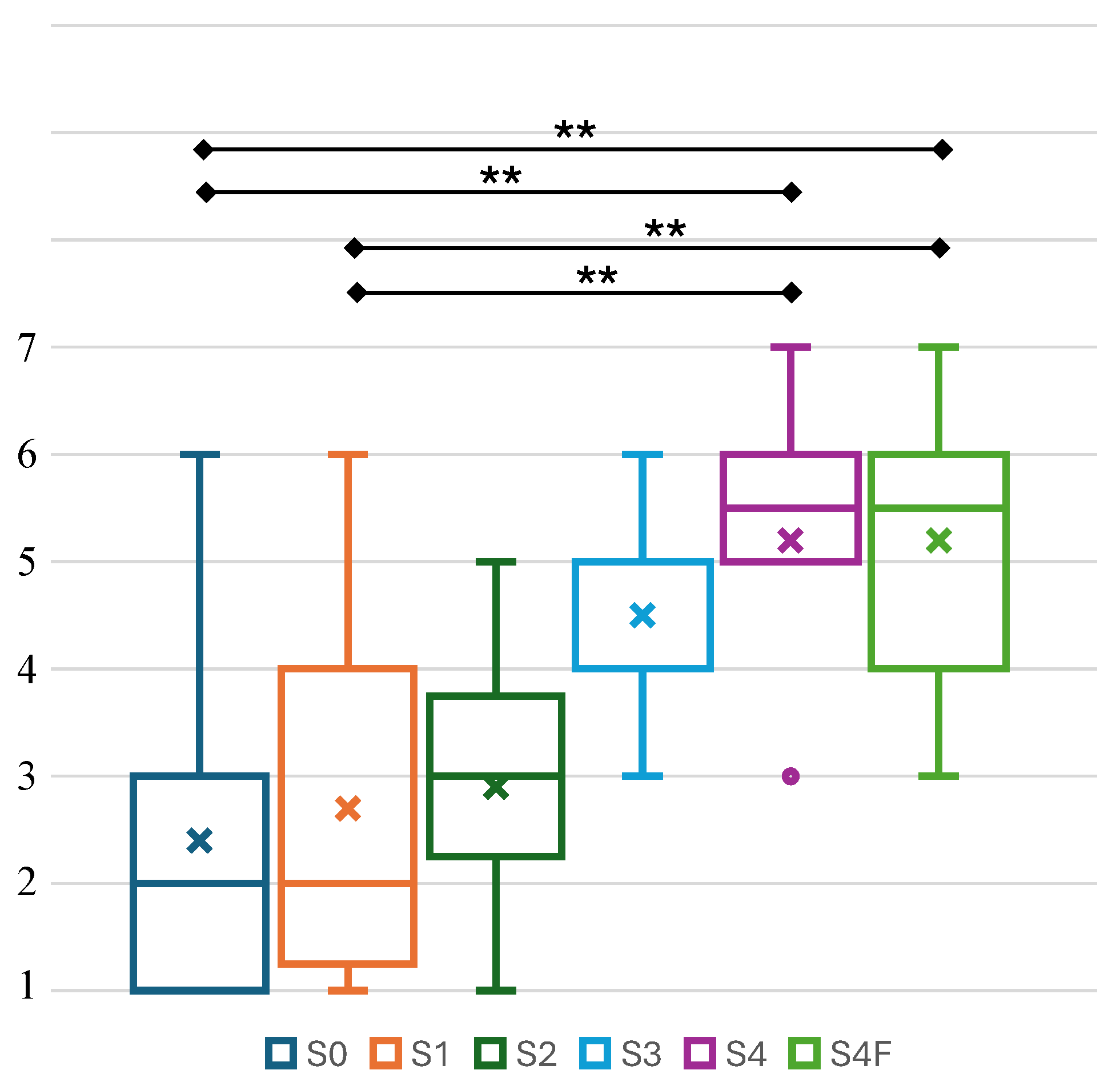
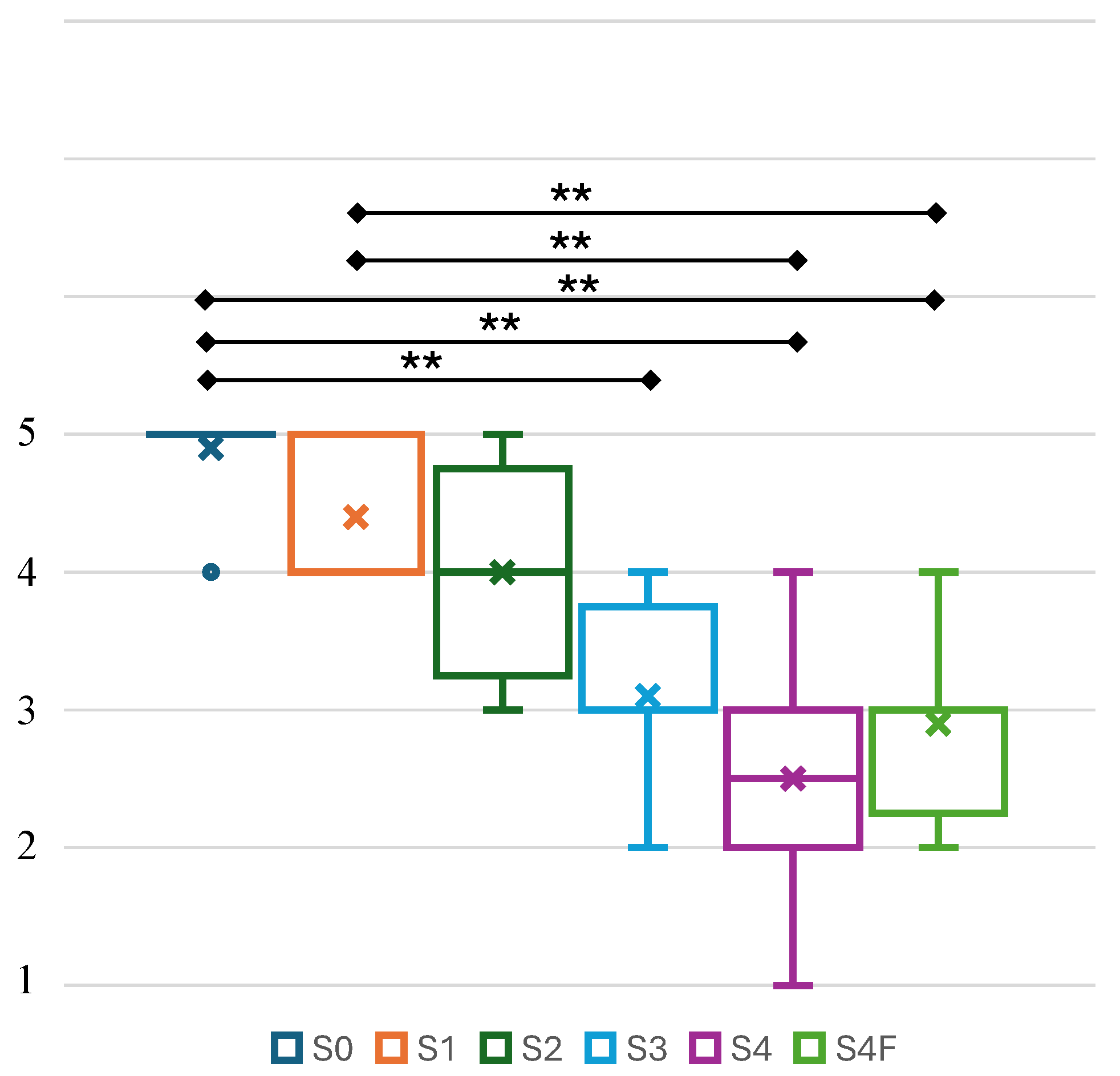
4.2. Perceived Body Representation and Electrical Stimulus Comfort
4.3. The Influence of the Individual Stimulation Order on the Perceived Body Representation
5. Discussion
5.1. Personalization of Electrical Intensity
5.2. Implication of Body Representation Perception and Electrical Stimulus Comfort Level
5.3. The Influence of Stimulation Order in Altering the Perceived Body Representation
5.4. Limitation and Future Works
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EMS | Electrical Muscle Stimulation |
| BMI | Body Mass Index |
| S0 | Stimulus Type 0 |
| S1 | Stimulus Type 1 |
| S2 | Stimulus Type 2 |
| S3 | Stimulus Type 3 |
| S4 | Stimulus Type 4 |
| S4F | Stimulus Type 4 Free Movement |
| UH | Unlimited Hand |
References
- Howard, G.; Goff, D.C. Population Shifts and the Future of Stroke: Forecasts of the Future Burden of Stroke. Ann. N. Y. Acad. Sci. 2012, 1268, 14–20. [Google Scholar] [CrossRef]
- Cheng, Y.; Lin, Y.; Shi, H.; Cheng, M.; Zhang, B.; Liu, X.; Shi, C.; Wang, Y.; Xia, C.; Xie, W. Projections of the Stroke Burden at the Global, Regional, and National Levels up to 2050 Based on the Global Burden of Disease Study 2021. J. Am. Heart Assoc. 2024, 13, e036142. [Google Scholar] [CrossRef] [PubMed]
- Joynt Maddox, K.E.; Elkind, M.S.V.; Aparicio, H.J.; Commodore-Mensah, Y.; de Ferranti, S.D.; Dowd, W.N.; Hernandez, A.F.; Khavjou, O.; Michos, E.D.; Palaniappan, L.; et al. Forecasting the Burden of Cardiovascular Disease and Stroke in the United States Through 2050—Prevalence of Risk Factors and Disease: A Presidential Advisory From the American Heart Association. Circulation 2024, 150, e65–e88. [Google Scholar] [CrossRef]
- Marciniak, C. Poststroke Hypertonicity: Upper Limb Assessment and Treatment. Top. Stroke Rehabil. 2011, 18, 179–194. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The Fugl-Meyer Assessment of Motor Recovery after Stroke: A Critical Review of Its Measurement Properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef]
- Dombovy, M.L.; Sandok, B.A.; Basford, J.R. Rehabilitation for Stroke: A Review. Stroke 1986, 17, 363–369. [Google Scholar] [CrossRef]
- Selohandono, A.; Zamroni, Z.; Budi Rahayu, A. Emerging Neuroplasticity-Based Therapies in Stroke Rehabilitation: Literature Review. Ahmad Dahlan Med. J. 2024, 5, 204–218. [Google Scholar] [CrossRef]
- Michielsen, M.E.; Selles, R.W.; van der Geest, J.N.; Eckhardt, M.; Yavuzer, G.; Stam, H.J.; Smits, M.; Ribbers, G.M.; Bussmann, J.B.J. Motor Recovery and Cortical Reorganization After Mirror Therapy in Chronic Stroke Patients. Neurorehabil. Neural Repair 2011, 25, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Thieme, H.; Mehrholz, J.; Pohl, M.; Behrens, J.; Dohle, C. Mirror Therapy for Improving Motor Function after Stroke. Cochrane Database Syst. Rev. 2018, 2018, 1–154. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, D.B.; Sterba, A.; Khatter, H.; Pandian, J.D. Mirror Therapy in Stroke Rehabilitation: Current Perspectives. Ther. Clin. Risk Manag. 2020, 16, 75–85. [Google Scholar] [CrossRef]
- Khan, M.; Mazhar, S.; Ur-Rahman, M.; Jan, M.B.A.; Khan, Y.; Rahman, Z.; Habib, S.H.; Huma, Z.; Ghandapur, M.S.J.; Ali, Y. The Effectiveness of Mirror Therapy in Stroke Rehabilitation: Current Perspective Review. EC Neurol. 2020, 12, 69–80. [Google Scholar]
- Gallagher, S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn. Sci. 2000, 4, 14–21. [Google Scholar] [CrossRef]
- Lisalde-Rodríguez, M.E.; Garcia-Fernández, J.A. Mirror Therapy in Hemiplegic Patient. Rev. Neurol. 2016, 62, 28–36. [Google Scholar]
- Casale, R.; Damiani, C.; Rosati, V. Mirror Therapy in the Rehabilitation of Lower-Limb Amputation. Am. J. Phys. Med. Rehabil. 2009, 88, 837–842. [Google Scholar] [CrossRef]
- Salhab, G.; Sarraj, A.R.; Saleh, S. Mirror Therapy Combined with Functional Electrical Stimulation for Rehabilitation of Stroke Survivors’ Ankle Dorsiflexion. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Xu, Q.; Guo, F.; Salem, H.M.A.; Chen, H.; Huang, X. Effects of Mirror Therapy Combined with Neuromuscular Electrical Stimulation on Motor Recovery of Lower Limbs and Walking Ability of Patients with Stroke: A Randomized Controlled Study. Clin. Rehabil. 2017, 31, 1583–1591. [Google Scholar] [CrossRef]
- Oh, Z.-H.; Liu, C.-H.; Hsu, C.-W.; Liou, T.-H.; Escorpizo, R.; Chen, H.-C. Mirror Therapy Combined with Neuromuscular Electrical Stimulation for Poststroke Lower Extremity Motor Function Recovery: A Systematic Review and Meta-Analysis. Sci. Rep. 2023, 13, 20018. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.J.; Chun, M.H.; Park, J.Y.; Kim, B.R. The Synergic Effects of Mirror Therapy and Neuromuscular Electrical Stimulation for Hand Function in Stroke Patients. Ann. Rehabil. Med. 2011, 35, 316–321. [Google Scholar] [CrossRef]
- Mathieson, S.; Parsons, J.; Kaplan, M.; Parsons, M. Combining Functional Electrical Stimulation and Mirror Therapy for Upper Limb Motor Recovery Following Stroke: A Randomised Trial. Eur. J. Physiother. 2018, 20, 244–249. [Google Scholar] [CrossRef]
- Kharka, M.; Singh, P. A Study to Compare the Effectiveness of Mirror Therapy and Neuromuscular Electrical Stimulation on Upper-Extremity Motor Recovery, Motor Function, and Quality of Life in Subacute Stroke Subjects. Med. J. Dr. D.Y. Patil Vidyapeeth 2021, 14, 318–326. [Google Scholar] [CrossRef]
- Paik, Y.-R.; Lee, J.-H.; Lee, D.-H.; Park, H.-S.; Oh, D.-H. Effect of Mirror Therapy and Electrical Stimulation on Upper Extremity Function in Stroke with Hemiplegic Patient: A Pilot Study. J. Phys. Ther. Sci. 2017, 29, 2085–2086. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J.S. Electrical Stimulation: Neurophysiological Basis and Application. Basic Appl. Myol. 2004, 14, 205–213. [Google Scholar]
- Kesselring, J. Neuroplasticity – Basis for Lifelong Learning. Eur. Neurol. Rev. 2015, 9, 143. [Google Scholar] [CrossRef]
- Puderbaugh, M.; Emmady, P.D. Neuroplasticity-StatPearls-NCBI Bookshelf. Available online: www.ncbi.nlm.nih.gov/books/NBK557811 (accessed on 30 July 2025).
- Singh, S. Neuroplasticity and Rehabilitation: Harnessing Brain Plasticity for Stroke Recovery and Functional Improvement. Univers. Res. Rep. 2024, 11, 50–56. [Google Scholar] [CrossRef]
- Luft, A.R. Rehabilitation and Plasticity. In Clinical Recovery from CNS Damage; Naritomi, H., Krieger, D.W., Eds.; S. Karger AG: Basel, Switzerland, 2013; pp. 88–94. [Google Scholar]
- Straudi, S.; Basaglia, N. Neuroplasticity-Based Technologies and Interventions for Restoring Motor Functions in Multiple Sclerosis. In Multiple Sclerosis: Bench to Bedside; Asea, A.A.A., Geraci, F., Kaur, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 171–185. [Google Scholar]
- Jarmolowska, J.; Miladinović, A.; Valvason, E.; Busan, P.; Ajčević, M.; Battaglini, P.P.; Accardo, A. Effects of Mirror Therapy on Motor Imagery Elicited ERD/S: An EEG Study on Healthy Subjects. In Proceedings of the 8th European Medical and Biological Engineering Conference (EMBEC), Portorož, Slovenia, 29 November–3 December 2020. [Google Scholar]
- Saavedra-García, A.; Moral-Munoz, J.A.; Lucena-Anton, D. Mirror Therapy Simultaneously Combined with Electrical Stimulation for Upper Limb Motor Function Recovery after Stroke: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Rehabil. 2021, 35, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Ikuno, K.; Morii, Y.; Tokuhisa, K.; Morimoto, S.; Shomoto, K. Feasibility Study of a Combined Treatment of Electromyography-Triggered Neuromuscular Stimulation and Mirror Therapy in Stroke Patients: A Randomized Crossover Trial. Neurorehabilit. Int. Interdiscip. J. 2014, 34, 235–244. [Google Scholar] [CrossRef]
- Hall, C.R.; Martin, K.A. Measuring Movement Imagery Abilities: A Revision of the Movement Imagery Questionnaire. J. Ment. Imag. 1997, 21, 143–154. [Google Scholar]
- Iwanami, J.; Mutai, H.; Sagari, A.; Sato, M.; Kobayashi, M. Relationship between Corticospinal Excitability While Gazing at the Mirror and Motor Imagery Ability. Brain Sci. 2023, 13, 463. [Google Scholar] [CrossRef]
- Kusano, K.; Hayashi, M.; Iwama, S.; Ushiba, J. Improved Motor Imagery Skills after Repetitive Passive Somatosensory Stimulation: A Parallel-Group, Pre-Registered Study. Front. Neural Circuits 2025, 18, 1510324. [Google Scholar] [CrossRef] [PubMed]
- Gerovasili, V.; Stefanidis, K.; Vitzilaios, K.; Karatzanos, E.; Politis, P.; Koroneos, A.; Chatzimichail, A.; Routsi, C.; Roussos, C.; Nanas, S. Electrical Muscle Stimulation Preserves the Muscle Mass of Critically Ill Patients: A Randomized Study. Crit. Care 2009, 13, R161. [Google Scholar] [CrossRef]
- H2L Inc. UnlimitedHand Developer’s Site. Available online: http://dev.unlimitedhand.com/devise/ (accessed on 7 August 2025).
- P, A.R.S.S.; Ohnishi, A.; Terada, T.; Tsukamoto, M. Estimation of Cylinder Grasping Contraction Force of Forearm Muscle in Home-Based Rehabilitation Using a Stretch-Sensor Glove. Appl. Sci. 2025, 15, 7534. [Google Scholar] [CrossRef]
- Images Scientific Instruments, Inc. Flexible Stretch Sensor. Available online: https://www.imagesco.com/sensors/stretch-sensor.html (accessed on 20 March 2025).
- Sachetti, A.; Carpes, M.F.; Dias, A.S.; Sbruzzi, G. Safety of Neuromuscular Electrical Stimulation among Critically Ill Patients: Systematic Review. Rev. Bras. Ter. Intensiv. 2018, 30, 219–225. [Google Scholar] [CrossRef]
- Gregg, M.; Hall, C.; Butler, A. The MIQ-RS: A Suitable Option for Examining Movement Imagery Ability. Evid. Based Complement. Altern. Med. 2010, 7, 249–257. [Google Scholar] [CrossRef]
- Miller, M.G.; Cheatham, C.C.; Holcomb, W.R.; Ganschow, R.; Michael, T.J.; Rubley, M.D. Subcutaneous Tissue Thickness Alters the Effect of NMES. J. Sport Rehabil. 2008, 17, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Petrofsky, J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med. Eng. Phys. 2008, 30, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.V.; Vieira, A.; Carregaro, R.; Bottaro, M.; Maffiuletti, N.; Durigan, J. Skinfold thickness affects the isometric knee extension torque evoked by Neuromuscular Electrical Stimulation. Braz. J. Phys. Ther. 2015, 19, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Bolfe, V.J.; Ribas, S.I.; Montevelo, M.I.L.; Guirro, R.R.J. Electrical Impedance Behavior of Biological Tissues During Transcutaneous Electrical Stimulation. Braz. J. Phys. Ther. 2007, 11, 135–140. [Google Scholar]
- Park, J.; Hopkins, J. Quadriceps activation normative values and the affect of subcutaneous tissue thickness. J. Sport Rehabil. 2011, 21, 136–140. [Google Scholar] [CrossRef]
- Kim, J.Y.; Noh, H.; Park, J. Effects of Functional Electrical Stimulation Intensity Level on Corticomuscular Coherence during Action Observation. J. Kor. Phys. Ther. 2020, 32, 307–311. [Google Scholar] [CrossRef]
- Insausti-Delgado, A.; López-Larraz, E.; Omedes, J.; Ramos-Murguialday, A. Intensity and Dose of Neuromuscular Electrical Stimulation Influence Sensorimotor Cortical Excitability. Front. Neurosci. 2021, 14, 593360. [Google Scholar] [CrossRef]
- Paillard, T. Neuromuscular or Sensory Electrical Stimulation for Reconditioning Motor Output and Postural Balance in Older Subjects? Front. Physiol. 2022, 12, 779249. [Google Scholar] [CrossRef]
- Shindo, M.; Isezaki, T.; Aoki, R. Electrical Stimulation Intensity to Induce Sensory Reweighting Dynamics While Standing on Balance Board. In Proceedings of the 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Sydney, Australia, 24–27 July 2023. [Google Scholar]
- Poso, K.; Goda, Y. Unraveling Mechanisms of Homeostatic Synaptic Plasticity. Neuron 2010, 66, 337–351. [Google Scholar] [CrossRef] [PubMed]

| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|
| Very hard to feel | Hard to feel | Somewhat hard to feel | Uncertain | Somewhat easy to feel | Easy to feel | Very easy to feel |
| 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Very uncomfortable | Uncomfortable | Uncertain | Comfortable | Very comfortable |
| Participant | BMI (kg/m2) | Forearm Circumference (cm) | S1 | S2 | S3 | S4 | Intensity Difference Variance |
|---|---|---|---|---|---|---|---|
| 1 | 25.06 | 24.55 | 5 | 6 | 7 | 8 | 0.00 |
| 2 | 17.40 | 24.30 | 2 | 3 | 5 | 8 | 0.67 |
| 3 | 32.91 | 28.85 | 6 | 7 | 9 | 11 | 0.22 |
| 4 | 29.86 | 27.60 | 2 | 5 | 7 | 9 | 0.22 |
| 5 | 17.31 | 22.40 | 4 | 6 | 9 | 10 | 0.67 |
| 6 | 17.21 | 22.40 | 4 | 7 | 10 | 11 | 0.89 |
| 7 | 19.88 | 22.20 | 1 | 3 | 5 | 7 | 0.00 |
| 8 | 20.20 | 23.60 | 2 | 4 | 5 | 6 | 0.22 |
| 9 | 23.11 | 24.70 | 2 | 4 | 6 | 7 | 0.22 |
| 10 | 21.80 | 24.40 | 6 | 9 | 11 | 12 | 0.67 |
| Stimulus Type | BMI | Forearm Circumference |
|---|---|---|
| S1 | 0.26 | 0.38 |
| S2 | 0.17 | 0.26 |
| S3 | 0.02 | 0.17 |
| S4 | 0.01 | 0.19 |
| Participant | Stimulation Order | Body Representation Perception | Electrical Stimulus Comfort | Correlation of Body Representation and Stimulus Comfort (ρ) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | S4 | S4F | S0 | S1 | S2 | S3 | S4 | S4F | |||
| 1 | S2-S4-S0-S3-S1-S4F | 1 | 2 | 2 | 6 | 6 | 6 | 5 | 4 | 4 | 2 | 1 | 3 | −0.94 ** |
| 2 | S4-S2-S0-S3-S1-S4F | 1 | 1 | 4 | 3 | 5 | 7 | 5 | 5 | 3 | 3 | 2 | 4 | −0.63 |
| 3 | S2-S4-S0-S3-S1-S4F | 1 | 2 | 3 | 3 | 3 | 3 | 5 | 4 | 4 | 3 | 3 | 3 | −0.82 * |
| 4 | S1-S3-S2-S4-S0-S4F | 6 | 4 | 4 | 5 | 7 | 7 | 5 | 5 | 5 | 4 | 4 | 4 | −0.70 |
| 5 | S1-S3-S2-S4-S0-S4F | 2 | 4 | 3 | 5 | 6 | 6 | 5 | 4 | 4 | 4 | 2 | 2 | −0.94 ** |
| 6 | S0-S2-S3-S4-S1-S4F | 3 | 2 | 3 | 4 | 5 | 5 | 4 | 4 | 4 | 4 | 3 | 2 | −0.84 * |
| 7 | S0-S2-S3-S4-S1-S4F | 3 | 6 | 5 | 5 | 6 | 4 | 5 | 4 | 3 | 2 | 1 | 2 | −0.40 |
| 8 | S3-S1-S2-S0-S4-S4F | 2 | 1 | 1 | 4 | 5 | 4 | 5 | 4 | 3 | 3 | 2 | 3 | −0.66 |
| 9 | S3-S1-S2-S0-S4-S4F | 1 | 1 | 1 | 5 | 6 | 4 | 5 | 5 | 5 | 3 | 4 | 3 | −0.79 |
| 10 | S4-S2-S0-S3-S1-S4F | 4 | 4 | 3 | 5 | 3 | 6 | 5 | 5 | 5 | 3 | 3 | 3 | −0.40 |
| Primary Outcome | Stimulus Types | S0 | S1 | S2 | S3 | S4 | S4F |
|---|---|---|---|---|---|---|---|
| Body Representation Perception | S0 | 1.000 | 1.000 | 1.000 | 0.145 | 0.008 | 0.009 |
| S1 | 1.000 | 1.000 | 1.000 | 0.412 | 0.031 | 0.036 | |
| S2 | 1.000 | 1.000 | 1.000 | 0.606 | 0.052 | 0.060 | |
| S3 | 0.145 | 0.412 | 0.606 | 1.000 | 1.000 | 1.000 | |
| S4 | 0.008 | 0.031 | 0.052 | 1.000 | 1.000 | 1.000 | |
| S4F | 0.009 | 0.036 | 0.060 | 1.000 | 1.000 | 1.000 | |
| Electrical Stimulus Comfort | S0 | 1.0000 | 1.0000 | 0.6382 | 0.0013 | 0.00003 | 0.0002 |
| S1 | 1.0000 | 1.0000 | 1.0000 | 0.0774 | 0.0042 | 0.0220 | |
| S2 | 0.6382 | 1.0000 | 1.0000 | 0.8702 | 0.0948 | 0.3391 | |
| S3 | 0.0013 | 0.0774 | 0.8702 | 1.0000 | 1.0000 | 1.0000 | |
| S4 | 0.00003 | 0.0042 | 0.0948 | 1.0000 | 1.0000 | 1.0000 | |
| S4F | 0.0002 | 0.0220 | 0.3391 | 1.0000 | 1.0000 | 1.0000 |
| S0 | S1 | S2 | S3 | S4 | Average |
|---|---|---|---|---|---|
| 0.14 | −0.03 | −0.17 | −0.14 | 0.46 | 0.05 |
| Stimulus Type | Interpretation |
|---|---|
| S0 | Small chance to induce higher body representation when located at the later order, and vice versa |
| S1 | Small chance to induce higher body representation when located at the earlier order, and vice versa |
| S2 | Small chance to induce higher body representation when located at the earlier order, and vice versa |
| S3 | Small chance to induce lower body representation when located at the later order, and vice versa |
| S4 | Moderate chance to induce lower body representation when located at the earlier order, and vice versa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
P, A.R.S.S.; Ohnishi, A.; Terada, T.; Tsukamoto, M. Enhancement of Visual Feedback Ownership in Hand Mirror Therapy Using Automated Control of Electrical Muscle Stimulation Based on Healthy Hand Movement. Appl. Sci. 2025, 15, 11179. https://doi.org/10.3390/app152011179
P ARSS, Ohnishi A, Terada T, Tsukamoto M. Enhancement of Visual Feedback Ownership in Hand Mirror Therapy Using Automated Control of Electrical Muscle Stimulation Based on Healthy Hand Movement. Applied Sciences. 2025; 15(20):11179. https://doi.org/10.3390/app152011179
Chicago/Turabian StyleP, Adhe Rahmatullah Sugiharto Suwito, Ayumi Ohnishi, Tsutomu Terada, and Masahiko Tsukamoto. 2025. "Enhancement of Visual Feedback Ownership in Hand Mirror Therapy Using Automated Control of Electrical Muscle Stimulation Based on Healthy Hand Movement" Applied Sciences 15, no. 20: 11179. https://doi.org/10.3390/app152011179
APA StyleP, A. R. S. S., Ohnishi, A., Terada, T., & Tsukamoto, M. (2025). Enhancement of Visual Feedback Ownership in Hand Mirror Therapy Using Automated Control of Electrical Muscle Stimulation Based on Healthy Hand Movement. Applied Sciences, 15(20), 11179. https://doi.org/10.3390/app152011179






