Surface Engineering of Enamel with Sodium Hypochlorite: Effects on Bond Strength and Etching Microstructure in Adhesive Applications
Abstract
Featured Application
Abstract
1. Introduction
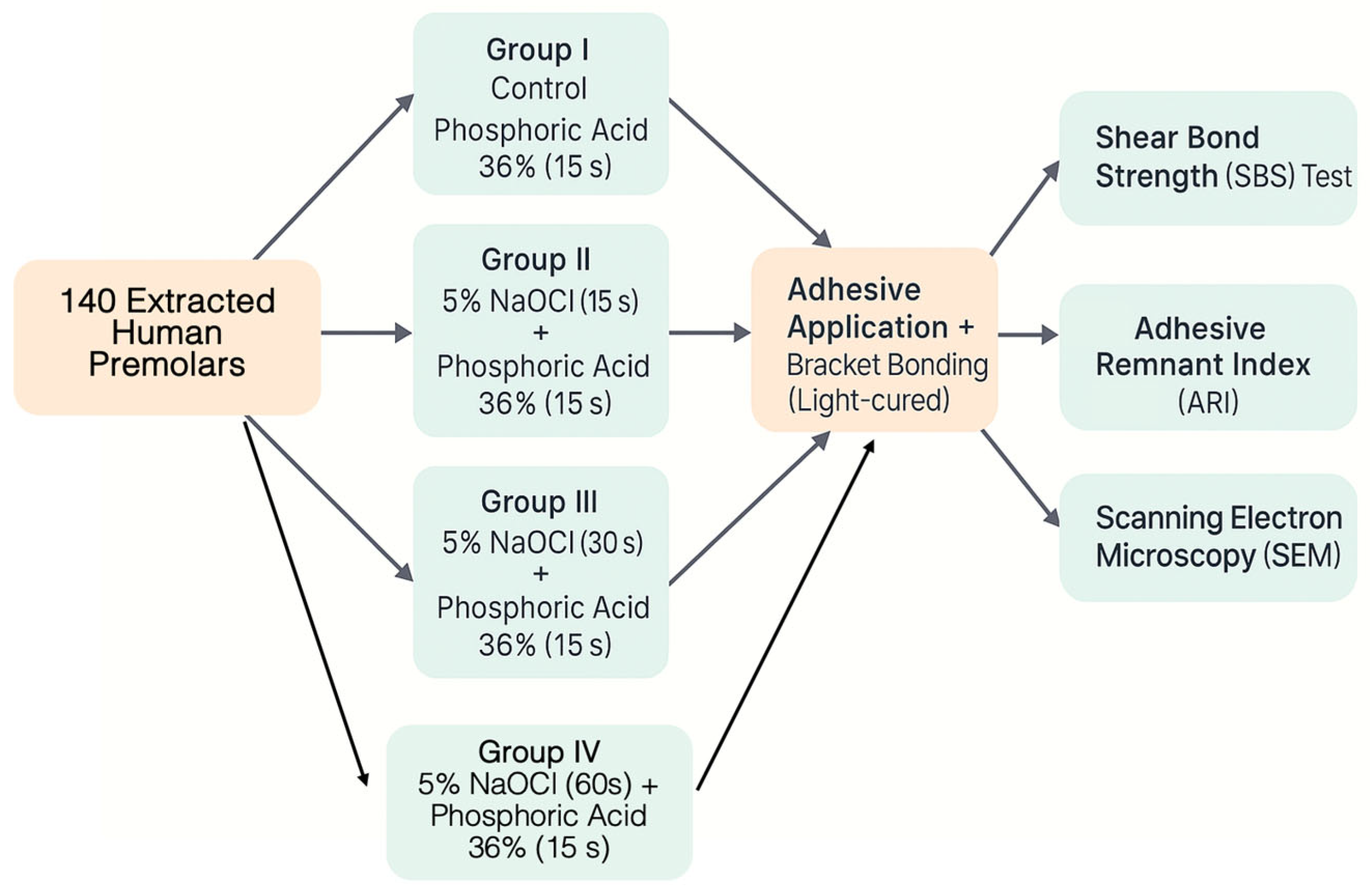
2. Materials and Methods
- -
- 120 teeth were randomly allocated into four experimental groups of 30 teeth each for shear bond strength (SBS) testing.
- -
- The remaining 20 teeth (5 per group) were designated exclusively for scanning electron microscopy (SEM) evaluation of enamel morphology.
2.1. Experimental Groups
- -
- Group I (Control): Phosphoric acid 36% etching (n = 30);
- -
- Group II: 5% NaOCl (15 s) + phosphoric acid etching (n = 30);
- -
- Group III: 5% NaOCl (30 s) + phosphoric acid etching (n = 30);
- -
- Group IV: 5% NaOCl (60 s) + phosphoric acid etching (n = 30).
2.2. Specialized Subgroup
2.3. Materials Used
- Etching agent: 36% orthophosphoric acid (Solventum, St. Paul, MN, USA).
- Deproteinizing agent: 5% sodium hypochlorite (NaOCl), freshly prepared by diluting commercial-grade NaOCl (Panreac, Spain) in distilled water.
- Adhesive system: Transbond™ XT primer and light-cured composite resin (Solventum, Monrovia, CA, USA).
- Brackets: Stainless steel MBT brackets (Victory Series™, Solventum) with a base area of 10.5 mm2.
- Light-curing unit: LED curing device (Ortholux™ XT, Solventum).
2.4. Surface Preparation and Bonding Protocol
2.5. Storage Before Testing
2.6. Adhesive Remnant Index (ARI)
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistical Analysis
2.9. Intra-Examiner Reliability
2.10. Ethical Considerations
3. Results
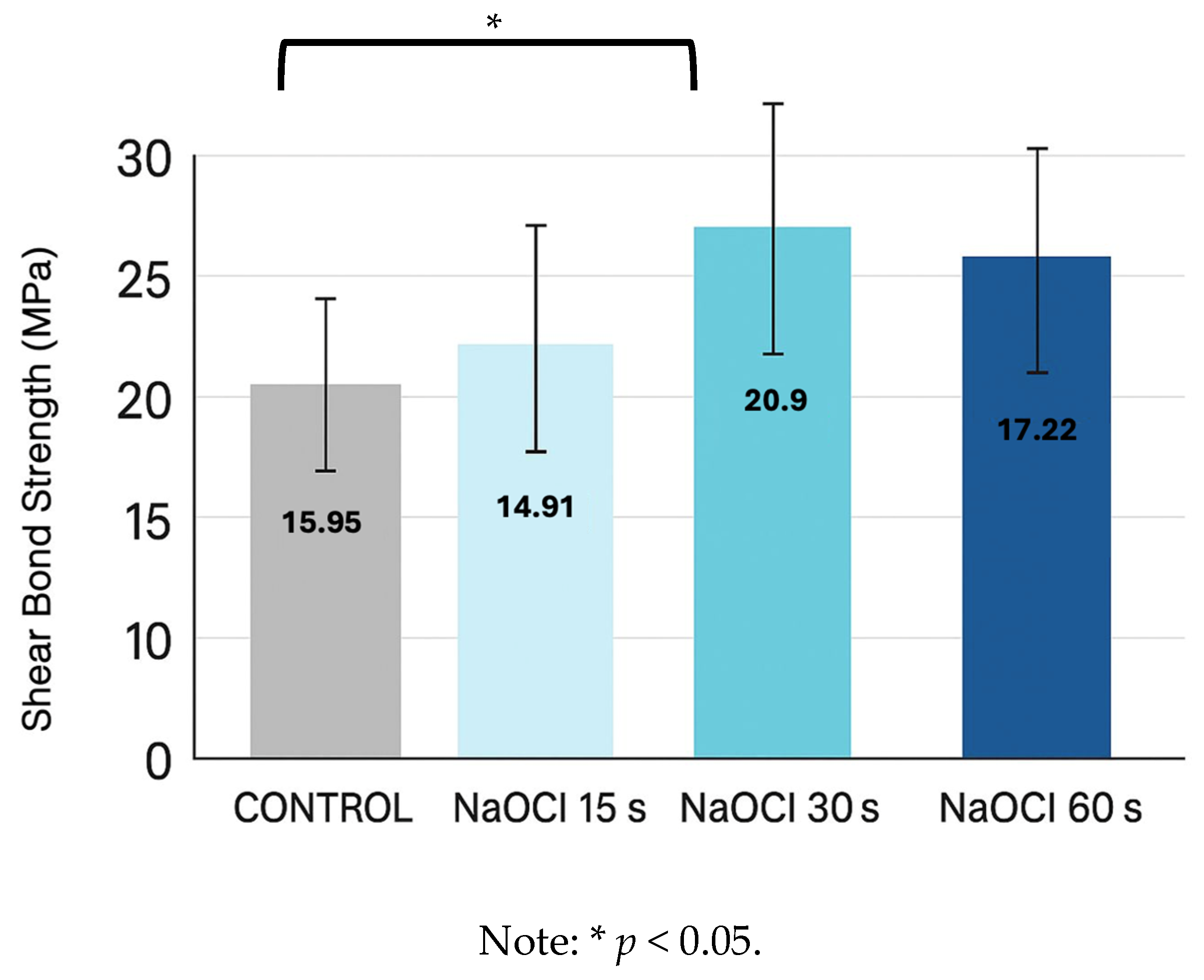
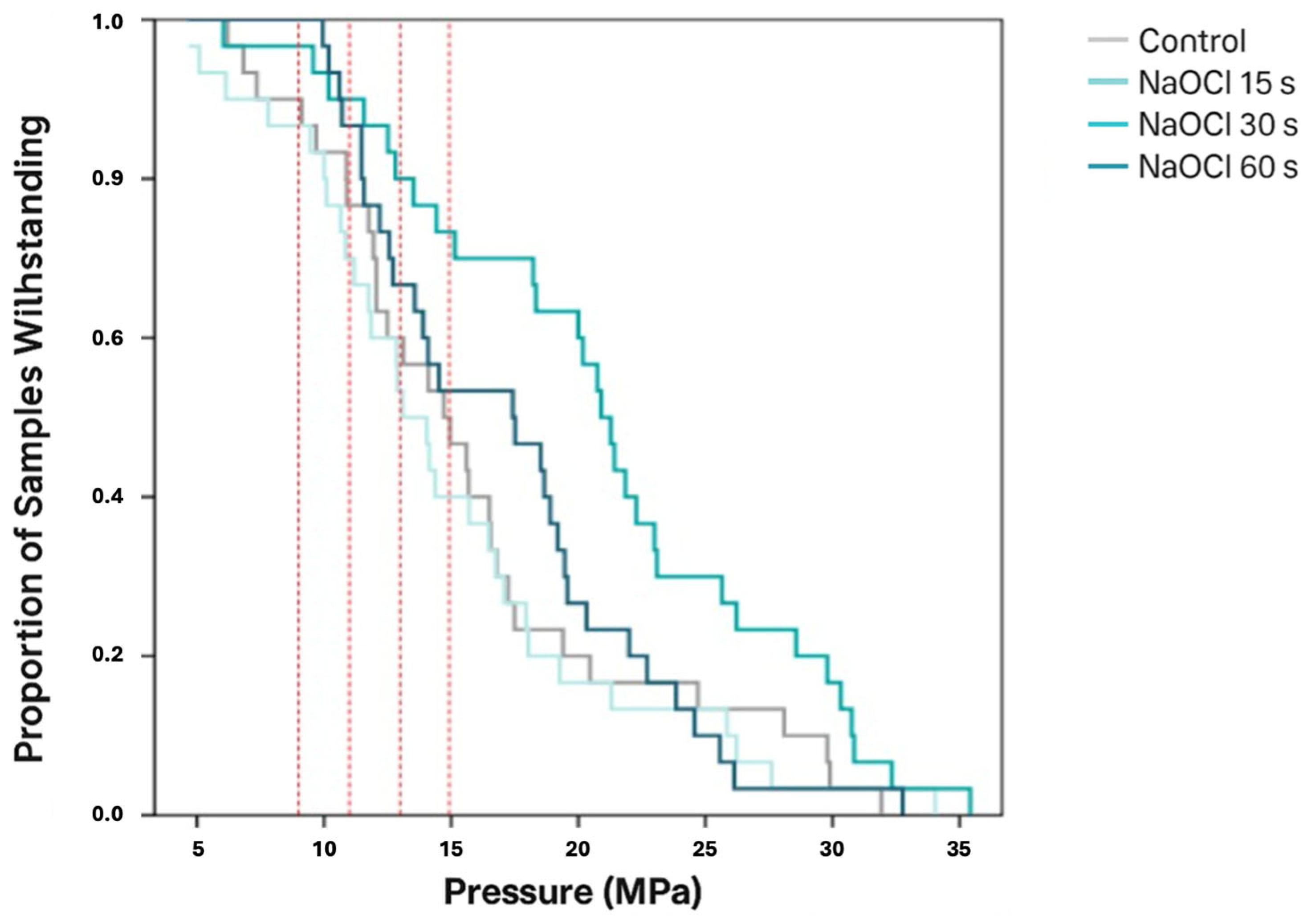
3.1. Shear Bond Strength
3.2. Adhesive Remnant Index (ARI)
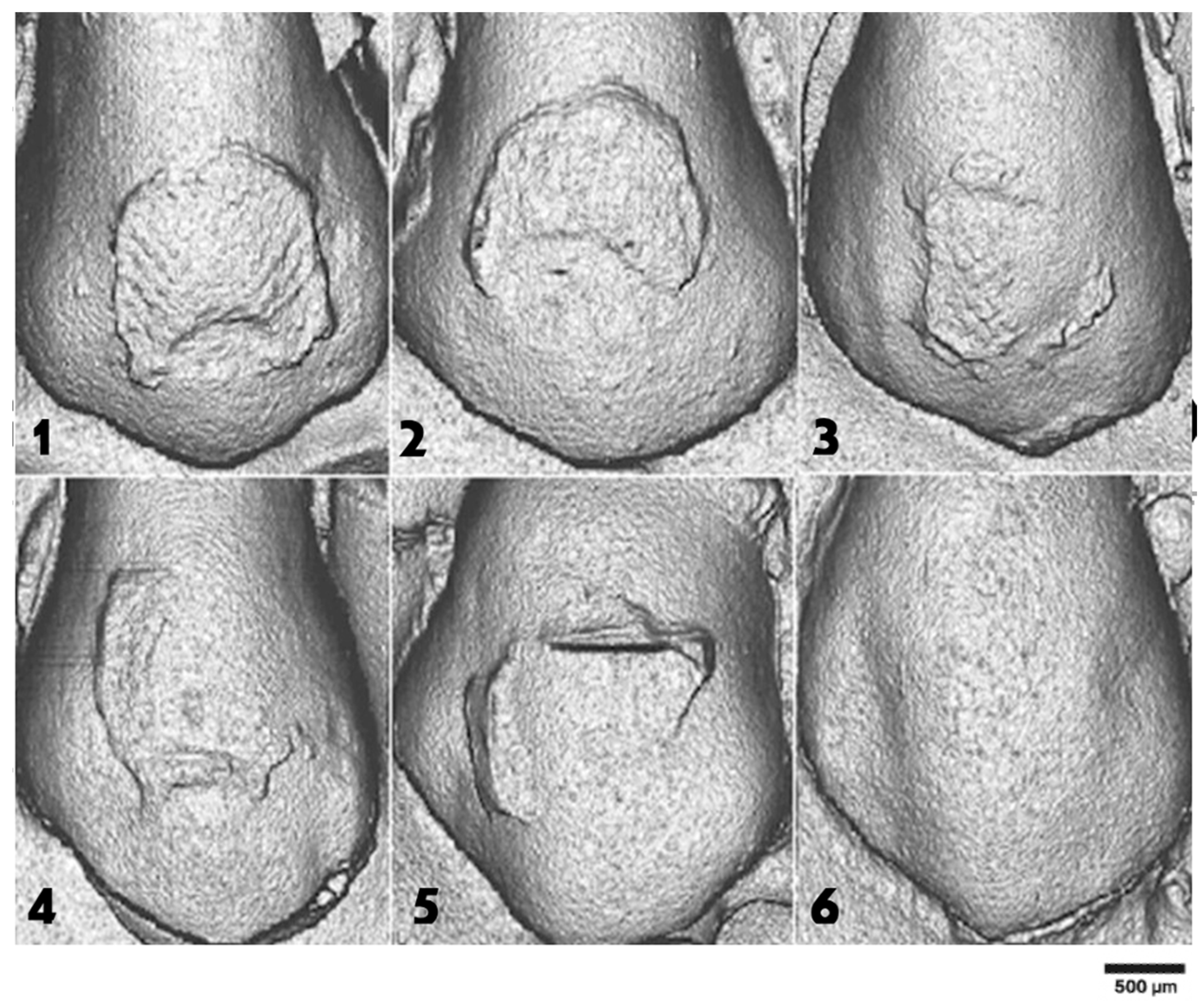
3.3. Scanning Electron Microscopy (SEM)
3.4. Etching Pattern Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soares, F.Z.; Rocha, R.O.; Raggio, D.P.; Sadek, F.T.; Cardoso, P.E. Microtensile bond strength of different adhesive systems to primary and permanent dentin. Pediatr. Dent. 2005, 27, 457–462. [Google Scholar]
- Armstrong, S.; Geraldeli, S.; Maia, R.; Raposo, L.H.; Soares, C.J.; Yamagawa, J. Adhesion to tooth structure: A critical review of “micro” bond strength test methods. Dent. Mater. 2010, 26, e50–e62. [Google Scholar] [CrossRef]
- Dos Santos, P.A.; Garcia, P.P.; Palma-Dibb, R.G. Shear bond strength of adhesive systems to enamel and dentin. J. Mater. Sci. Mater. Med. 2005, 16, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Scougall-Vilchis, R.J.; Zárate-Díaz, C.; Kusakabe, S.; Yamamoto, K. Bond strengths of different orthodontic adhesives after enamel conditioning with the same self-etching primer. Aust. Orthod. J. 2010, 26, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Gwinnett, A.J. The effect of various surface treatments on the enamel of human teeth. J. Dent. Res. 1967, 46, 1401–1404. [Google Scholar]
- Espinosa, R.; Valencia, R.; Uribe, M.; Ceja, I.; Cruz, J.; Saadia, M. Resin replica in enamel deproteinization and its effect on acid etching. J. Clin. Pediatr. Dent. 2010, 35, 47–51. [Google Scholar] [CrossRef]
- Pithan, S.A.; Hallgren, A.; Cordeiro, R.; Pithan, J.C.; Carlsson, J.; Pithan, E.G.T. The effect of sodium hypochlorite on the bond strength of an adhesive system to dental enamel. Pesqui. Odontol. Bras. 2003, 17, 349–353. [Google Scholar]
- Estrela, C.; Estrela, C.R.; Barbin, E.L.; Spanó, J.C.; Marchesan, M.A.; Pécora, J.D. Mechanism of action of sodium hypochlorite. Braz. Dent. J. 2002, 13, 113–117. [Google Scholar] [CrossRef]
- Justus, R.; Cubero, T.; Ondarza, R.; Morales, F. A new technique with sodium hypochlorite to increase bracket shear bond strength. Semin. Orthod. 2010, 16, 66–75. [Google Scholar] [CrossRef]
- Kiryk, S.; Kiryk, J.; Matys, J.; Dobrzyński, M. The Influence of Resin Infiltration on the Shear Bond Strength of Orthodontic Brackets: A Systematic Review and Meta-Analysis. Biomater. Investig. Dent. 2025, 16, 32. [Google Scholar] [CrossRef]
- Pereira, T.B.; Jansen, W.C.; Pithon, M.M.; Souki, B.Q.; Tanaka, O.M.; Oliveira, D.D. Effects of enamel deproteinization on bracket bonding. Eur. J. Orthod. 2013, 35, 442–446. [Google Scholar] [CrossRef]
- Elnafar, A.A.; Alam, M.K.; Hasan, R. The impact of surface preparation on shear bond strength. J. Orthod. 2014, 41, 201–207. [Google Scholar] [CrossRef]
- Peloso, R.M.; Cotrin, P.; Oliveira, R.C.G.; Valarelli, F.P.; Freitas, K.M.S. Evaluation of enamel deproteinization in bond strength. Am. J. Orthod. Dentofac. Orthop. 2022, 162, 443–450. [Google Scholar] [CrossRef]
- Sun, Z.; You, X.; Xu, J.; Chen, L.; Li, S.; Zhang, Z.; Guo, L. Effectiveness of sodium hypochlorite treatment. Dent. Mater. J. 2022, 41, 660–667. [Google Scholar] [CrossRef]
- Alrai, S.; Al-Madi, A.; Al-Khalifa, K.; Al-Ghamdi, H.; Bukhary, S.; Bawazir, S.; Alqahtani, A.; Alharbi, A.; Alotaibi, R.; Alghamdi, F. Effect of Enamel Deproteinization on Bonding Orthodontic Brackets: A systematic review and meta-analysis. J. Clin. Exp. Dent. 2025, 17, e122–e129. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, Y.; Wang, H.; Li, X.; Huang, Y.; Chen, L. Effect of Sodium Hypochlorite and EDTA Pretreatment on Enamel Surface for Resin Infiltration. Front. Dent. Med. 2024, 5, 11600858. [Google Scholar]
- Basalamah, A.; Maher, A.; Whba, A.H.; Scribante, A.; Sfondrini, M.F.; Montasser, M.A. Effects of fluorosed enamel on orthodontic bracket bonding. J. Orofac. Orthop. 2023, 84, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Daou, C.; Akl, R.; Mati, M.; Kassis, A.; Ghoubril, J.; Khoury, E. Effects of enamel deproteinization with different application times on the shear bond strength of a self-etching primer: An in vitro study. Int. Orthod. 2021, 19, 505–511. [Google Scholar] [CrossRef]
- Alharbi, A.; Smith, J.; Johnson, L. Effect of sodium hypochlorite deproteinization on bond strength in fluorotic enamel. Angle Orthod. 2023, 93, 421–428. [Google Scholar]
- Cordeiro, P.; Neves, J.G.; Tsuzuki, M.; Facury, A.G.F.; Correr-Sobrinho, L.; Costa, A.R. Enamel deproteinization with sodium hypochlorite: Effects on ceramic bracket bond strength. Braz. Dent. J. 2025, 36, e246106. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.B.; Souza, R.A.; Mendes, J.F.; Araújo, M.V.; Figueiredo, D.S.; Pithon, M.M.; Oliveira, D.D. Clinical evaluation of sodium hypochlorite pretreatment on bracket failure. Am. J. Orthod. Dentofac. Orthop. 2023, 165, 45–52. [Google Scholar]
- Poggio, C.; Beltrami, R.; Colombo, M.; Chiesa, M.; Scribante, A. Influence of dentin pretreatment on bond strength. Acta Biomater. Odontol. Scand. 2017, 3, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, F.S.F.; Armas-Vega, A.; Izquierdo-Bucheli, A.; Pinto, T.F.; Hanzen, T.A.; Bauer, J.; Cardenas, A.F.M.; Loguercio, A.D. Does the Conditioning Mode and Duration of Universal Adhesives Affect the Bonding Effectiveness to Fluorotic Enamel? J. Adhes. Dent. 2019, 21, 525–536. [Google Scholar] [PubMed]
- Mas-López, A.C.; Robles-Ruiz, J.; Arriola, L.E. Effect of sodium hypochlorite as a deproteinizing agent on the bond strength of metal brackets. J. Clin. Pediatr. Dent. 2024, 16, e947–e952. [Google Scholar] [CrossRef]
- Eliades, T.; Hiskia, A.; Eliades, G.; Athanasiou, A.E. Surface characterization of retrieved orthodontic brackets. Eur. J. Orthod. 2007, 29, 350–357. [Google Scholar]
- Nilgun, A.; Usumez, A.; Ozturk, B.; Usumez, S. Influence of different light sources on microleakage. J. Oral Rehabil. 2004, 31, 500–504. [Google Scholar] [CrossRef]
- ISO/TS 11405:2015; Dental Materials-Testing of Adhesion to Tooth Structure. International Organization for Standardization: Geneva, Switzerland, 2015.
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Silverstone, L.M.; Saxton, C.A.; Dogon, I.L.; Fejerskov, O. Variation in the pattern of acid etching of enamel surfaces: An electron microscopic study. Br. Dent J. 1975, 138, 69–76. [Google Scholar]
- Iglesias, A.; Flores, T.; Moyano, J.; Artés, M.; Gil, F.J.; Puigdollers, A. In vitro study of shear bond strength in direct and indirect bonding with three types of adhesive systems. Materials 2020, 13, 2644. [Google Scholar] [CrossRef]
- Blöcher, S.; Frankenberger, R.; Hellak, A.; Schauseil, M.; Roggendorf, M.J.; Korbmacher-Steiner, H.M. Effect on enamel shear bond strength of adding microsilver and nanosilver particles to the primer of an orthodontic adhesive. BMC Oral Health 2015, 15, 42. [Google Scholar] [CrossRef]
- Da Rocha, J.M.; Gravina, M.A.; da Silva Campos, M.J.; Quintão, C.C.; Elias, C.N.; Vitral, R.W. Shear bond resistance and enamel surface comparison after the bonding and debonding of ceramic and metallic brackets. Dent. Press J. Orthod. 2014, 19, 77–85. [Google Scholar] [CrossRef]
- Ayman, M.; Bakry, N.; El-Kest, A.S. Effects of sodium hypochlorite enamel deproteinization on bonding efficacy. Dent. Med. Probl. 2022, 59, 223–229. [Google Scholar]
- López-Luján, N.A.; Munayco-Pantoja, E.R.; Torres-Ramos, G.; Blanco-Victorio, D.J.; Siccha-Macassi, A.; López-Ramos, R.P. Deproteinization of primary enamel with sodium hypochlorite before etching. Acta Odontol. Latinoam. 2019, 32, 29–35. [Google Scholar]
- Ahuja, B.; Yeluri, R.; Baliga, S.; Munshi, A.K. Enamel deproteinization before acid etching: A scanning electron microscopic observation. J. Clin. Pediatr. Dent. 2010, 35, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Söderholm, K.J.; Roberts, M.J. Variables influencing the bond strength of dentin adhesives. J. Dent. Res. 1990, 69, 844–851. [Google Scholar]
- Al-Daher, M.S.; Sultan, K.; Hajeer, M.Y.; Burhan, A.S. Enamel deproteinization or sandblasting for enamel reconditioning. Cureus 2024, 16, e66210. [Google Scholar] [CrossRef]
- Perdigão, J.; Gomes, G.; Duarte, S., Jr.; Lopes, M.M. Enamel bond strengths of pairs of adhesives from the same manufacturer. Oper. Dent. 2005, 30, 492–499. [Google Scholar] [PubMed]
- Cardoso, M.V.; De Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current aspects on bonding effectiveness and stability in adhesive dentistry. Aust. Dent. J. 2011, 56 (Suppl. S1), 31–44. [Google Scholar] [CrossRef]
- Wang, Y.; Spencer, P. Hybridization efficiency of the adhesive/dentin interface. J. Dent. Res. 2003, 82, 141–145. [Google Scholar] [CrossRef]
- Billoo, M.S.; Khan, M.A.K.; Khalid, T.; Kazmi, S.M.R. Effect of storage medium of extracted human teeth on the result of in vitro studies—A scoping review. Biomater Investig Dent. 2025, 12, 44183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammadi, A.; Nili, M.; Yazdi, F.M.; Taghavi, R. The Effect of Sodium Hypochlorite Enamel Pretreatment on Improving the Bond Strength of Resin-Modified Glass Ionomer Cement. Dent. Res. J. 2021, 18, 13. [Google Scholar] [CrossRef]

| Material | Commercial Name | Manufacturer (City, Country) | Purpose in Study |
|---|---|---|---|
| Phosphoric acid 36% | Scotchbond™ | Solventum, St. Paul, MN, USA | Etching agent for enamel conditioning |
| Sodium hypochlorite 5% | Prepared from commercial-grade NaOCl | Panreac, Barcelona, Spain | Deproteinising agent prior to etching |
| Adhesive primer | Transbond™ XT Primer | Solventum, Monrovia, CA, USA | Primer for enhancing resin bonding to enamel |
| Light-cured composite resin | Transbond™ XT Composite | Solventum, Monrovia, CA, USA | Resin material for bracket bonding |
| Stainless steel MBT brackets | Victory Series™ | Solventum, Monrovia, CA, USA | Orthodontic brackets for SBS testing |
| LED curing unit | Ortholux™ XT | Solventum, Monrovia, CA, USA | Light source for curing adhesive materials |
| Control | NaOCl 15 s | NaOCl 30 s | NaOCl 60 s | |
|---|---|---|---|---|
| Control | — | — | — | — |
| NaOCl 15 s | 0.00001 *** | — | — | — |
| NaOCl 30 s | 0.0003 ** | 0.137 | — | — |
| NaOCl 60 s | 0.00001 *** | 0.412 | 0.412 | — |
| Etching Pattern Type | Control Group (H3PO4) | NaOCl 15 s | NaOCl 30 s | NaOCl 60 s |
|---|---|---|---|---|
| Type I | 25% | 25% | 75% | – |
| Type II | 25% | 50% | 20% | 25% |
| Type III | 25% | 25% | 5% | 75% |
| Type IV | 25% | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrella-Girbes, M.; Arias-Luxán, S.; Guinot-Barona, C.; Marqués-Martínez, L.; García-Miralles, E.; Aura-Tormos, J.I. Surface Engineering of Enamel with Sodium Hypochlorite: Effects on Bond Strength and Etching Microstructure in Adhesive Applications. Appl. Sci. 2025, 15, 10952. https://doi.org/10.3390/app152010952
Torrella-Girbes M, Arias-Luxán S, Guinot-Barona C, Marqués-Martínez L, García-Miralles E, Aura-Tormos JI. Surface Engineering of Enamel with Sodium Hypochlorite: Effects on Bond Strength and Etching Microstructure in Adhesive Applications. Applied Sciences. 2025; 15(20):10952. https://doi.org/10.3390/app152010952
Chicago/Turabian StyleTorrella-Girbes, Mar, Santiago Arias-Luxán, Clara Guinot-Barona, Laura Marqués-Martínez, Esther García-Miralles, and Juan Ignacio Aura-Tormos. 2025. "Surface Engineering of Enamel with Sodium Hypochlorite: Effects on Bond Strength and Etching Microstructure in Adhesive Applications" Applied Sciences 15, no. 20: 10952. https://doi.org/10.3390/app152010952
APA StyleTorrella-Girbes, M., Arias-Luxán, S., Guinot-Barona, C., Marqués-Martínez, L., García-Miralles, E., & Aura-Tormos, J. I. (2025). Surface Engineering of Enamel with Sodium Hypochlorite: Effects on Bond Strength and Etching Microstructure in Adhesive Applications. Applied Sciences, 15(20), 10952. https://doi.org/10.3390/app152010952






