Effect of Surgical Tightening Torque on the Pull-Out Strength of Screws in Vertebral Body Tethering
Abstract
1. Introduction
2. Methodology
2.1. Sample Preparation
2.2. Pull-Out Test
2.3. Geometric Model
2.4. Mechanical Properties
2.5. Mesh
2.6. Model Formulation
2.6.1. Load Cases
2.6.2. Boundary Conditions
3. Results and Discussion
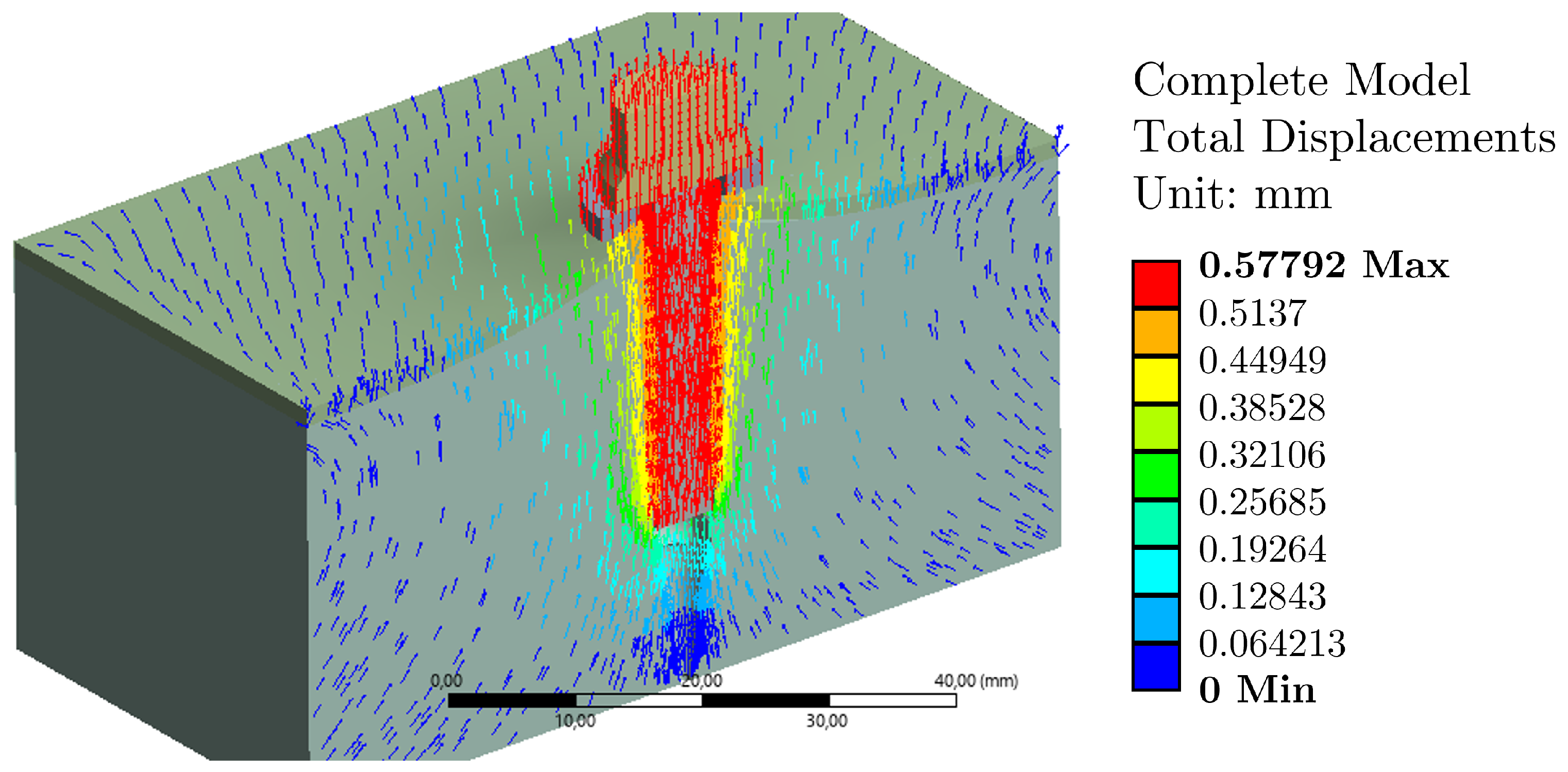
3.1. Load Case 1: Effect of Tightening Torque
3.2. Load Case 2: Plus Tightening Torque/Effect of Pull-Out Load
3.3. Limitations of the Model and Future Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ames, R.J.; Samdani, A.F.; Betz, R.R. Anterior scoliosis correction in immature patients with idiopathic scoliosis. Oper. Tech. Orthop. 2016, 26, 247–257. [Google Scholar] [CrossRef]
- Driscoll, M.; Aubin, C.E.; Moreau, A.; Parent, S. Biomechanical comparison of fusionless growth modulation corrective techniques in pediatric scoliosis. Med. Biol. Eng. Comput. 2011, 49, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Cunin, V. Early-onset scoliosis–current treatment. Orthop. Traumatol. Surg. Res. 2015, 101, S109–S118. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, H.; Little, J.P.; Adam, C.J. Intra-operative measurement of applied forces during anterior scoliosis correction. Clin. Biomech. 2016, 40, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.L.; Cristofolini, L.; Davico, G.; Viceconti, M. Computational modelling of the scoliotic spine: A literature review. Int. J. Numer. Methods Biomed. Eng. 2021, 37, e3503. [Google Scholar] [CrossRef]
- Cobetto, N.; Parent, S.; Aubin, C.E. 3D correction over 2 years with anterior vertebral body growth modulation: A finite element analysis of screw positioning, cable tensioning and postoperative functional activities. Clin. Biomech. 2018, 51, 26–33. [Google Scholar] [CrossRef]
- Yaszay, B.; Doan, J.D.; Parvaresh, K.C.; Farnsworth, C.L. Risk of Implant Loosening after Cyclic Loading of Fusion-less Growth Modulation Techniques: Nitinol Staples vs Flexible Tether. Spine 2017, 42, 443–449. [Google Scholar] [CrossRef]
- Brink, R.C.; Schlösser, T.P.; van Stralen, M.; Vincken, K.L.; Kruyt, M.C.; Hui, S.C.; Viergever, M.A.; Chu, W.C.; Cheng, J.C.; Castelein, R.M. Anterior-posterior length discrepancy of the spinal column in adolescent idiopathic scoliosis—A 3D CT study. Spine J. 2018, 18, 2259–2265. [Google Scholar] [CrossRef]
- Helenius, I.; Remes, V.; Yrjönen, T.; Ylikoski, M.; Schlenzka, D.; Helenius, M.; Poussa, M. Harrington and Cotrel-Dubousset instrumentation in adolescent idiopathic scoliosis: Long-term functional and radiographic outcomes. J. Bone Joint Surg. Am. 2003, 85, 2303–2309. [Google Scholar] [CrossRef]
- Helenius, L.; Diarbakerli, E.; Grauers, A.; Lastikka, M.; Oksanen, H.; Pajulo, O.; Löyttyniemi, E.; Manner, T.; Gerdhem, P.; Helenius, I. Back pain and quality of life after surgical treatment for adolescent idiopathic scoliosis at 5-year follow-up: Comparison with healthy controls and patients with untreated idiopathic scoliosis. J. Bone Jt. Surg. Am. 2019, 101, 1460–1466. [Google Scholar] [CrossRef]
- Marks, M.C.; Bastrom, T.P.; Petcharaporn, M.; Shah, S.A.; Betz, R.R.; Samdani, A.; Lonner, B.; Miyanji, F.; Newton, P.O. The effect of time and fusion length on motion of the unfused lumbar segments in adolescent idiopathic scoliosis. Spine Deform. 2015, 3, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Parsch, D.; Gaertner, V.; Brocai, D.; Carstens, C. The effect of spinal fusion on the long-term outcome of idiopathic scoliosis: A case-control study. J. Bone Surg. Brit. 2001, 83, 1133–1136. [Google Scholar] [CrossRef]
- Keenan, B.E.; Izatt, M.T.; Askin, G.N.; Labrom, R.D.; Pettet, G.J.; Pearcy, M.J.; Adam, C.J. Segmental torso masses in adolescent idiopathic scoliosis. Clin Biomech. 2014, 29, 773–779. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.P.; Vázquez-Albornoz, J.H.; Vázquez-Silva, E.; Hidalgo-Bravo, A.J.; Torres-Jara, P.B.; Moya-Loaiza, D.P. 3D Printing and Virtual Surgical Planning in Craniofacial and Thoracic Surgery: Applications to Personalised Medicine. J. Pers. Med. 2025, 15, 397. [Google Scholar] [CrossRef]
- Vázquez-Silva, E.; Bohorquez-Vivas, D.; Peña-Tapia, P.; Moncayo-Matute, F.; Torres-Jara, P.; Moya-Loaiza, D. Oculopalpebral prosthesis prototype design using the additive manufacturing technique: A case study. JPRAS Open 2024, 39, 228–236. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.P.; Peña-Tapia, P.G.; Vázquez-Silva, E.; Torres-Jara, P.B.; Moya-Loaiza, D.P.; Abad-Farfán, G.; Andrade-Galarza, A.F. Surgical planning and finite element analysis for the neurocraneal protection in cranioplasty with PMMA: A case study. Heliyon 2022, 8, e10706. [Google Scholar] [CrossRef]
- Desroches, G.; Aubin, C.E.; Sucato, D.J.; Rivard, C.H. Simulation of an anterior spine instrumentation in adolescent idiopathic scoliosis using a flexible multi-body model. Med. Biol. Eng. Comput. 2007, 45, 759–768. [Google Scholar] [CrossRef]
- Wall, E.J.; Jain, V.V.; Crawford, A.H.; Bylski-Austrow, D.I.; Reynolds, J.E. Spine growth modulation in early adolescent idiopathic scoliosis: Prospective US FDA IDE pilot study of titanium clip-screw implant at two to five years. Spine Deform. 2019, 7, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Ahmed, E.B.; Tsirikos, A.I. Adolescent idiopathic scoliosis: A comprehensive approach to aetiology, diagnostic assessment and treatment. Orthop. Trauma 2017, 31, 343–349. [Google Scholar] [CrossRef]
- Cobetto, N.; Aubin, C.E.; Parent, S. Surgical planning and follow-up of anterior vertebral body growth modulation in pediatric idiopathic scoliosis using a patient-specific finite element model integrating growth modulation. Spine Deform. 2018, 6, 344–350. [Google Scholar] [CrossRef]
- Burwell, R.G.; Dangerfield, P.H.; Moulton, A.; Grivas, T.B. Adolescent idiopathic scoliosis (AIS), environment, exposome and epigenetics: A molecular perspective of postnatal normal spinal growth and the etiopathogenesis of AIS with consideration of a network approach and possible implications for medical therapy. Scoliosis 2011, 6, 26. [Google Scholar] [CrossRef]
- Fogel, G.R.; Parikh, R.D.; Ryu, S.I.; Turner, A.W. Biomechanics of lateral lumbar interbody fusion constructs with lateral and posterior plate fixation. J. Neurosurg. Spine 2014, 20, 291–297. [Google Scholar] [CrossRef]
- Cappuccino, A.; Cornwall, G.B.; Turner, A.W.; Fogel, G.R.; Duong, H.T.; Kim, K.D.; Brodke, D.S. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine 2010, 35, S361–S367. [Google Scholar] [CrossRef]
- Nicolini, L.F.; Beckmann, A.; Laubach, M.; Hildebrand, F.; Kobbe, P.; de Mello Roesler, C.R.; Fancello, E.A.; Markert, B.; Stoffel, M. An experimental-numerical method for the calibration of finite element models of the lumbar spine. Med. Eng. Phys. 2022, 107, 103854. [Google Scholar] [CrossRef]
- Jung, R.E.; Pjetursson, B.E.; Glauser, R.; Zembic, A.; Zwahlen, M.; Lang, N.P. A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clin. Oral Implants Res. 2008, 19, 119–130. [Google Scholar] [CrossRef]
- Cleek, T.M.; Reynolds, K.J.; Hearn, T.C. Effect of screw torque level on cortical bone pullout strength. J. Orthop. Trauma 2007, 21, 117–123. [Google Scholar] [CrossRef]
- Krishnan, V.; Varghese, V.; Kumar, G.S. Comparative analysis of effect of density, insertion angle and reinsertion on pull-out strength of single and two pedicle screw constructs using synthetic bone model. Asian Spine J. 2016, 10, 414. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.K.; Lee, D.M.; Li, Y.D.; Peng, C.C.; Tsai, T.T.; Lai, P.L.; Chen, W.P.; Tai, C.L. Biomechanical evaluation of position and bicortical fixation of anterior lateral vertebral screws in a porcine model. Sci. Rep. 2023, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.P.; Tan, K.B.; Liu, G.R. Application of finite element analysis in implant dentistry: A review of the literature. J. Prosthet. Dent. 2001, 85, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Silva, E.; Abad-Farfán, G.; Pena-Tapia, P.G.; Torres-Jara, P.B.; Moncayo-Matute, F.P.; Viloria-Avila, T.J.; Vergara-Paredes, M.J. Composites and hybrid materials used for implants and bone reconstruction: A state of the art. Contemp. Eng. Sci. 2022, 15, 105–135. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.P.; Pena-Tapia, P.G.; Vázquez-Silva, E.; Torres-Jara, P.B.; Abad-Farfán, G.; Moya-Loaiza, D.P.; Andrade-Galarza, A.F. Description and application of a comprehensive methodology for custom implant design and surgical planning. Interdiscip. Neurosurg. 2022, 29, 101585. [Google Scholar] [CrossRef]
- Singh, H.; Sonkesriya, S.; Patel, B.J.; Rathod, V.; Gupta, B.; Vyas, T.; Sawhney, A.; Makkad, R.S. Implant abutment using hand drivers versus torque wrench. Bioinformation 2023, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Park, J.S.; Choi, B.W.; Lee, J.S.; Lee, C.S. Computational investigation for biomechanical characteristics of lumbar spine with various porous Ti–6Al–4V implant systems. Appl. Sci. 2021, 11, 8023. [Google Scholar] [CrossRef]
- Le Cann, S.; Cachon, T.; Viguier, E.; Miladi, L.; Odent, T.; Rossi, J.M.; Chabrand, P. Pedicle screw fixation study in immature porcine spines to improve pullout resistance during animal testing. PLoS ONE 2015, 10, e0127463. [Google Scholar] [CrossRef]
- Conrad, B.P.; Cordista, A.G.; Horodyski, M.; Rechtine, G.R. Biomechanical evaluation of the pullout strength of cervical screws. Clin. Spine Surg. 2005, 18, 506–510. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Bravo, M.; Tello, S.; Cornejo, E.; Paredes, Y.; Paltan, C.A.; Fajardo, J.I. Fracture Resistance Comparative Analysis of Milled-Derived vs. 3D-Printed CAD/CAM Materials for Single-Unit Restorations. Polymers 2023, 15, 3773. [Google Scholar] [CrossRef]
- Vázquez-Silva, E.; Pintado-Pintado, J.A.; Moncayo-Matute, F.P.; Torres-Jara, P.B.; Moya-Loaiza, D.P. Effect of Infill Density on the Mechanical Properties of Natural Peek Processed by Additive Manufacturing. Polymers 2025, 17, 347. [Google Scholar] [CrossRef]
- Matute, F.P.M.; Alonso, R.C.C.; Terán, J.V.B.; Tanarro, E.C. Influencia del Par de Apriete en las Cargas Axiales de Extracción de Tornillos Quirúrgicos de Cuerpos Vertebrales en Técnicas Vbt. In Proceedings of the XVI Congreso Iberoamericano de Ingeniería Mecánica (CIBIM 2024), Concepción, Chile, 22–24 October 2024. [Google Scholar]
- UNE-EN ISO 7500-1:2018; Metallic Materials—Calibration and Verification of Static Uniaxial Testing Machines—Part 1: Tension/Compression Testing Machines—Calibration and Verification of the Force-Measuring System. International Organization for Standardization: Geneva, Switzerland, 2018.
- Moncayo-Matute, F.P.; Vázquez-Silva, E.; Peña-Tapia, P.G.; Torres-Jara, P.B.; Moya-Loaiza, D.P.; Viloria-Ávila, T.J. Finite Element Analysis of Patient-Specific 3D-Printed Cranial Implant Manufactured with PMMA and PEEK: A Mechanical Comparative Study. Polymers 2023, 15, 3620. [Google Scholar] [CrossRef]
- Balladares, A.O.; Abad-Coronel, C.; Ramos, J.C.; Fajardo, J.I.; Paltán, C.A.; Martín Biedma, B.J. Comparative Study of the Influence of Heat Treatment on Fracture Resistance of Different Ceramic Materials Used for CAD/CAM Systems. Materials 2024, 17, 1246. [Google Scholar] [CrossRef] [PubMed]
- Mejía Rodríguez, M.; González-Estrada, O.A.; Villegas-Bermúdez, D.F. Finite Element Analysis of Patient-Specific Cranial Implants under Different Design Parameters for Material Selection. Designs 2024, 8, 31. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.; Vázquez-Silva, E.; Torres-Jara, P.; Peña-Tapia, P.; Moya-Loayza, D.; Abad-Farán, G. Mechanical analysis for personalized implant for neurocranial protection manufactured with Polymethylmethacrylate. J. Phys. Conf. Ser. 2023, 2516, 012005. [Google Scholar] [CrossRef]
- Torres-Jara, P.; Vázquez-Silva, E.; Moncayo-Matute, F.; Peña-Tapia, P.; Moya-Loayza, D.; Abad-Farán, G. Application of a comprehensive methodology for the development of personalized bone implants: Exemplification through three cases. J. Phys. Conf. Ser. 2023, 2516, 012004. [Google Scholar] [CrossRef]
- Bermejillo Barrera, M.D.; Franco-Martínez, F.; Díaz Lantada, A. Artificial Intelligence Aided Design of Tissue Engineering Scaffolds Employing Virtual Tomography and 3D Convolutional Neural Networks. Materials 2021, 14, 5278. [Google Scholar] [CrossRef]
- Cobos, C.M.; Ferrandiz, S.; Garzón, L.; López, J.; Rayon, E. Exploring the mechanical response of PLA/MWCNT and PLA/HNT composites obtained by additive manufacturing. Rapid Prototyp. J. 2025, 31, 179–188. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Córdova, J.; Merchán, A.; Larriva, J.; Bravo, A.; Bernal, B.; Paltán, C.A.; Fajardo, J.I. Comparative Analysis of the Fracture Resistance of a Polymeric Material for 3D Printing and a Milled Polymethylmethacrylate Material as Interim Material for Fixed Partial Dentures: New Material Updated. Designs 2023, 7, 118. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Freire Bonilla, C.; Vidal, S.; Rosero, F.; Encalada Abad, C.; Mena Córdova, N.; Paltán, C.A.; Fajardo, J.I.; Aliaga, P. Evaluating a Novel 3D-Printed Resin for Dental Restorations: Fracture Resistance of Restorations Fabricated by Digital Press Stereolithography. Polymers 2025, 17, 2322. [Google Scholar] [CrossRef]
- Abad-Coronel, C.; Calle, C.; Abril, G.; Paltán, C.A.; Fajardo, J.I. Fracture Resistance Analysis of CAD/CAM Interim Fixed Prosthodontic Materials: PMMA, Graphene, Acetal Resin and Polysulfone. Polymers 2023, 15, 1761. [Google Scholar] [CrossRef]
- Kocis, J.; Navrat, T.; Florian, Z.; Wendsche, P. Biomechanical testing of spinal segment fixed by thoracolumbar spine locking plate on the swine lumbar spine. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2010, 154, 345–354. [Google Scholar] [CrossRef]
- Lv, Q.B.; Gao, X.; Pan, X.X.; Jin, H.M.; Lou, X.T.; Li, S.M.; Yan, Y.Z.; Wu, C.C.; Lin, Y.; Ni, W.F.; et al. Biomechanical properties of novel transpedicular transdiscal screw fixation with interbody arthrodesis technique in lumbar spine: A finite element study. J. Orthop. Transl. 2018, 15, 50–58. [Google Scholar] [CrossRef]
- Zanetti, E.M.; Salaorno, M.; Grasso, G.; Audenino, A.L. Parametric analysis of orthopedic screws in relation to bone density. Open Med. Inform. J. 2009, 3, 19. [Google Scholar] [CrossRef]
- Carson, W.L.; Duffield, R.C.; Arendt, M.; Ridgely, B.J.; Gaines, R.W., Jr. Internal forces and moments in transpedicular spine instrumentation the effect of pedicle screw angle and transfixation—The 4R-4Bar linkage concept. Spine 1990, 15, 893–901. [Google Scholar] [CrossRef]
- Fan, W.; Guo, L.X.; Zhao, D. Stress analysis of the implants in transforaminal lumbar interbody fusion under static and vibration loadings: A comparison between pedicle screw fixation system with rigid and flexible rods. J. Mater. Sci. Mater. Med. 2019, 30, 118. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.P.; Gerardo Peña-Tapia, P.; Vázquez-Silva, E.; Torres-Jara, P.B.; Patricia Moya-Loaiza, D.; Abad-Farfán, G. Surgical planning for the removal of cranial espheno-orbitary meningioma through the use of personalized polymeric prototypes obtained with an additive manufacturing technique. In Proceedings of the 2023 3rd International Conference on Electrical, Computer, Communications and Mechatronics Engineering (ICECCME), Online, 20–21 July 2023; pp. 1–7. [Google Scholar]
- Torres-Jara, P.B.; Bohorquez Vivas, D.D.; Moya-Loaiza, D.P.; Peña-Tapia, P.G. Análisis de Elementos Finitos de un Implante Personalizado a Base de PMMA/PEEK: Acople Con el Hueso Craneal y Sistema de Anclaje; Universidad de Concepción: Concepción, Chile, 2024. [Google Scholar]
- Burguete, R.L.; Johns, R.B.; King, T.; Patterson, E.A. Tightening characteristics for screwed joints in osseointegrated dental implants. J. Prosthet. Dent. 1994, 71, 592–599. [Google Scholar] [CrossRef]
- Chen, S.I.; Lin, R.M.; Chang, C.H. Biomechanical investigation of pedicle screw–vertebrae complex: A finite element approach using bonded and contact interface conditions. Med. Eng. Phys. 2003, 25, 275–282. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.P.; Torres-Jara, P.B.; Vázquez-Silva, E.; Peña-Tapia, P.G.; Moya-Loaiza, D.P.; Abad-Farfán, G. Finite element analysis of a customized implant in PMMA coupled with the cranial bone. J. Mech. Behav. Biomed. Mater. 2023, 146, 106046. [Google Scholar] [CrossRef] [PubMed]
- Straumal, B.; Gornakova, A.; Kiselevskiy, M.; Anisimova, N.Y.; Nekrasov, A.; Kilmametov, A.; Strug, R.; Rabkin, E. Optimal surface roughness of Ti6Al4V alloy for the adhesion of cells with osteogenic potential. J. Mater. Res. 2022, 37, 2661–2674. [Google Scholar] [CrossRef]
- Harrison, N.M.; McDonnell, P.F.; O’Mahoney, D.C.; Kennedy, O.D.; O’Brien, F.J.; McHugh, P.E. Heterogeneous linear elastic trabecular bone modelling using micro-CT attenuation data and experimentally measured heterogeneous tissue properties. J. Biomech. 2008, 41, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, C.A.; Fonseca, E.M.; Jorge, R.N. Bio-lubricant properties analysis of drilling an innovative design of bioactive kinetic screw into bone. Designs 2023, 7, 21. [Google Scholar] [CrossRef]
- Solórzano-Requejo, W.; Martinez Cendrero, A.; Altun, A.A.; Nohut, S.; Ojeda, C.; Garcia Molleja, J.; Molina-Aldareguia, J.; Schwentenwein, M.; Diaz Lantada, A. Topology optimisation and lithography-based ceramic manufacturing of short-stem hip prostheses with enhanced biomechanical and mechanobiological performance. Virtual Phys. Prototyp. 2024, 19, e2387280. [Google Scholar] [CrossRef]
- Bonney, H.; Colston, B.; Goodman, A. Regional variation in the mechanical properties of cortical bone from the porcine femur. Med. Eng. Phys. 2011, 33, 513–520. [Google Scholar] [CrossRef]
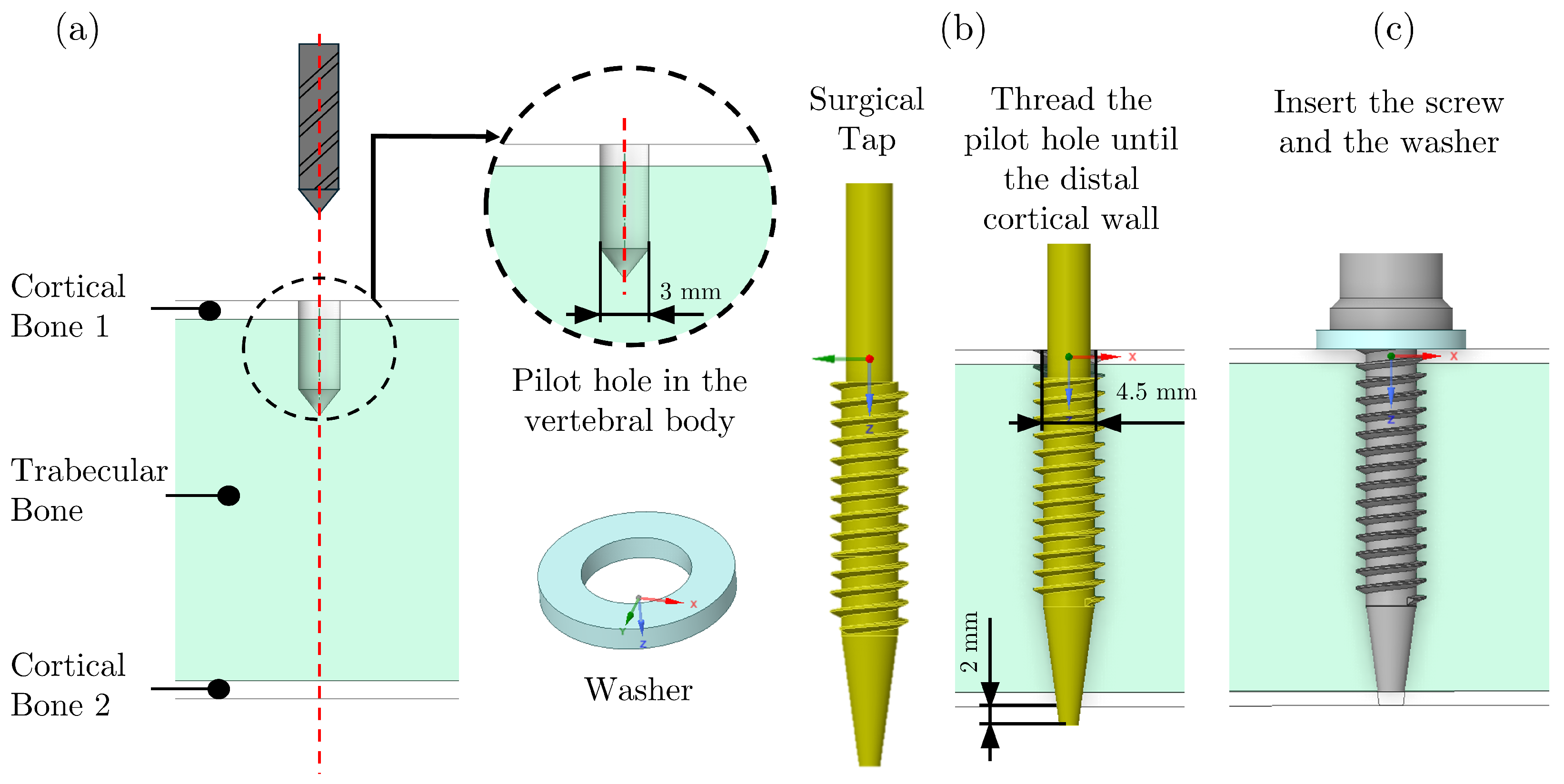












| (mm) | (mm) | (°) | (°) | (mm) | (mm) | s (mm) | p (mm) | L (mm) |
|---|---|---|---|---|---|---|---|---|
| 5.4 | 6.5 | 65 | 25 | 1.2 | 0.8 | 0.2 | 1.8 | 37.5 |
| Washer | ||
|---|---|---|
| (mm) | (mm) | e (mm) |
| 6.5 | 5.4 | 5 |
| Structures | Mechanical Strength (MPa) | Young’s Modulus (MPa) | Poisson Ratio | References |
|---|---|---|---|---|
| Porcine Cortical Bone | 80–120 | 13,500 | 0.3 | [9,50,51,52] |
| Porcine Trabecular Bone | 5–15 | 200 | 0.2 | [28,32,51,52] |
| Washer (Structural Steel—A36) | 400–450 | 210,000 | 0.3 | [8] |
| Surgical Screw (Ti-6Al-4V) | 895 | 107,000 | 0.3 | [8] |
| Structure | Size (mm) | Number of Nodes | Number of Elements |
|---|---|---|---|
| Cortical Bone | 0.5 | 82,650 | 55,474 |
| Trabecular Bone | 0.1 | 83,858 | 77,985 |
| Surgical Screw | 0.3 | 78,556 | 49,481 |
| Round Washer | 0.1 | 79,758 | 38,958 |
| Specimen | Exp. Torque (Nm) | Exp. Pull-Out (kN) | von Mises Cortical (MPa) | von Mises Trabecular (MPa) | von Mises Cortical (MPa) | von Mises Cortical (MPa) |
|---|---|---|---|---|---|---|
| Tightening only | Tightening only | Tightening + pull-out | Tightening + pull-out | |||
| E1 | 7.0 | 2.36 | 14.24 | 2.17 | 141.56 | 19.77 |
| E2 | 5.2 | 1.78 | 16.12 | 2.05 | 141.60 | 19.27 |
| E3 | 6.5 | 1.83 | 16.95 | 2.02 | 140.23 | 19.90 |
| E4 | 5.4 | 1.99 | 16.57 | 2.13 | 140.80 | 20.09 |
| E5 | 5.4 | 2.57 | 16.61 | 2.11 | 141.32 | 19.74 |
| Mean ± SD | 5.90 ± 0.80 | 2.1 ± 0.31 | 16.1 ± 0.86 | 2.1 ± 0.13 | 141.1 ± 0.70 | 19.7 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moncayo-Matute, F.P.; Claramunt, R.; Guzmán-Bautista, Á.; Torres-Jara, P.B.; Chacón-Tanarro, E. Effect of Surgical Tightening Torque on the Pull-Out Strength of Screws in Vertebral Body Tethering. Appl. Sci. 2025, 15, 11074. https://doi.org/10.3390/app152011074
Moncayo-Matute FP, Claramunt R, Guzmán-Bautista Á, Torres-Jara PB, Chacón-Tanarro E. Effect of Surgical Tightening Torque on the Pull-Out Strength of Screws in Vertebral Body Tethering. Applied Sciences. 2025; 15(20):11074. https://doi.org/10.3390/app152011074
Chicago/Turabian StyleMoncayo-Matute, Freddy Patricio, Rafael Claramunt, Álvaro Guzmán-Bautista, Paúl Bolívar Torres-Jara, and Enrique Chacón-Tanarro. 2025. "Effect of Surgical Tightening Torque on the Pull-Out Strength of Screws in Vertebral Body Tethering" Applied Sciences 15, no. 20: 11074. https://doi.org/10.3390/app152011074
APA StyleMoncayo-Matute, F. P., Claramunt, R., Guzmán-Bautista, Á., Torres-Jara, P. B., & Chacón-Tanarro, E. (2025). Effect of Surgical Tightening Torque on the Pull-Out Strength of Screws in Vertebral Body Tethering. Applied Sciences, 15(20), 11074. https://doi.org/10.3390/app152011074






