Abstract
This study examined sarcopenia severity effects on body composition, physical performance, and mechanical properties of gait-related muscles in older women. Forty-one women aged ≥70 years participated and were classified by the following criteria: non-sarcopenia (NS, n = 15), functional sarcopenia (FS, n = 10), sarcopenia (SP, n = 9), and severe sarcopenia (SS, n = 7). Assessments included body composition, physical performance, and muscle tone, stiffness, and elasticity of the tibialis anterior (TA) and gastrocnemius medialis (GM). Group differences were analyzed using one-way ANOVA with Bonferroni post hoc tests (α = 0.05). SP and SS groups had lower body weight, BMI, appendicular skeletal muscle mass, and calf circumference compared with NS. FS demonstrated poorer physical performance than SP across all variables, with six-meter gait speed lower than SS (p < 0.05). SP exhibited significantly higher TA muscle tone, GM muscle tone and GM stiffness than NS (p < 0.05, p < 0.01, p < 0.05, respectively), while TA elasticity was significantly lower in SP (p < 0.01). These findings indicate that sarcopenia severity negatively influences body composition, muscle function, and mechanical properties, with functional sarcopenia showing the greatest impairment in performance. Early detection and targeted interventions are therefore critical to mitigate functional decline in older women.
1. Introduction
With the global rise in life expectancy, both physical and psychological age-related health challenges have become major concerns for individuals and society alike [1]. Among these, sarcopenia has emerged as a particularly pressing health concern in the aging population. Sarcopenia is typically characterized by age-related reductions in muscle mass, strength, and physical performance [2]. In Korea, the prevalence of sarcopenia among older adults ranges from 4% to 46.8%, depending on the diagnostic criteria, and recent studies have highlighted its progressive increase with age as well as the need for reliable diagnostic tools [2]. Notably, prevalence is consistently higher in women than in men [3], a disparity linked not only to estrogen deficiency post-menopause and lower baseline muscle mass, but also to lifestyle factors such as reduced physical activity levels, nutritional differences, and impaired protein metabolism. A recent meta-analysis of 151 studies comprising 692,056 individuals with a mean age of 68.5 years reported that the global prevalence of sarcopenia ranges from 10% to 27%, with the highest prevalence observed in Oceania. When stratified by age, prevalence was 8–36% in adults younger than 60 years and 10–27% in those aged 60 years and older. Analyses based on the International Working Group on Sarcopenia (IWGS) criteria further demonstrated a higher prevalence in women than in men (17% vs. 12%). Moreover, the prevalence of severe sarcopenia was reported to range from 2% to 9% [4]. Consequently, a multidimensional approach to managing sarcopenia in older women has gained increasing emphasis [5,6].
Sarcopenia is strongly associated with functional decline, increased risk of falls, disability, higher hospitalization rates, and mortality, and is therefore regarded as a condition requiring early diagnosis and systematic management [7]. Importantly, recent evidence [8,9] suggests that even functional sarcopenia, characterized by reduced muscle strength and performance without muscle mass loss, may independently predict adverse outcomes such as greater fall risk and impaired mobility. In recognition of its clinical significance, sarcopenia was incorporated into the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) in 2016 [10], and in Korea, it was formally registered under code M 62.5 in the 8th Revision of the Korean Standard Classification of Diseases (KCD-8) in 2021. The Korean Working Group on Sarcopenia (KWGS) proposed a classification of sarcopenia into three categories: severe sarcopenia, sarcopenia, and functional sarcopenia [8]. This classification revises the criteria established by the Asian Working Group for Sarcopenia (AWGS) [6], which had previously recognized only severe sarcopenia and sarcopenia, by adding functional sarcopenia as an additional category. Severe sarcopenia and sarcopenia both require reduced appendicular skeletal muscle mass (ASM; men < 7.0 kg/m2, women < 5.7 kg/m2) as an essential diagnostic criterion, whereas functional sarcopenia is defined by low muscle strength (handgrip strength: men < 28 kg, women < 18 kg) and reduced physical performance (Short Physical Performance Battery (SPPB) ≤ 9 points, gait speed < 1 m/s over 6 m, or five-time chair stand ≥ 12 s) without a reduction in ASM. This reflects both the demographic characteristics of the Korean population and the evolving diagnostic framework in Asia [6,8].
Importantly, functional sarcopenia can result in clinical outcomes comparable to those of severe sarcopenia, including an increased risk of falls, gait limitations, and higher mortality, despite the absence of reduced muscle mass [9]. Declines in muscle strength, particularly handgrip strength, are strongly correlated with both reduced muscle mass and impaired physical performance, and are further associated with a higher relative risk of depressive symptoms among older adults. These findings underscore handgrip strength as a valuable indicator for the early detection and management of sarcopenia [10]. The SPPB, comprising assessments of balance, gait speed, and chair stand performance, is widely used to evaluate fall risk and to predict physical disability in older adults [8,10]. Impaired physical function is also closely linked to altered gait patterns, characterized by reduced gait speed, shortened stride length, decreased step length, and diminished single-leg support time compared with healthy older adults [11].
Efficient gait requires precise coordination and synchronization of the lower extremity muscles, which generate ground reaction forces to propel the body forward, maintain balance to minimize fall risk, and distribute excessive loads across the joints [12]. Among these muscles, the gastrocnemius and tibialis anterior are particularly critical, as they contribute to both stability and propulsion [12,13]. Specifically, the gastrocnemius facilitates propulsion during the push-off phase, whereas the tibialis anterior stabilizes the foot and ensures smooth landing during the heel-strike phase. Their coordinated activation enhances gait stability and reduces the likelihood of falls [13]. Although muscle coordination is essential for propulsion and stability, physical performance is determined not only by neural activation and coordination but also by the intrinsic mechanical properties of the muscles. These properties include tone (the level of muscle tension), stiffness (resistance to external force without length change), and elasticity (the ability to return to the original state after deformation). Optimal physical function is achieved when these properties are well balanced [14,15,16].
Previous studies have reported that increased muscle stiffness and decreased elasticity are associated with impaired motor function [17], whereas reductions in stiffness are linked to improvements in functional tasks such as walking, running, and jumping [18]. Moreover, age-related increases in collagen concentration, decreases in elastin, and fat infiltration contribute to greater stiffness and diminished elasticity, thereby weakening the connectivity between fascia, skeletal muscle, and neural systems and ultimately impairing motor control [19]. Such adverse alterations in the mechanical properties of the lower extremity muscles in older women with sarcopenia result in slower gait speed and reduced gait stability, which in turn lead to secondary consequences such as increased fall risk and lower levels of physical activity [20]. Notably, older adults with functional sarcopenia, whose ASM levels remain within the normal range despite impaired physical performance, are at risk of being misclassified as healthy. As a result, they may be excluded from appropriate exercise and nutritional interventions for sarcopenia, leaving them particularly vulnerable to secondary complications [8]. Despite recognition of these clinical implications, few studies have investigated how the severity of sarcopenia affects the mechanical properties of gait-related muscles in older women, particularly when assessed using myotonometry. Addressing this research gap may provide important insights into the mechanisms by which sarcopenia severity contributes to gait impairment and increased fall risk.
Therefore, the present study aimed to examine the impact of sarcopenia severity on body composition, physical performance, and the mechanical properties of gait-related muscles in older women. Specifically, we examined whether the severity of sarcopenia, as defined by current diagnostic criteria, exerts differential impacts on these outcomes. By addressing this aim, the study seeks to provide scientific evidence to support the development of exercise prescriptions tailored to varying levels of sarcopenia severity in this population.
2. Materials and Methods
2.1. Participants
A total of 62 individuals were recruited between March and June 2025 through flyers posted at senior welfare centers in Gyeonggi-do, and all applicants provided written informed consent at the time of application. All applicants subsequently underwent clinical prescreening by a board-certified family medicine physician. Twenty-one individuals with orthopedic conditions (e.g., musculoskeletal pain, degenerative joint disease), neurological disorders (e.g., nerve injury), or psychiatric disorders (e.g., cognitive impairment, dementia, depression) were excluded to minimize secondary complications during testing and to ensure the reliability of the study (Figure 1). Consequently, a total of 41 community-dwelling older women (mean age: 80.12 ± 5.53 years) were finally included in the study (Table 1). This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board of Jung-Ang University (approval number: 1041078-20250731-HR-263).
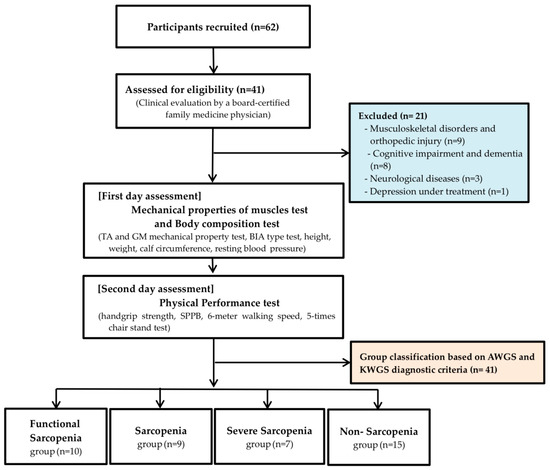
Figure 1.
Consort flowchart. TA: Tibialis Anterior, GM: Gastrocnemius Medialis, BIA: Bioelectrical Impedance Analysis, SPPB: Short Physical Performance Battery, AWGS: Asian Working Group for Sarcopenia: KWGS, Korean Working Group for Sarcopenia.

Table 1.
Subject characteristics.
2.2. Study Procedures
Following the diagnostic algorithms proposed by the AWGS [5] and the KWGS [8], participants were classified into four groups: functional sarcopenia (FS; n = 10), sarcopenia (SP; n = 9), severe sarcopenia (SS; n = 7), and non-sarcopenia (NS; n = 15). The NS group was defined as individuals with skeletal muscle mass, muscle strength, and physical performance all above the diagnostic thresholds (Figure 2). All participants underwent assessments of physical performance and the mechanical properties of gait-related muscles. To minimize measurement errors due to unfamiliarity with the procedures, participants received standardized instructions and completed three preliminary trials prior to the main assessments. In addition, intra-rater reliability for myotonometric measurements was assessed in a subset of participants, showing excellent reproducibility (Intraclass Correlation Coefficient; ICC > 0.90).
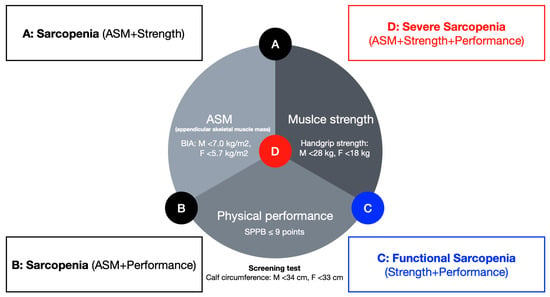
Figure 2.
Diagnostic criteria for different severity levels of sarcopenia in older women. ASM: appendicular skeletal muscle mass, SPPB: short physical performance battery.
2.3. Measurement
2.3.1. Body Composition
Height and weight were measured using an automatic stadiometer and scale (BSM 330, InBody, Seoul, Republic of Korea), and body mass index (BMI) was subsequently calculated. ASM and body fat percentage were assessed using a bioelectrical impedance analyzer (BIA; S10, InBody, Seoul, Republic of Korea) with participants standing with their feet shoulder-width apart and their arms positioned at a 45 degrees angle from the trunk. Calf circumference was measured with participants standing upright, feet positioned approximately 20 cm apart, at the thickest point between the knee and ankle using a non-elastic tape (Lufkin executive thinline, Apex Tool Group, Huntersville, NC, USA) placed horizontally. Resting blood pressure, including systolic and diastolic values (mmHg), was recorded using an automated sphygmomanometer (Bpbio320, InBody, Seoul, Republic of Korea). To ensure measurement accuracy, participants were instructed to refrain from eating, drinking fluids (including caffeine and alcohol), and engaging in vigorous physical activity for at least 5 h before testing [4,6,8].
2.3.2. Physical Performance
Muscle strength was assessed using a handgrip dynamometer (Smedley type; TKK 5401, Takei Scientific Instruments Co., Ltd., Tokyo, Japan). For measurement, participants stood upright with their feet shoulder-width apart and elbows fully extended, with the grip handle adjusted to the second phalanx of the fingers. Participants were instructed to exert maximal force for 5 s. Each hand was tested twice, and the highest value was recorded to the nearest 0.1 kg [21]. Overall physical performance was evaluated using the SPPB, which comprises three domains balance, gait speed, and chair stand each scored up to 4 points, for a total of 12 points [22]. Gait function was additionally assessed by measuring 6-m walking speed, while lower extremity endurance was evaluated by recording the time required to complete five consecutive chair stands.
2.3.3. Mechanical Properties of Gait-Related Muscles
The mechanical properties of gait-related muscles were assessed using a handheld myotonometer (Myoton Pro, Myoton AS, Tallinn, Estonia) targeting the tibialis anterior (TA) and gastrocnemius medialis (GM). For the TA, measurements were obtained in the supine position at a point 12 cm distal to the lateral condyle of the femur, whereas for the GM, measurements were performed in the prone position at a point 30 cm proximal to the posterior border of the medial malleolus. During each assessment, the probe was placed perpendicularly at the midpoint of the muscle belly to induce mechanical oscillations, and all measurements were conducted at the same anatomical site under identical conditions. Measurements were performed in multi-scan mode, with three mechanical impulses delivered at intervals of 0.8 s, each applied at a velocity of 15 m/s. The outcomes included frequency (Hz), stiffness (N/m), and decrement (log), corresponding to muscle tone, muscle stiffness, and muscle elasticity, respectively. The mean of three impulses was used for subsequent analyses. In cases where motion artifacts produced outlier values, additional scans were performed, and the most consistent set of values was retained [23].
2.4. Statistical Analysis
All statistical analyses were performed using SPSS software, version 21.0 for Windows (IBM Corp., Armonk, NY, USA). Descriptive statistics were applied to calculate the mean and standard deviation of physical performance and related variables. To examine group differences in body composition, physical performance, and the mechanical properties of gait-related muscles according to sarcopenia severity, one-way analysis of variance (ANOVA) was conducted. For ANOVA, effect sizes were reported as partial eta-squared (η2), with large effects defined as η2 ≥ 0.13 [24]. For variables that demonstrated statistically significant differences, post-hoc comparisons were performed using the Bonferroni correction. The significance level was set at α = 0.05.
3. Results
3.1. Differences in Body Composition by Sarcopenia Severity in Older Women
Body weight differed significantly across groups defined by sarcopenia severity (p < 0.01, partial η2 = 0.376), with both the severe SS and SP groups showing lower values than the NS group (all p < 0.01).
BMI also differed significantly across groups (p < 0.01, partial η2 = 0.275), being significantly lower in the SS and SP groups compared with the NS group (all p < 0.05).
ASM differed significantly across groups (p < 0.001, partial η2 = 0.681). The SP group had lower ASM than both the FS and NS groups (p < 0.01 and p < 0.001, respectively), while the SS group exhibited lower ASM than both the FS and NS groups (all p < 0.001). However, no significant difference was observed between the SS and SP groups.
Calf circumference also differed significantly across groups (p < 0.001, partial η2 = 0.436), with both the SS and SP groups showing smaller values than the NS group (p < 0.01 and p < 0.001, respectively).
All dependent variables (body weight, ASM, calf circumference) exhibited identical degrees of freedom, with between-groups df = 3 and within-groups df = 37 (Table 2).

Table 2.
Comparison of body composition by sarcopenia levels in older women.
3.2. Differences in Physical Performance by Sarcopenia Severity in Older Women
Handgrip strength differed significantly across groups defined by sarcopenia severity (p < 0.001, partial η2 = 0.565). The FS group showed lower values than both the SP and NS groups (all p < 0.001), and the SS group also demonstrated lower values than the NS group (p < 0.05).
The SPPB score also differed significantly across groups (p < 0.05), with the FS group scoring lower than the SP group (p < 0.05, partial η2 = 0.229).
6 m gait speed showed significant differences across groups (p < 0.01, partial η2 = 0.168). The FS group exhibited slower gait speed than the SS, SP, and NS groups (p < 0.05, p < 0.05, and p < 0.01, respectively).
The 5-time chair stand test also revealed significant differences across groups (p < 0.01, partial η2 = 0.355), with the FS group requiring longer completion times than the SP and NS groups (all p < 0.05).
All dependent variables (handgrip strength, SPPB score, 6 m gait speed, 5-time chair stand test) exhibited identical degrees of freedom, with between-groups df = 3 and within-groups df = 37 (Table 3).

Table 3.
Comparison of physical performance according to sarcopenia levels in older women.
3.3. Differences in Mechanical Properties of Gait-Related Muscles by Sarcopenia Severity in Older Women
Muscle tone of the TA differed significantly across groups defined by sarcopenia severity (p < 0.05, partial η2 = 0.234), with the SP group showing higher values than the NS group (p < 0.05).
Elasticity of the TA also differed significantly across groups (p < 0.01, partial η2 = 0.311), being lower in the SP group compared with the NS group (p < 0.01).
Tone of the GM differed significantly across groups (p < 0.01, partial η2 = 0.297), with the SP group showing higher values than the NS group (p < 0.01).
Stiffness of the GM also differed significantly across groups (p < 0.05, partial η2 = 0.206), with the SP group exhibiting higher values than the NS group (p < 0.05).
All dependent variables (TA muscle tone, TA elasticity, GM muscle tone, GM stiffness) exhibited identical degrees of freedom, with between-groups df = 3 and within-groups df = 37 (Table 4).

Table 4.
Comparison of mechanical properties of gait-related muscles according to sarcopenia levels in older women.
4. Discussion
This study investigated the effects of sarcopenia severity on body composition, physical performance, and the mechanical properties of gait-related muscles in women aged 70 years and older. The findings highlight the clinical characteristics associated with different stages of sarcopenia and underscore the importance of early intervention, thereby providing essential evidence for exercise prescription tailored to sarcopenia severity.
When body composition was compared across sarcopenia severity levels, a progressive decline in ASM and calf circumference was observed in the order of SS, SP, and FS. Specifically, the SS and SP groups showed significantly lower body weight, BMI, ASM, and calf circumference compared with the NS group, and both had significantly lower ASM than the FS group, indicating a more adverse body composition profile. These findings are consistent with the general characteristics of patients with sarcopenia and are in agreement with the diagnostic criteria of the AWGS [6] which emphasize reduced muscle mass as a primary criterion [25]. Moreover, the accelerated decline in ASM among older adults with advanced sarcopenia may reflect an imbalance between muscle protein synthesis and degradation. The reduction in calf circumference, observed here, is consistent with prior evidence linking muscle loss and an increased risk of falls [26]. Consistent with this, Takagi [27], who examined the relationship between limb circumference and muscle mass in 74 men and women, reported a strong correlation between muscle mass and calf circumference and recommended calf circumference as a practical clinical marker to predict sarcopenia. The present findings therefore suggest that the reduced ASM and smaller calf circumference observed in the SS and SP groups may increase vulnerability to falls and related complications. In contrast, no significant differences were observed in body fat percentage between groups. This finding differs from prior systematic reviews of sarcopenic obesity [28], which reported that aging is typically accompanied by increased body fat, along with declines in muscle mass and function, thereby contributing to the onset of sarcopenia. This discrepancy suggests that the relationship between muscle mass and body fat may vary depending on individual factors, including metabolic state, hormonal changes, inflammatory status, and lifestyle behaviors [29].
When physical performance was compared by severity, the NS group outperformed the FS, SP, and SS groups. Among the sarcopenia groups, the FS group performed significantly worse than the SP group across all performance tests, including handgrip strength, SPPB, 6-m walking speed, and the 5-time chair stand (p < 0.001, p < 0.05, p < 0.05, and p < 0.05, respectively). Notably, gait speed in the FS group tended to be slower than in the SS group. These findings suggest that the pronounced decline in physical performance observed in FS, which is often regarded as a relatively mild stage of sarcopenia, may accelerate its progression to more severe stages if further age-related muscle loss occurs. Moreover, although muscle mass was preserved in the FS group, their physical performance was lower than that of the SP and SS groups. This may be explained by the markedly low physical activity levels observed in the FS group, which could have induced neuromuscular impairments, such as diminished motor unit recruitment, thereby reducing physical performance irrespective of muscle mass [30]. These results indicate that the assessment of sarcopenia severity should take into account not only muscle mass but also functional deficits and physical activity levels. Sarcopenia is strongly associated with physical performance and overall activity levels. Maintaining higher levels of physical activity reduces the risk of sarcopenia [31], whereas persistently low activity contributes to sarcopenic obesity and impaired daily activities, ultimately reducing quality of life and life expectancy [32]. Gait speed is considered a particularly sensitive marker for classifying sarcopenia severity and has been reported to correlate significantly with fall risk [33] and cognitive impairment [34]. Specifically, gait speeds of ≤1.0 m/s are associated with a 3.7-fold increased risk of falls. In addition, other factors such as depressive symptoms, polypharmacy, and low educational attainment have also been identified as contributors to reduced gait speed in older adults [33]. In the present study, the mean gait speed in the FS group was 0.74 m/s, below the established fall-risk threshold, suggesting that older women with FS are highly vulnerable to falls. Given the elevated risk of progression to severe sarcopenia and secondary complications such as falls, immediate exercise interventions, nutritional management, and lifestyle modifications are urgently required for this population.
Gait speed depends on the coordinated activation of lower-limb muscles, particularly during ground contact and propulsion [35]. In older adults with sarcopenia, gait often involves impaired mechanical function of the lower extremity muscles, which should be recognized as a key determinant of physical performance. Nevertheless, most existing studies have primarily focused on reductions in ASM, SPPB scores, and gait speed, whereas investigations into impaired coordination and the mechanical properties of the lower limb muscles remain limited. Di Nardo et al. [36] reported that TA regulates ankle dorsiflexion during gait, whereas the GM controls plantarflexion, with both muscles acting complementarily to maintain dynamic stability. Similarly, Maharaj et al. [37] demonstrated that the tendon structures of the TA absorb energy during foot strike to prevent muscle fiber damage and subsequently release this stored energy during propulsion to accelerate dorsiflexion. Furthermore, De Visser et al. [38] compared patients who underwent limb-sparing surgery following tumor-related muscle damage with healthy controls, and found significant differences in gait symmetry and phase transition patterns in the electromyographic (EMG) activity of the TA and GM. Collectively, these findings underscore the essential role of the TA and GM in maintaining ankle function and dynamic stability, suggesting that evaluation of the mechanical properties of these muscles is essential for understanding gait impairments in older women with sarcopenia.
In this study, we found that the FS, SP, and SS groups showed higher tone and stiffness and lower elasticity compared with the NS group. Moreover, the SP group exhibited higher tone and stiffness in both muscles, whereas the SS group showed a tendency toward lower elasticity, indicating adverse mechanical characteristics. Specifically, the SP group exhibited significantly higher TA and GM tone (p < 0.05 and p < 0.01, respectively) and significantly higher GM stiffness (p < 0.05) compared with the NS group. These findings are consistent with previous reports showing that aging increases muscle tone and stiffness [39] and that sarcopenia induces changes in muscle spasticity and collagen matrix composition, leading to structural alterations and the activation of neuromuscular compensatory mechanisms that elevate muscle tone [40,41]. Persistent increases in TA and GM tone may compromise muscle blood flow, predisposing individuals to musculoskeletal disorders such as myofascial pain syndrome [42]. In our study, although no participants reported severe musculoskeletal pain, some did complain of mild fatigue and stiffness during physical activity, which may support this physiological interpretation. Such conditions, disrupt the balance between muscle contraction and relaxation and ultimately impair gait function [36,37,39,40,41,42]. In addition, increased GM stiffness may impair ankle dorsiflexion flexibility, reduce shock absorption, and restrict the storage and release of elastic energy required for propulsion. These alterations compromise gait stability, reduce endurance, and increase fall risk [36,43].
In contrast, TA elasticity was significantly lower in the SP group compared with the NS group (p < 0.01), suggesting seemingly favorable elastic properties. This finding contrasts with prior studies reporting age-related declines in elasticity [39]. Although the SP group appeared to exhibit enhanced TA elasticity, this phenomenon may be explained by the pathological characteristics of sarcopenia, in which reductions in type II fibers result in a relative increase in type I fibers. Given that type I fibers are characterized by lower decrement ratios, the apparent increase in elasticity observed in the SP group may result from such fiber-type shifts [44,45]. While this interpretation is plausible, it should be noted that elasticity measurements provide only indirect insights into muscle fiber composition, and further research is warranted to clarify these mechanisms.
Furthermore, the relatively greater fat infiltration and fibrosis commonly observed in sarcopenic muscles may increase shock absorption and load distribution, creating the appearance of greater elasticity [46]. Since sarcopenia adversely affects the mechanical properties of both the TA and GM, targeted exercise interventions are required to mitigate these changes. Resistance training at approximately 70% of one-repetition maximum (1RM), performed three times per week with three sets of eight repetitions, has been recommended as an effective strategy to stimulate muscle protein synthesis, promote hypertrophy of type II fibers, enhance muscle strength, and improve overall physical performance [45]. However, such initial high-intensity training may be difficult to implement for older adults, particularly those with sarcopenia. In this regard, Villareal et al. [47] recommended resistance exercise three times per week on non-consecutive days, with each session lasting about 60 min and consisting of flexibility training (10 min), resistance training (40 min), and balance training (10 min). Their protocol emphasized nine multi-joint exercises involving both the upper and lower limbs, beginning at approximately 65% 1RM with 1–2 sets of 8–12 repetitions, and progressively increasing the load to about 85% 1RM with 2–3 sets. Such a progressive approach provides a safer and more practical alternative for older adults while still improving muscle function and physical performance.
For very old women with sarcopenia (≥85 years), multicomponent exercise programs that combine high-velocity resistance training, balance training, and gait training are particularly recommended to improve muscle function and increase muscle mass [48].
In summary, differences in body composition (body weight, BMI, ASM, calf circumference) and in the mechanical properties of gait-related muscles (TA tone, TA elasticity, GM tone, GM stiffness) were most pronounced in the SP and SS groups, whereas physical performance was poorest in the FS group. Although not recognized in international guidelines [6], FS is classified by the KWGS as functional sarcopenia [8]. These findings suggest that older women with FS are at high risk of progression to more severe sarcopenia.
This study, however, has certain limitations, including the relatively small sample size, insufficient control of potential confounding factors such as comorbidities and nutritional status, and the recruitment of participants limited to rural Korean women. These factors may limit the generalizability of the findings to diverse ethnic groups, populations from other regions, and individuals with comorbid conditions. In addition, in this study including a comparison between patients with severe sarcopenia and healthy older adults, differences in body weight and height between groups may have indirectly influenced anthropometric indices such as BMI and calf circumference. Future studies should minimize these differences to reduce confounding and enhance the accuracy of result interpretation.
Despite these limitations, the findings provide valuable insights into the clinical management of sarcopenia, and early identification of older adults with functional sarcopenia through physical performance assessments in clinical practice, along with timely exercise interventions, is strongly recommended.
5. Conclusions
This study investigated the effects of sarcopenia severity on body composition, physical performance, and the mechanical properties of gait-related muscles in older women, offering preliminary evidence for exercise prescriptions tailored to different stages of sarcopenia.
First, older women with sarcopenia and severe sarcopenia showed greater deterioration in body composition and in the mechanical properties of gait-related muscles, specifically the tibialis anterior and gastrocnemius, compared with those with functional sarcopenia.
Second, functional sarcopenia was associated with more pronounced declines in physical performance than either sarcopenia or severe sarcopenia. It may be associated with adverse outcomes, and early diagnosis and timely exercise interventions are essential to prevent further decline. Importantly, these findings highlight that functional sarcopenia should not be underestimated, and that early intervention may be warranted even in the absence of muscle mass loss.
Finally, follow-up longitudinal studies are required to directly elucidate the associations over time and potential causal relationships in older adults with functional sarcopenia, which will provide more conclusive evidence.
Author Contributions
G.B. and K.-H.L. drafted the manuscript and critically revised the paper. G.B. and K.-H.L. designed the study and collected the data. K.-H.L. analyzed and interpreted the data and performed the statistical analysis. G.B., B.H. and K.-H.L. discussed the results and contributed to the final version of the article. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support or the research, authorship, and publication of this article.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Jung-Ang University (approval number: 1041078-20250731-HR-263, approval date: 31 July 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions and can be shared upon approval by the Institutional Review Board (IRB) of Jung-Ang University.
Acknowledgments
The authors would like to express their sincere gratitude to BrownSpring Co., Ltd. (Seongnam, Republic of Korea), the official distributor of the Myoton Pro in Korea, for their generous equipment support and technical training provided for this study. The authors also extend their appreciation to all participants for their valuable contributions and cooperation.
Conflicts of Interest
The authors have no potential conflicts of interest to declare with respect to the research, authorship, and publication of this article.
References
- Khan, H.T.A.; Addo, K.M.; Findlay, H. Public Health Challenges and Responses to the Growing Ageing Populations. Public Health Chall. 2024, 3, e213. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Lee, E.; Jung, H.W.; Jang, I.Y. Geriatrics Fact Sheet in Korea 2021. Ann. Geriatr. Med. Res. 2021, 25, 65–71. [Google Scholar] [CrossRef]
- Kim, S.; Ha, Y.C.; Kim, D.Y.; Yoo, J.I. Recent Update on the Prevalence of Sarcopenia in Koreans: Findings from the Korea National Health and Nutrition Examination Survey. J. Bone Metab. 2024, 31, 150–161. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Messier, V.; Rabasa-Lhoret, R.; Barbat-Artigas, S.; Elisha, B.; Karelis, A.D.; Aubertin-Leheudre, M. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 2011, 68, 331–336. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jung, H.W.; Kim, K.M.; Kim, M.; Park, C.Y.; Lee, K.P.; Lee, S.Y.; Jang, I.Y.; Jeon, O.H.; Lim, J.Y. Korean Working Group on Sarcopenia Guideline: Expert Consensus on Sarcopenia Screening and Diagnosis by the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society. Ann. Geriatr. Med. Res. 2023, 27, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Shim, G.Y.; Jang, H.C.; Kim, K.W.; Lim, J.Y. Impact of Sarcopenia on Falls, Mobility Limitation, and Mortality Using the Diagnostic Criteria Proposed in the Korean Working Group on Sarcopenia Guideline. Ann. Geriatr. Med. Res. 2025, 29, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Choi, Y.; Jung, S.J.; Kwak, H.B. Role of exercise in estrogen deficiency-induced sarcopenia. J. Exerc. Rehabil. 2022, 18, 2–9. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, B.; Huang, G.; Zhang, G.; Ding, Z.; Li, Z.; Sinclair, J.; Fan, Y. Sarcopenia: Body Composition and Gait Analysis. Front. Aging Neurosci. 2022, 14, 909551. [Google Scholar] [CrossRef]
- Moissenet, F.; Cheze, L.; Dumas, R. Individual muscle contributions to ground reaction and to joint contact, ligament and bone forces during normal gait. Multibody Syst. Dyn. 2017, 40, 193–211. [Google Scholar] [CrossRef]
- Kirkwood, R.N.; Trede, R.G.; Moreira Bde, S.; Kirkwood, S.A.; Pereira, L.S. Decreased gastrocnemius temporal muscle activation during gait in elderly women with history of recurrent falls. Gait Posture 2011, 34, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Needle, A.R.; Baumeister, J.; Kaminski, T.W.; Higginson, J.S.; Farquhar, W.B.; Swanik, C.B. Neuromechanical coupling in the regulation of muscle tone and joint stiffness. Scand. J. Med. Sci. Sports 2014, 24, 737–748. [Google Scholar] [CrossRef]
- Schleip, R.; Naylor, I.L.; Ursu, D.; Melzer, W.; Zorn, A.; Wilke, H.J.; Lehmann-Horn, F.; Klingler, W. Passive muscle stiffness may be influenced by active contractility of intramuscular connective tissue. Med. Hypotheses 2006, 66, 66–71. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Kjaer, M.; Mackey, A.L. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand. J. Med. Sci. Sports 2011, 21, 749–757. [Google Scholar] [CrossRef]
- Chuang, L.L.; Wu, C.Y.; Lin, K.C. Reliability, validity, and responsiveness of myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef]
- Park, G.Y.; Kwon, D.R. Sonoelastographic evaluation of medial gastrocnemius muscles intrinsic stiffness after rehabilitation therapy with botulinum toxin a injection in spastic cerebral palsy. Arch. Phys. Med. Rehabil. 2012, 93, 2085–2089. [Google Scholar] [CrossRef]
- Zullo, A.; Fleckenstein, J.; Schleip, R.; Hoppe, K.; Wearing, S.; Klingler, W. Structural and Functional Changes in the Coupling of Fascial Tissue, Skeletal Muscle, and Nerves During Aging. Front. Physiol. 2020, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Nakamura, M.; Shima, H.; Asakawa, Y.; Ichihashi, N. Association between walking ability and trunk and lower-limb muscle atrophy in institutionalized elderly women: A longitudinal pilot study. J. Physiol. Anthropol. 2015, 34, 31. [Google Scholar] [CrossRef] [PubMed]
- McGrath, R.; Cawthon, P.M.; Clark, B.C.; Fielding, R.A.; Lang, J.J.; Tomkinson, G.R. Recommendations for Reducing Heterogeneity in Handgrip Strength Protocols. J. Frailty Aging 2022, 11, 143–150. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef]
- Ramazanoğlu, E.; Usgu, S.; Yakut, Y. Assessment of the mechanical characteristics of the lower extremity muscles with myotonometric measurements in healthy individuals. Physiother. Q. 2020, 28, 1–12. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Villegas, G.; Parodi, J.; Merino-Taboada, A.; Perez-Agüero, C.; Castro-Viacava, G.; Runzer-Colmenares, F.M. Calf circumference and risk of falls among Peruvian older adults. Eur. Geriatr. Med. 2016, 7, 543–546. [Google Scholar] [CrossRef]
- Takagi, D. Relationships among limb circumferences and appendicular. muscle and fat masses using bioelectrical impedance analysis. Int. J. Phys. Ther. Rehab. 2018, 4, 2. [Google Scholar] [CrossRef]
- Prado, C.M.; Batsis, J.A.; Donini, L.M.; Gonzalez, M.C.; Siervo, M. Sarcopenic obesity in older adults: A clinical overview. Nat. Rev. Endocrinol. 2024, 20, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Leung, J.; Morley, J.E. Defining sarcopenia in terms of incident adverse outcomes. J. Am. Med. Dir. Assoc. 2015, 16, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Petnehazy, N.; Barnes, H.N.; Newman, A.B.; Kritchevsky, S.B.; Cummings, S.R.; Hepplen, R.T.; Cawthon, P.M. Muscle Mass, Strength, Power and Physical Performance and Their Association with Quality of Life in Older Adults, the Study of Muscle, Mobility and Aging (SOMMA). J. Frailty Aging 2024, 13, 384–390. [Google Scholar] [CrossRef]
- Ryu, M.; Jo, J.; Lee, Y.; Chung, Y.S.; Kim, K.M.; Baek, W.C. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013, 42, 734–740. [Google Scholar] [CrossRef]
- Rolland, Y.; Lauwers-Cances, V.; Cristini, C.; Abellan van Kan, G.; Janssen, I.; Morley, J.E.; Vellas, B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: The EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am. J. Clin. Nutr. 2009, 89, 1895–1900. [Google Scholar] [CrossRef]
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. J. Res. Clin. Phys. Ther. 2019, 24, e1743. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W. Sarcopenia Is Associated with Cognitive Impairment Mainly Due to Slow Gait Speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 2019, 16, 1491. [Google Scholar] [CrossRef]
- Murley, G.S.; Menz, H.B.; Landorf, K.B. Electromyographic patterns of tibialis posterior and related muscles when walking at different speeds. Gait Posture 2014, 39, 1080–1085. [Google Scholar] [CrossRef]
- Di Nardo, F.; Ghetti, G.; Fioretti, S. Assessment of the activation modalities of gastrocnemius lateralis and tibialis anterior during gait: A statistical analysis. J. Electromyogr. Kinesiol. 2013, 23, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, J.N.; Cresswell, A.G.; Lichtwark, G.A. Tibialis anterior tendinous tissue plays a key role in energy absorption during human walking. J. Exp. Biol. 2019, 222, jeb191247. [Google Scholar] [CrossRef] [PubMed]
- De Visser, E.; Veth, R.P.; Schreuder, H.W.; Duysens, J. Altered phase-transitions in tibialis anterior and medial gastrocnemius during walking after limbsaving surgery. Clin. Neurophysiol. 2005, 116, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Measurement of ageing effects on muscle tone and mechanical properties of rectus femoris and biceps brachii in healthy males and females using a novel hand-held myometric device. Arch. Gerontol. Geriatr. 2016, 62, 59–67. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular Changes with Aging and Sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar] [CrossRef]
- Kang, C.; Jung, C.-H.; Baek, J.-H. Trigger Point Injection for the Treatment of Myofascial Pain Syndrome. J. Korean Orthop. Assoc. 2024, 59, 247–255. [Google Scholar] [CrossRef]
- Kim, N.; Park, J.; Shin, H.; Bae, Y. Gastrocnemius Medial Head Stiffness Is Associated with Potential Fall Risk in Community-Dwelling Older Adults. Healthcare 2022, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, W.H. Clinical and physiopathological mechanism of sarcopenia. Korean J. Med. 2012, 83, 444–454. [Google Scholar] [CrossRef]
- Heo, J.-W.; No, M.-H.; Min, D.-H.; Kang, J.-H.; Kwak, H.-B. Aging-induced Sarcopenia and Exercise. Off. J. Korean Acad. Kinesiol. 2017, 19, 43–59. [Google Scholar] [CrossRef]
- Wang, Z.; Taniguchi, M.; Saeki, J.; Yagi, M.; Murota, N.; Nakazato, K.; Niiya, N.; Ichihashi, N. Intramuscular fat infiltration influences mechanical properties during muscle contraction in older women. Appl. Physiol. Nutr. Metab. 2024, 49, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Cadore, E.L.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Idoate, F.; Millor, N.; Gómez, M.; Rodriguez-Mañas, L.; Izquierdo, M. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age 2014, 36, 773–785. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).