Design and Verification of a New Fixture for Machining of Porous Blocks for Medical CAD/CAM Systems
Abstract
1. Introduction
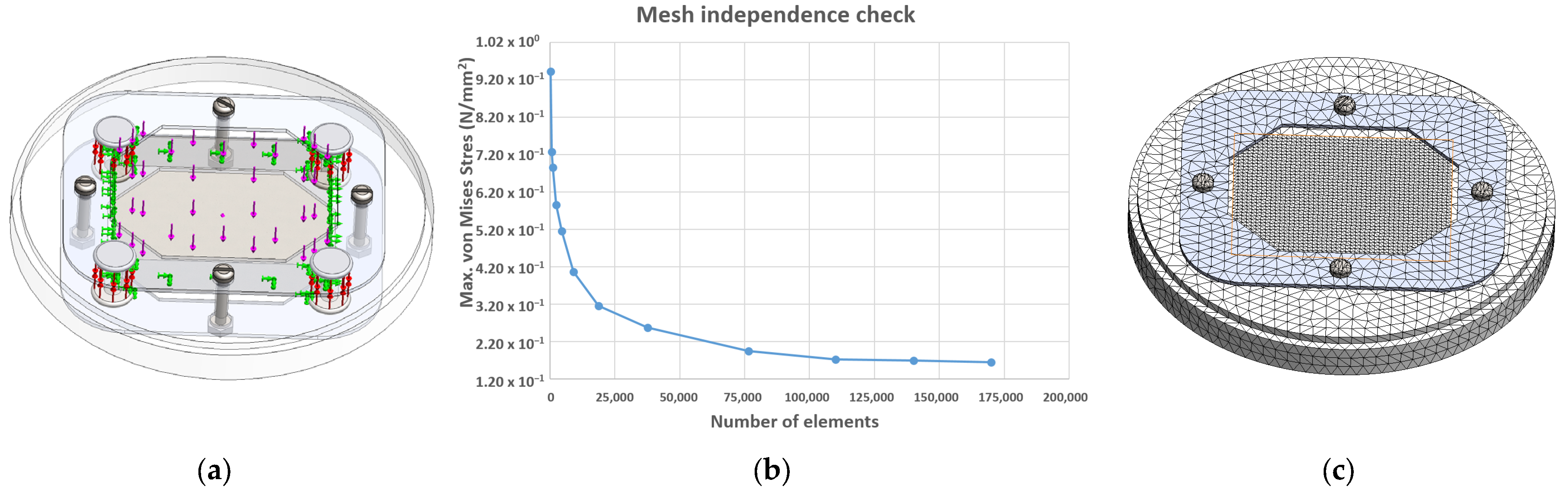
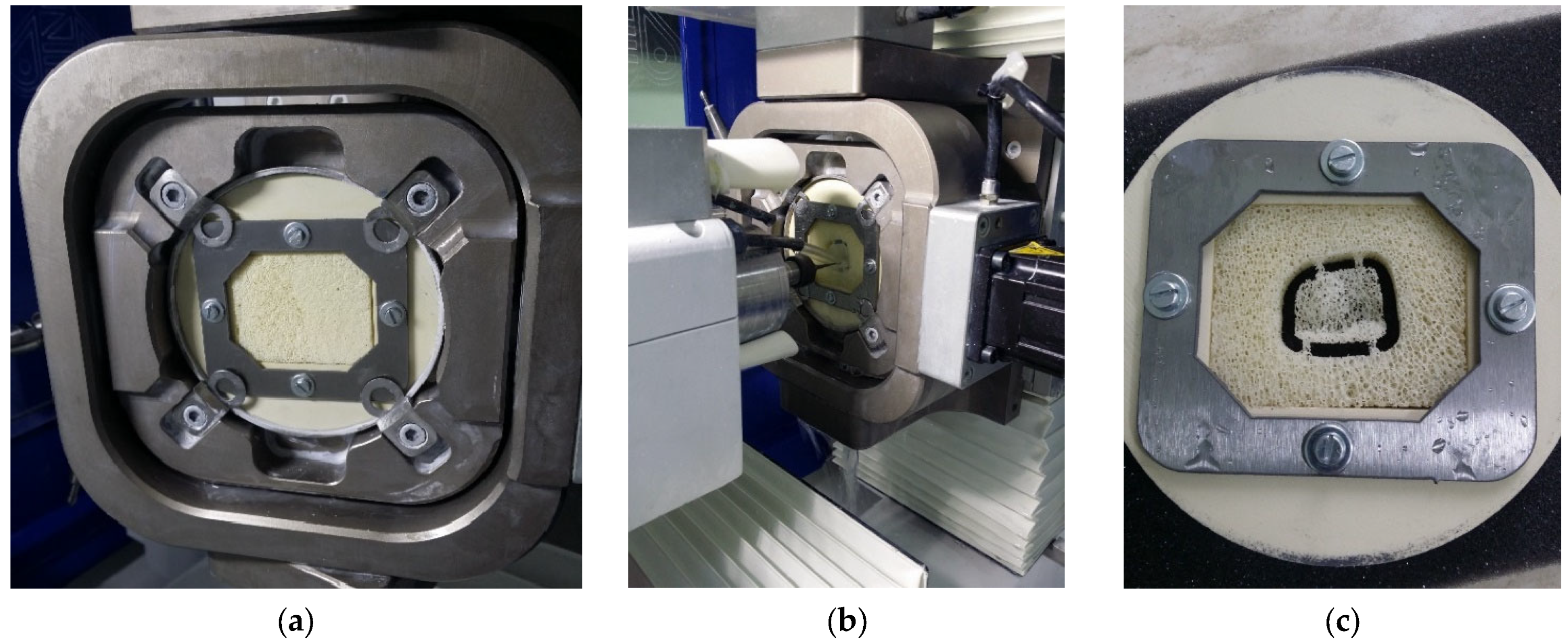
2. Methodology
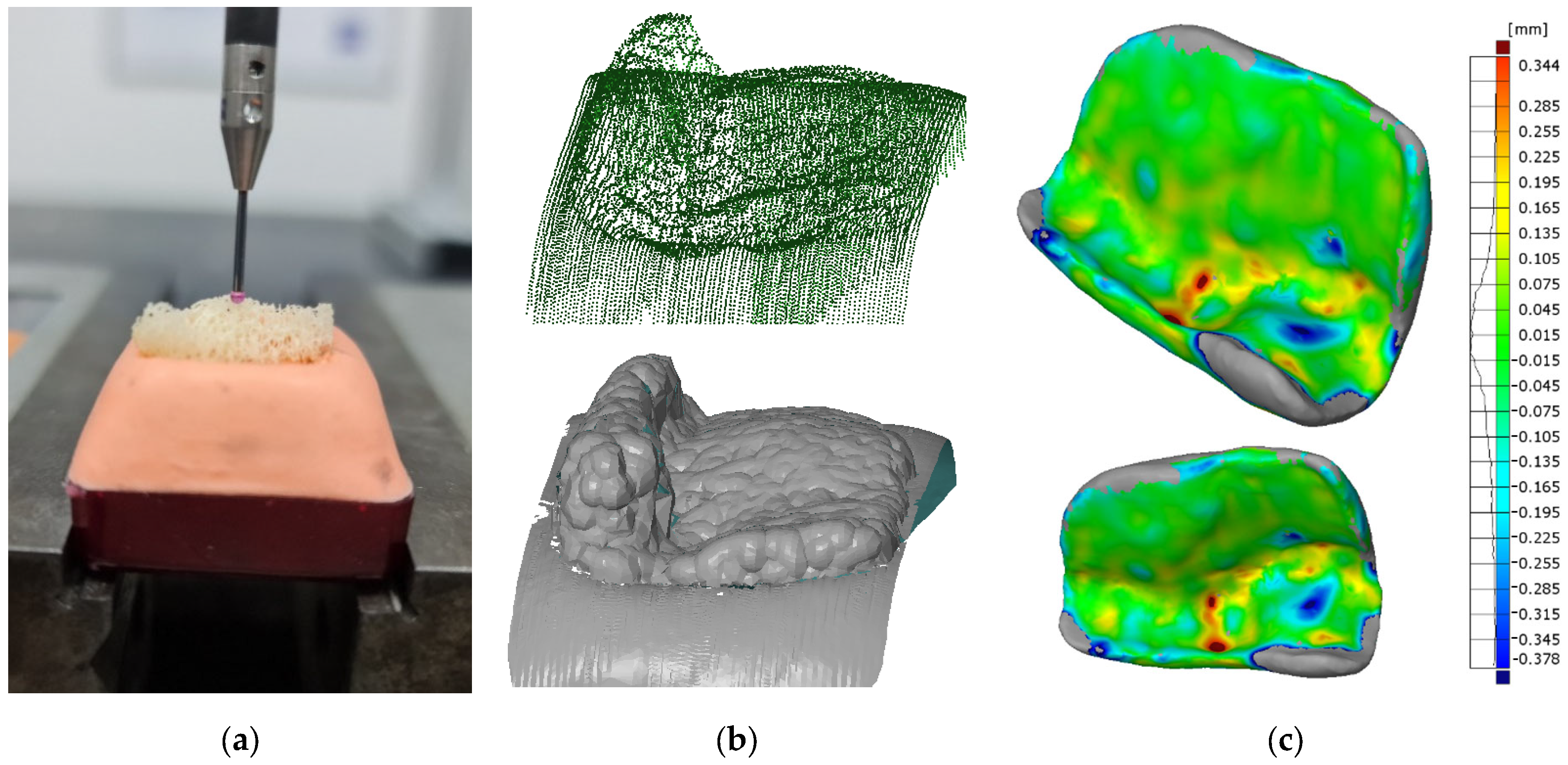
3. Results
4. Discussion
5. Conclusions
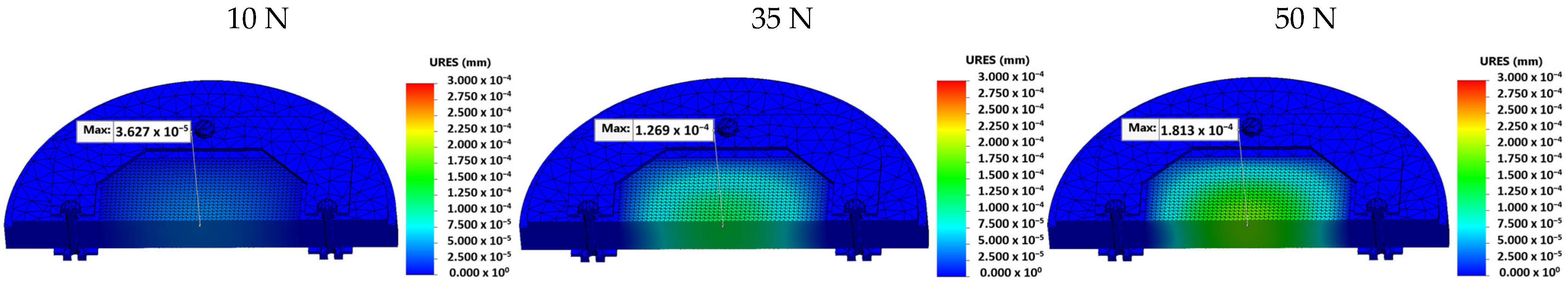
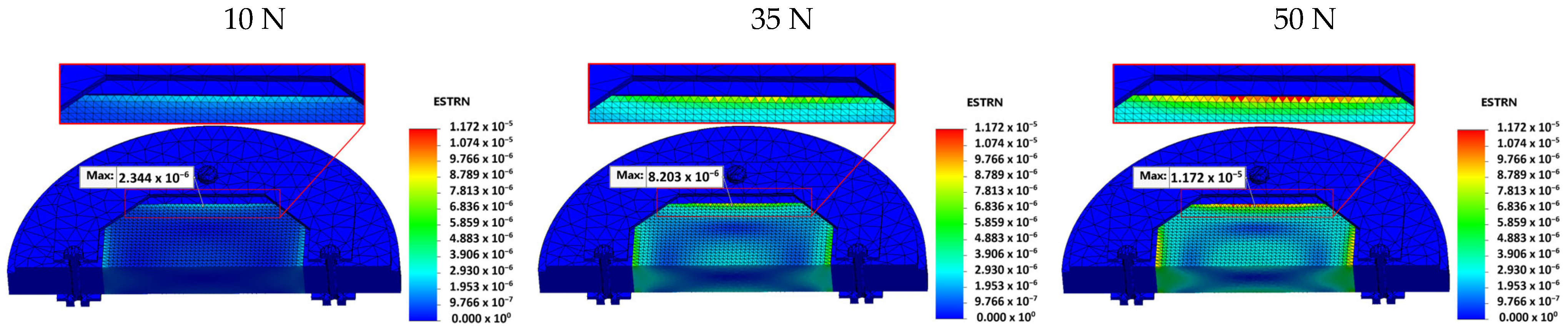
- Stress, displacement, and strain values were calculated for three different preload conditions (10 N, 35 N, and 50 N).
- At 10 N load, the stress was 4.856 × 10−2 MPa. For 35 N and 50 N loads, stress concentrations on the longer edge of the porous block were 1.700 × 10−1 MPa and 2.428 × 10−1 MPa, respectively.
- Displacement at 35 N was 1.269 × 10−4 mm, and at 50 N, it was 1.813 × 10−4 mm, while at 10 N was negligible (it was 3.627 × 10−5 mm).
- Strain values were 2.344 × 10−6 at 10 N, 8.203 × 10−6 at 35 N, and 1.172 × 10−5 at 50 N.
- Dimensional deviations of the machined bone graft were found to be within the range of ±0.135 mm, with a peak concentration of +0.015 mm.
- Clamping and machining performance showed that the fixture design reduced the risk of material breakage and deformation, ensuring stability during machining.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyle, I.; Rong, Y.; Brown, D.C. A review and analysis of current computer-aided fixture design approaches. Robot. Comput. Integr. Manuf. 2011, 27, 1–12. [Google Scholar] [CrossRef]
- Gameros, A.; Lowth, S.; Axinte, D.; Nagy-Sochacki, A.; Craig, O.; Siller, H.R. State-of-the-art in fixture systems for the manufacture and assembly of rigid components: A review. Int. J. Mach. Tools Manuf. 2017, 123, 1–21. [Google Scholar] [CrossRef]
- Pandit, H.C. Jigs and Fixtures in Manufacturing. Int. J. Eng. Res. Appl. 2022, 12, 50–55. [Google Scholar]
- Chai, S.; Ouyang, L.; Bi, Q.; Yu, J.; Zhang, Y. An adaptive fixture for suppress vibrations and measuring workpiece deformation of thin-walled casings. Procedia CIRP 2021, 101, 322–325. [Google Scholar] [CrossRef]
- Bijelic, I.; Milovanovic, V.; Vukelic, D.; Simunovic, G.; Prica, M.; Sokac, M.; Tadic, B. Application of Natrium Silicate as a Phase Change Material in Fixture Design. Teh. Vjesn.—Tech. Gaz. 2023, 30, 465–473. [Google Scholar] [CrossRef]
- Feng, Q.; Maier, W.; Braun, S.; Möhring, H.-C. Dynamic modeling of the workpiece-fixture contact behavior for intelligent fixture design. Procedia CIRP 2023, 119, 58–65. [Google Scholar] [CrossRef]
- Vukelic, D.; Simunovic, G.; Tadic, B.; Buchmeister, B.; Saric, T.; Simeunovic, N. Intelligent design and optimization of machining fixtures. Teh. Vjesn.—Tech. Gaz. 2016, 23, 1325–1334. [Google Scholar] [CrossRef]
- Fiedler, F.; Ehrenstein, J.; Höltgen, C.; Blondrath, A.; Schäper, L.; Göppert, A.; Schmitt, R. Jigs and fixtures in production: A systematic literature review. J. Manuf. Syst. 2024, 72, 373–405. [Google Scholar] [CrossRef]
- Halabi, O.; Halwani, Y. Design and implementation of haptic virtual fixtures for preoperative surgical planning. Displays 2018, 54, 9–19. [Google Scholar] [CrossRef]
- Jones, S.A.; Mayich, M.; Mayich, D.J.; Harrison, M.; Deluzio, K.J.; Mechefske, C.K. Design and evaluation of a novel fixture to optimally support a Lisfranc injured foot during CT scanning. Measurement 2013, 46, 3010–3018. [Google Scholar] [CrossRef]
- Naeem, A.; Pasha, R.A.; Muneeb, M. A novel milling fixture pallet system for production growth of alligator forceps: Design, manufacturing, and testing. Results Eng. 2022, 16, 100668. [Google Scholar] [CrossRef]
- Budak, I.; Mirkovic, S.; Sokac, M.; Santosi, Z.; Puskar, T.; Vukelic, D. An Approach to Modelling of Personalized Bone Grafts Based on Advanced Technologies. Int. J. Simul. Model. 2016, 15, 637–648. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, J.-E.; Kim, E.; Park, S.; Kang, I.; Kwon, Y.-D. Stability of the Implant–Alveolar Bone Complex According to the Peri-Implant Bone Loss and Bone Quality: A Finite Element Analysis Study. Appl. Sci. 2024, 14, 11674. [Google Scholar] [CrossRef]
- Burca, B.A.; Popa, C.F.; Galatanu, S.V. Numerical analysis of osteosynthesis plates used in the reconstruction of the mandible. Mater. Today Proc. 2022, 78, 337–342. [Google Scholar] [CrossRef]
- Moncayo-Matute, F.P.; Torres-Jara, P.B.; Vázquez-Silva, E.; Peña-Tapia, P.G.; Moya-Loaiza, D.P.; Abad-Farfán, G. Finite element analysis of a customized implant in PMMA coupled with the cranial bone. J. Mech. Behav. Biomed. Mater. 2023, 146, 106046. [Google Scholar] [CrossRef] [PubMed]
- Tatli, Z.; Bretcanu, O.; Çalışkan, F.; Dalgarno, K. Fabrication of porous apatite-wollastonite glass ceramics using a two steps sintering process. Mater. Today Commun. 2022, 30, 103216. [Google Scholar] [CrossRef]
- Kawachi, G.; Misumi, H.; Fujimori, H.; Goto, S.; Ohtsuki, C.; Kamitakahara, M.; Ioku, K. Fabrication of porous blocks of calcium phosphate through hydrothermal processing under glycine coexistence. J. Ceram. Soc. Japan 2010, 118, 559–563. [Google Scholar] [CrossRef]
- Okada, K.; Kameya, T.; Ishino, H.; Hayakawa, T. A novel technique for preparing dental CAD/CAM composite resin blocks using the filler press and monomer infiltration method. Dent. Mater. J. 2014, 33, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Putri, T.S.; Hayashi, K.; Ishikawa, K. Fabrication of three-dimensional interconnected porous blocks composed of robust carbonate apatite frameworks. Ceram. Int. 2020, 46, 20045–20049. [Google Scholar] [CrossRef]
- Blume, O.; Donkiewicz, P.; Back, M.; Born, T. Bilateral maxillary augmentation using CAD/CAM manufactured allogenic bone blocks for restoration of congenitally missing teeth: A case report. J. Esthet. Restor. Dent. 2019, 31, 171–178. [Google Scholar] [CrossRef]
- Blume, O.; Back, M.; Born, T.; Donkiewicz, P. Reconstruction of a Unilateral Alveolar Cleft Using a Customized Allogenic Bone Block and Subsequent Dental Implant Placement in an Adult Patient. J. Oral Maxillofac. Surg. 2019, 77, 2127.e1–2127.e11. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, S.; Alizargar, J.; Smith, F. Design and Analyses of Passive Continuous Distraction Osteogenesis Device for Oral and Maxillofacial Reconstruction. Appl. Sci. 2024, 14, 9279. [Google Scholar] [CrossRef]
- Yu, H.; Pistol, C.; Franklin, R.; Barborica, A. Clinical Accuracy of Customized Stereotactic Fixtures for Stereoelectroencephalography. World Neurosurg. 2018, 109, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, A.M.; Ashtankar, K.M. Sacrificial Support Fixture Optimisation for the CNC Rapid Machining of Medical Parts with Thin Ends. Trans. Famena. 2019, 43, 65–82. [Google Scholar] [CrossRef]
- Jindal, P.; Chaitanya; Bharadwaja, S.S.S.; Rattra, S.; Pareek, D.; Gupta, V.; Breedon, P.; Reinwald, Y.; Juneja, M. Optimizing cranial implant and fixture design using different materials in cranioplasty. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2023, 237, 107–121. [Google Scholar] [CrossRef]
- Sindhu, V.; Soundarapandian, S. Additive Manufacturing Fixture Box for Bone Measurement. Procedia Eng. 2017, 184, 1–9. [Google Scholar] [CrossRef]
- Bregoli, C.; Stacchiotti, F.; Fiocchi, J.; Ferrari, R.; Biffi, C.A.; Morellato, K.; Gruppioni, E.; Tuissi, A. A biomechanical study of osseointegrated patient-matched additively manufactured implant for treatment of thumb amputees. Med. Eng. Phys. 2023, 118, 104019. [Google Scholar] [CrossRef] [PubMed]
- Alizad, A.; Walch, M.; Greenleaf, J.F.; Fatemi, M. Vibrational Characteristics of Bone Fracture and Fracture Repair: Application to Excised Rat Femur. J. Biomech. Eng. 2006, 128, 300–308. [Google Scholar] [CrossRef]
- Pashley, J.; Blunt, L.; Bills, P.; Racasan, R. Development of fused metrology methods for the analysis of hip implant tribology. Surf. Topogr. Metrol. Prop. 2023, 11, 024003. [Google Scholar] [CrossRef]
- Bae, J.-S.; Jeong, H.-Y. Effects of material properties and hole designs of the jig on the fatigue life of dental implants. J. Mech. Sci. Technol. 2012, 26, 759–766. [Google Scholar] [CrossRef]
- Pertici, G.; Carinci, F.; Carusi, G.; Epistatus, D.; Villa, T.; Crivelli, F.; Rossi, F.; Perale, G. Composite Polymer-Coated Mineral Scaffolds for Bone Regeneration: From Material Characterization To Human Studies. J. Biol. Regul. Homeost. Agents. 2015, 29, 136–148. [Google Scholar] [PubMed]
- Ayhan, M. Evaluation of the Temperature Values in the Use of Different Types of Burs. Med. Rec. 2023, 5, 342–348. [Google Scholar] [CrossRef]
- Khasnis, N.; Dhatrak, P.; Kurup, A. A review on artificial bone modelling: Materials and manufacturing techniques. Mater. Today Proc. 2021, 39, 114–120. [Google Scholar] [CrossRef]
- Grottoli, C.F.; Cingolani, A.; Zambon, F.; Villa, T.; Perale, G. Simulated Performance of a Xenohybrid Bone Graft (SmartBone®) in the Treatment of Acetabular Prosthetic Reconstruction. J. Funct. Biomater. 2019, 10, 53. [Google Scholar] [CrossRef]
- Chen, S.; Espadas-Escalante, J.J.; Isaksson, P. Numerical analysis of crack path stability in brittle porous materials. Eng. Fract. Mech. 2022, 275, 108811. [Google Scholar] [CrossRef]
- Gumirova, V.; Razumovskaya, I.; Apel, P.; Bedin, S.; Naumov, A. The Influence of Mechanical Stress Micro Fields around Pores on the Strength of Elongated Etched Membrane. Membranes 2022, 12, 1168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.; Chen, Y.; Feng, W.; Chen, X. Novel Design and Finite Element Analysis of Diamond-like Porous Implants with Low Stiffness. Materials 2021, 14, 6918. [Google Scholar] [CrossRef] [PubMed]
- Cioată, V.G.; Alexa, V.; Rațiu, S.A. Study of the stiffness of modular fixtures using the finite element method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 393, 012036. [Google Scholar] [CrossRef]
- Tabar, R.S.; Zheng, H.; Litwa, F.; Paetzold-Byhain, K.; Lindkvist, L.; Wärmefjord, K.; Söderberg, R. Digital Twin-Based Clamping Sequence Analysis and Optimization for Improved Geometric Quality. Appl. Sci. 2024, 14, 510. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Espana, F.; Balla, V.K.; Bose, S.; Ohgami, Y.; Davies, N.M. Influence of Porosity on Mechanical Properties and In vivo Response of Ti6Al4V Implants. Acta Biomater. 2010, 6, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Castillo, S.M.; Torres, Y. Different models for simulation of mechanical behaviour of porous materials. J. Mech. Behav. Biomed. Mater. 2018, 80, 88–96. [Google Scholar] [CrossRef]
- Simske, S.J.; Ayers, R.A.; Bateman, T.A. Porous Materials for Bone Engineering. Mater. Sci. Forum. 1997, 250, 151–182. [Google Scholar] [CrossRef]
- Otawa, N.; Sumida, T.; Kitagaki, H.; Sasaki, K.; Fujibayashi, S.; Takemoto, M.; Nakamura, T.; Yamada, T.; Mori, Y.; Matsushita, T. Custom-made titanium devices as membranes for bone augmentation in implant treatment: Modeling accuracy of titanium products constructed with selective laser melting. J. Cranio-Maxillofac. Surg. 2015, 43, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, A.; Waran, V.; Solati-Hashjin, M.; Rahman, Z.A.A.; Asadi, M.; Osman, N.A.A. Effect of layer printing delay on mechanical properties and dimensional accuracy of 3D printed porous prototypes in bone tissue engineering. Ceram. Int. 2015, 41, 8320–8330. [Google Scholar] [CrossRef]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Mateos, A.; Casuso, M.; Rivero, A.; Ukar, E.; Lamikiz, A. Vibrations characterization in milling of low stiffness parts with a rubber-based vacuum fixture. Chin. J. Aeronaut. 2021, 34, 54–66. [Google Scholar] [CrossRef]
- Jelitto, H.; Schneider, G.A. Fracture toughness of porous materials—Experimental methods and data. Data Br. 2019, 23, 103709. [Google Scholar] [CrossRef] [PubMed]
- Axinte, D.; Guo, Y.; Liao, Z.; Shih, A.J.; M’Saoubi, R.; Sugita, N. Machining of biocompatible materials—Recent advances. CIRP Ann. 2019, 68, 629–652. [Google Scholar] [CrossRef]
- Kim, N.; Yang, B.; On, S.; Kwon, I.; Ahn, K.; Lee, J.; Byun, S. Customized three-dimensional printed ceramic bone grafts for osseous defects: A prospective randomized study. Sci. Rep. 2024, 14, 3397. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Li, X.; Fu, G.; Chen, D.; Huang, Y. Research on the dimensional accuracy of customized bone augmentation combined with 3D-printing individualized titanium mesh: A retrospective case series study. Clin. Implant Dent. Relat. Res. 2021, 23, 5–18. [Google Scholar] [CrossRef]





| Young Modulus E (GPa) | Poisson Ratio m | Density Q (kg/m3) | Source | |
|---|---|---|---|---|
| Steel plate (steel 316) | 192.9 | 0.27 | 8000 | * SW database |
| Bone block | 13.7 | 0.3 | 480 | [31,32] |
| PEEK disc | 3.9 | 0.4 | 1310 | SW database |
| Silicone tabs | 112.4 | 0.28 | 2330 | SW database |
| No. | Operations | Used Tool | Spindle Speed (rpm) | Feed Rate (mm/min) | Total Machining Time (min:s) |
|---|---|---|---|---|---|
| 1. | External rough Milling (one side) | end mill, ball tip D = 2 mm, L = 50 mm | 1000 | 1200 | 5:59 |
| 2. | External rough Milling (other side) | end mill, ball tip D = 2 mm, L = 50 mm | 1000 | 1200 | 10:54 |
| 3. | External finish Milling (one side) | end mill, ball tip D = 1 mm, L = 50 mm | 1000 | 1000 | 15:46 |
| 4. | External finish Milling (other side) | end mill, ball tip D = 1 mm, L = 50 mm | 1000 | 1000 | 20:15 |
| 5. | Profile milling | end mill, ball tip D = 0.6 mm, L = 50 mm | 1000 | 500 | 20:31 |
| 6. | External rough milling of connectors | end mill, ball tip D = 0.6 mm, L = 50 mm | 1000 | 600 | 21:13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokac, M.; Milosevic, A.; Santosi, Z.; Vukelic, D.; Budak, I. Design and Verification of a New Fixture for Machining of Porous Blocks for Medical CAD/CAM Systems. Appl. Sci. 2025, 15, 794. https://doi.org/10.3390/app15020794
Sokac M, Milosevic A, Santosi Z, Vukelic D, Budak I. Design and Verification of a New Fixture for Machining of Porous Blocks for Medical CAD/CAM Systems. Applied Sciences. 2025; 15(2):794. https://doi.org/10.3390/app15020794
Chicago/Turabian StyleSokac, Mario, Aleksandar Milosevic, Zeljko Santosi, Djordje Vukelic, and Igor Budak. 2025. "Design and Verification of a New Fixture for Machining of Porous Blocks for Medical CAD/CAM Systems" Applied Sciences 15, no. 2: 794. https://doi.org/10.3390/app15020794
APA StyleSokac, M., Milosevic, A., Santosi, Z., Vukelic, D., & Budak, I. (2025). Design and Verification of a New Fixture for Machining of Porous Blocks for Medical CAD/CAM Systems. Applied Sciences, 15(2), 794. https://doi.org/10.3390/app15020794







