The Potential of Bilberry and Blackcurrant Juices as a Source of Colorants in Intelligent Pectin Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fruit Juices
2.3. Film Preparation
2.4. Thickness
2.5. Color
2.6. Opacity
2.7. Mechanical Properties
2.8. Water Vapor Permeability (WVP)
2.9. Thermal Properties
2.10. pH Change
2.11. Morphology of the Film
2.12. Statistical Analysis
3. Results and Discussion
3.1. The Optical Properties of Film-Forming Solutions and Film Characteristics
3.2. The Effect of Apple Pectin and Fruit Juice Concentrations on the Optical Properties of Edible Films
3.3. The Effect of Apple Pectin and Fruit Juice Concentrations on the Mechanical Properties of Films
3.4. The Effect of Apple Pectin and Fruit Juice Concentrations on the Water Vapor Permeability of Films
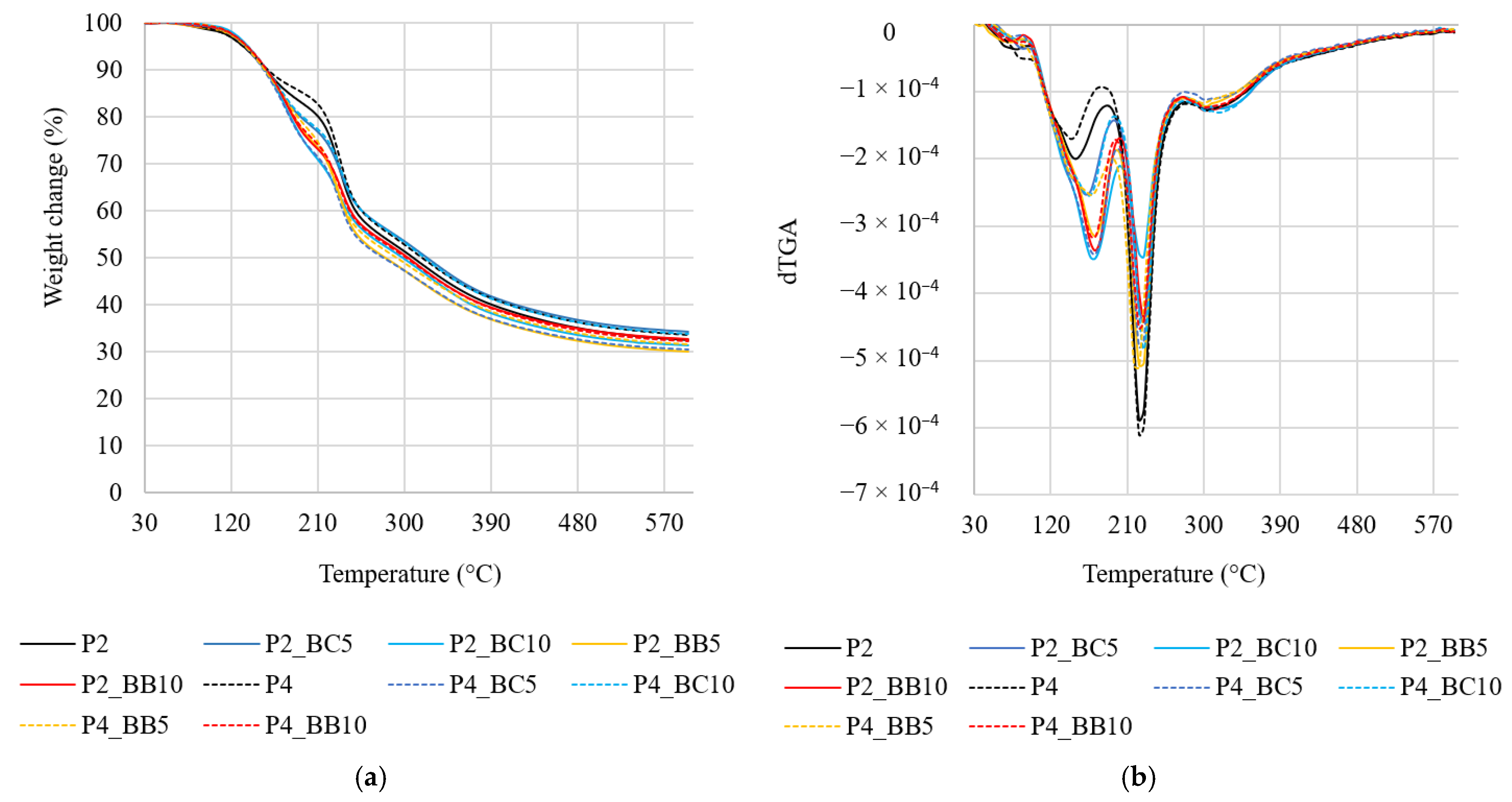
3.5. The Effect of Apple Pectin and Fruit Juice Concentrations on the Thermal Properties of Films
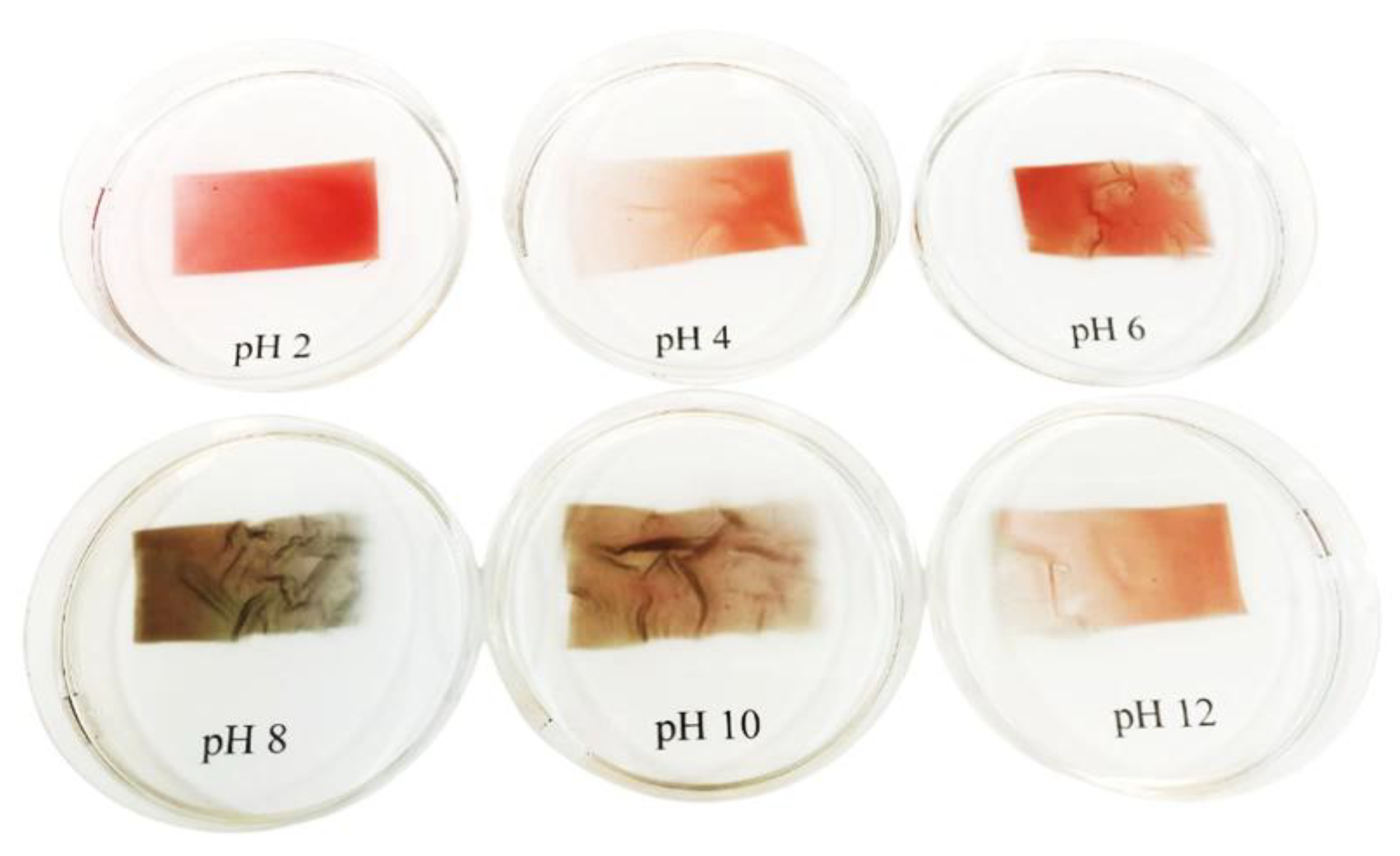
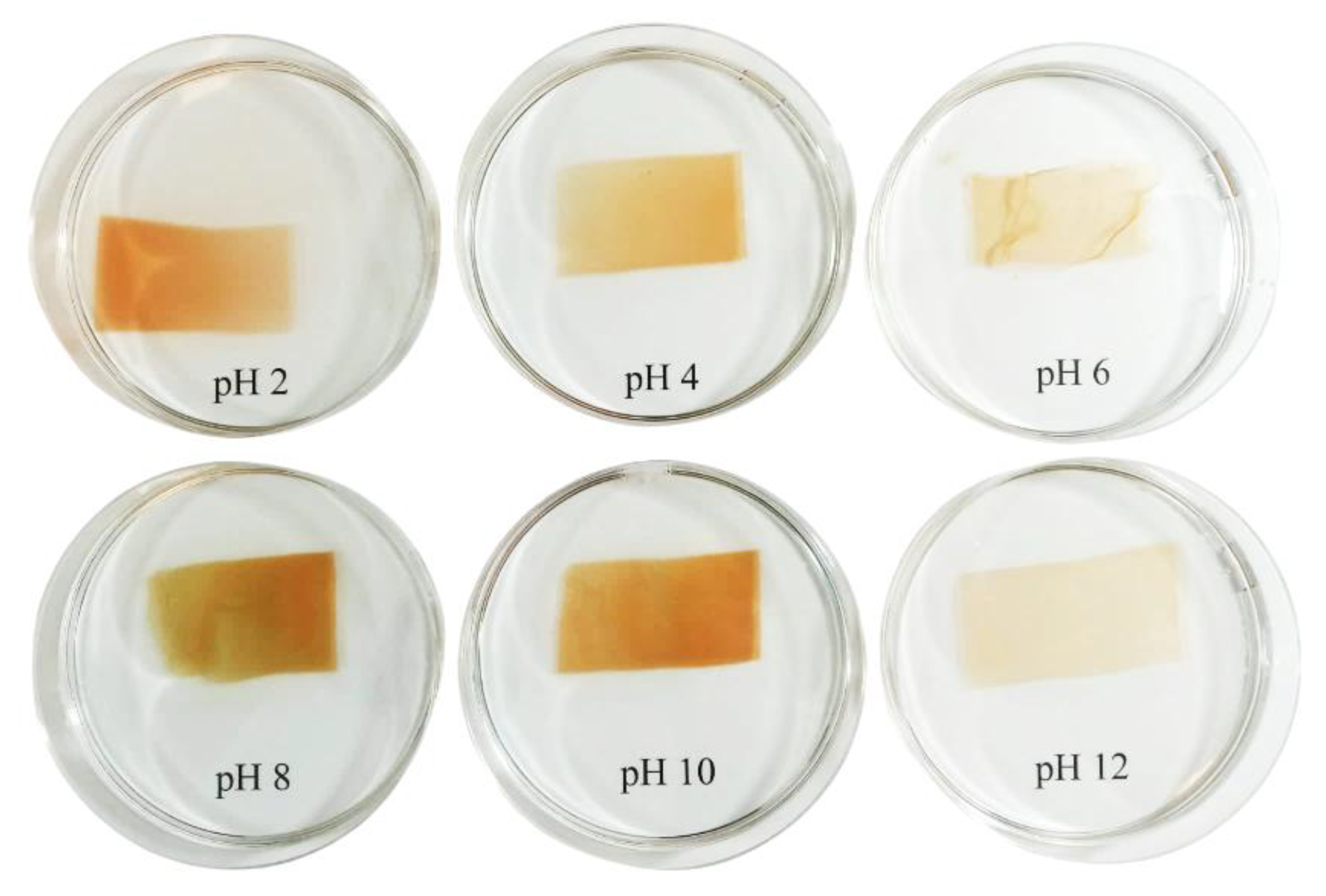
3.6. The Effect of Apple Pectin and Fruit Juice Concentrations on the Visual Changes of Films Under pH
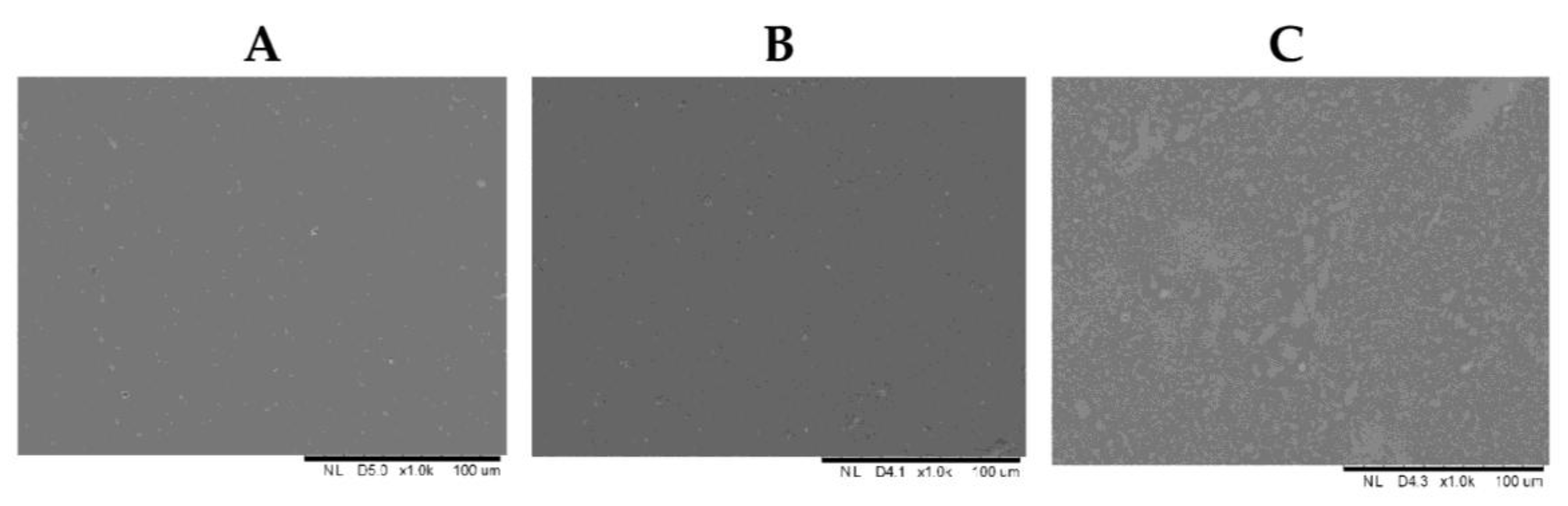
3.7. The Effect of Fruit Juices on the Morphology of the Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 23 September 2025).
- Talib, A.; Samad, A.; Hossain, M.J.; Muazzam, A.; Anwar, B.; Atique, R.; Hwang, Y.-H.; Joo, S.-T. Modern Trends and Techniques for Food Preservation. Food Life 2024, 2024, 19–32. [Google Scholar] [CrossRef]
- Khan, S.; Monteiro, J.K.; Prasad, A.; Filipe, C.D.M.; Li, Y.; Didar, T.F. Material Breakthroughs in Smart Food Monitoring: Intelligent Packaging and On-Site Testing Technologies for Spoilage and Contamination Detection. Adv. Mater. 2024, 36, e2300875. [Google Scholar] [CrossRef]
- Mkhari, T.; Adeyemi, J.O.; Fawole, O.A. Recent Advances in the Fabrication of Intelligent Packaging for Food Preservation: A Review. Processes 2025, 13, 539. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Xiang, H.; Yu, D.; Gao, M.; Yan, R.; Zhang, D. A Nature PH Indicator with High Colorimetric Response Sensitivity for Pork Freshness Monitoring. Food Biosci. 2024, 57, 103519. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Ren, D.; Wu, X.; Xu, D. Improved Sensitivity of Freshness Indicator Based on Purple Sweet Potato Anthocyanins through PH Optimization and Its Application in Flesh Food Monitoring during Logistics. Innov. Food Sci. Emerg. Technol. 2025, 100, 103929. [Google Scholar] [CrossRef]
- Nastasi, J.R.; Hay, T.O.; Fitzgerald, M.A.; Kontogiorgos, V. Design and Evaluation of PH-Sensitive Pectin Films Infused with Anthocyanin-Rich Extracts from Australian Native Fruits for Intelligent Food Packaging Applications. Food Biophys. 2025, 20, 1. [Google Scholar] [CrossRef]
- Teixeira, S.C.; de Oliveira, T.V.; Assis Silva, R.R.; Ribeiro, A.R.C.; Stringheta, P.C.; Rigolon, T.C.B.; Pinto, M.R.M.R.; Soares, N. de F.F. Colorimetric Indicators of Açaí Anthocyanin Extract in the Biodegradable Polymer Matrix to Indicate Fresh Shrimp. Food Biosci. 2022, 48, 101808. [Google Scholar] [CrossRef]
- Shi, S.; Xu, X.; Feng, J.; Ren, Y.; Bai, X.; Xia, X. Preparation of NH3- and H2S-Sensitive Intelligent PH Indicator Film from Sodium Alginate/Black Soybean Seed Coat Anthocyanins and Its Use in Monitoring Meat Freshness. Food Packag. Shelf Life 2023, 35, 100994. [Google Scholar] [CrossRef]
- Kwak, M.; Min, S.C. Monitoring Meat Freshness with Intelligent Colorimetric Labels Containing Red Cabbage Anthocyanins Copigmented with Gelatin and Gallic Acid. Foods 2024, 13, 3464. [Google Scholar] [CrossRef]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Chapter 17—Spoilage Bacteria and Meat Quality. In Meat Quality Analysis; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 307–334. ISBN 978-0-12-819233-7. [Google Scholar]
- Shaik, M.I.; Azhari, M.F.; Sarbon, N.M. Gelatin-Based Film as a Color Indicator in Food-Spoilage Observation: A Review. Foods 2022, 11, 3797. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, L.R.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Towards Intelligent Packaging: BCP-EVOH@ Optode for Milk Freshness Measurement. Talanta 2022, 241, 123230. [Google Scholar] [CrossRef]
- Cherian, E.; TS, K.; KN, S.; KS, A.; Poothicote, N.G. Investigation into Pectin Extraction and Technological Implementations in the Food Industry. J. Sci. Food Agric. 2024, 104, 9102–9110. [Google Scholar] [CrossRef]
- Syarifuddin, A.; Muflih, M.H.; Izzah, N.; Fadillah, U.; Ainani, A.F.; Dirpan, A. Pectin-Based Edible Films and Coatings: From Extraction to Application on Food Packaging towards Circular Economy—A Review. Carbohydr. Polym. Technol. Appl. 2025, 9, 100680. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Dai, J.; Cui, H.; Lin, L. Edible Films of Pectin Extracted from Dragon Fruit Peel: Effects of Boiling Water Treatment on Pectin and Film Properties. Food Hydrocoll. 2024, 147, 109324. [Google Scholar] [CrossRef]
- Ke, F.; Yang, M.; Ji, W.; Liu, D. Functional PH-Sensitive Film Based on Pectin and Whey Protein for Grape Preservation and Shrimp Freshness Monitoring. Food Chem. 2025, 463, 141092. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Al-Ola, K.A.A.; Al-Senani, G.M. Infiltration of Anthocyanidin and Cellulose-Reinforced Polylactic Acid into Delignified Wood toward Smart Packaging. Sustain. Chem. Pharm. 2024, 42, 101800. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting Antimicrobial Packaging Films and Measuring Their Physical Properties and Antimicrobial Activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- ASTM D882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2002. Available online: https://store.astm.org/d0882-02.html (accessed on 4 October 2025).
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity Homogeneity and Structure Affect Water Vapor Permeability of Model Edible Films. J. Food Sci. 1993, 58, 426–429. [Google Scholar] [CrossRef]
- Mikus, M.; Galus, S.; Ciurzyńska, A.; Janowicz, M. Development and Characterization of Novel Composite Films Based on Soy Protein Isolate and Oilseed Flours. Molecules 2021, 26, 3738. [Google Scholar] [CrossRef] [PubMed]
- Mujanović, I.; Balijagić, J.; Bajagić, M.; Poštić, D.; Đurović, S. Variations in Polyphenol Content and Anthocyanin Composition in Bilberry Populations (Vaccinium myrtillus L.) Due to Environmental Factors. J. Food Compos. Anal. 2024, 136, 106732. [Google Scholar] [CrossRef]
- Wagner, A.; Dussling, S.; Nowak, A.; Zimmermann, L.; Bach, P.; Ludwig, M.; Kumar, K.; Will, F.; Schweiggert, R.; Steingass, C.B. Investigations into the Stability of Anthocyanins in Model Solutions and Blackcurrant Juices Produced with Various Dejuicing Technologies. Eur. Food Res. Technol. 2023, 249, 1771–1784. [Google Scholar] [CrossRef]
- Giusti, M.M.; Gordillo, B.; González-Miret, M.L. Color Analysis. In Nielsen’s Food Analysis; Ismail, B.P., Nielsen, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 509–522. ISBN 978-3-031-50643-7. [Google Scholar]
- Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Repajić, M.; Klepac, D.; Valić, S.; Debeaufort, F.; Galić, K. Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material. Molecules 2021, 26, 2569. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Z.; Huo, R.; Cui, Z. Preparation of an Indicator Film Based on Pectin, Sodium Alginate, and Xanthan Gum Containing Blueberry Anthocyanin Extract and Its Application in Blueberry Freshness Monitoring. Heliyon 2023, 9, e14421. [Google Scholar] [CrossRef] [PubMed]
- Medeiros Silva, V.D.; Coutinho Macedo, M.C.; Rodrigues, C.G.; Neris dos Santos, A.; de Freitas e Loyola, A.C.; Fante, C.A. Biodegradable Edible Films of Ripe Banana Peel and Starch Enriched with Extract of Eriobotrya Japonica Leaves. Food Biosci. 2020, 38, 100750. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of Gelatin and Casein Additions on Starch Edible Biodegradable Films for Fruit Surface Coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Leocádio, J.; Mendes, C.V.T.; Cardeira, M.; Fernández, N.; Matias, A.; Carvalho, M.G.V.S.; Braga, M.E.M. Biodegradable Film Production from Agroforestry and Fishery Residues with Active Compounds. Food Packag. Shelf Life 2021, 28, 100661. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical Properties of Chitosan Films Incorporated with Natural Antioxidants. Ind. Crop. Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Wu, Q.; Gu, Y.; Kan, J.; Jin, C. Effect of Protocatechuic Acid Incorporation on the Physical, Mechanical, Structural and Antioxidant Properties of Chitosan Film. Food Hydrocoll. 2017, 73, 90–100. [Google Scholar] [CrossRef]
- Nazari, M.; Matusiak, B.; Yoshizawa, N.; Takase, K. Comparative Study of Color Shift Metrics in Architectural Lighting: Impact of Tinted Glazing on View Color Perception. LEUKOS 2025, 2515558. [Google Scholar] [CrossRef]
- Datta, D.; Mahto, S.; Das, B. Biodegradability and Property Assessment of Different Categorized Polymeric Films. Mater. Today Proc. 2021, 43, 2014–2024. [Google Scholar] [CrossRef]
- Sahoo, R.; Sundara, R.; Venkatachalam, S. Morphology Dependent EMI Shielding Performance of Ag-Ni Core-Shell Nanowires. J. Alloys Compd. 2024, 981, 173693. [Google Scholar] [CrossRef]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-Isothermal Pyrolysis of Pectin: A Thermochemical and Kinetic Approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Oancea, S. A Review of the Current Knowledge of Thermal Stability of Anthocyanins and Approaches to Their Stabilization to Heat. Antioxidants 2021, 10, 1337. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Lezama, A.Y.; Castaño, J.; Velázquez, G.; Carrillo-Navas, H.; Alvarez-Ramírez, J. Effect of Nopal Mucilage Addition on Physical, Barrier and Mechanical Properties of Citric Pectin-Based Films. J. Food Sci. Technol. 2018, 55, 3739–3748. [Google Scholar] [CrossRef] [PubMed]
- Gharanjig, H.; Iri, M.; Hosseinnezhad, M.; Gharanjig, K.; Jafari, S.M. Enhanced Thermal Stability of Anthocyanins through Natural Polysaccharides from Angum Gum and Cress Seed Gum. J. Food Sci. 2022, 87, 585–598. [Google Scholar] [CrossRef]
- Wang, C.; Cai, W.-D.; Yao, J.; Wu, L.-X.; Li, L.; Zhu, J.; Yan, J.-K. Conjugation of Ferulic Acid onto Pectin Affected the Physicochemical, Functional and Antioxidant Properties. J. Sci. Food Agric. 2020, 100, 5352–5362. [Google Scholar] [CrossRef]
- De Oliveira, A.C.S.; Ferreira, L.F.; de Oliveira Begali, D.; Ugucioni, J.C.; de Sena Neto, A.R.; Yoshida, M.I.; Borges, S.V. Thermoplasticized Pectin by Extrusion/Thermo-Compression for Film Industrial Application. J. Polym. Environ. 2021, 29, 2546–2556. [Google Scholar] [CrossRef]
- Wani, K.M.; Uppaluri, R.V.S. Characterization of Pectin Extracted from Pomelo Peel Using Pulsed Ultrasound Assisted Extraction and Acidic Hot Water Extraction Process. Appl. Food Res. 2023, 3, 100345. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of Characterization and Antioxidant Activity of Different Citrus Peel Pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Vieira, R.P.; Jamróz, E.; Anjos, C.A.R. Furcellaran: An Innovative Biopolymer in the Production of Films and Coatings. Carbohydr. Polym. 2021, 252, 117221. [Google Scholar] [CrossRef]
- Ahmad, N.; Tayyeb, D.; Ali, I.; Alruwaili, N.K.; Ahmad, W.; ur Rehman, A.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548. [Google Scholar] [CrossRef]
- Wu, L.-T.; Tsai, I.-L.; Ho, Y.-C.; Hang, Y.-H.; Lin, C.; Tsai, M.-L.; Mi, F.-L. Active and Intelligent Gellan Gum-Based Packaging Films for Controlling Anthocyanins Release and Monitoring Food Freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef]

| Film | Pectin (P) (%) | Glycerol (%) | Blackcurrant (BC) (%) | Bilberry (BB) (%) |
|---|---|---|---|---|
| P2 | 2 | 50 | - | - |
| P2_BC5 | 2 | 50 | 5 | - |
| P2_BC10 | 2 | 50 | 10 | - |
| P2_BB5 | 2 | 50 | - | 5 |
| P2_BB10 | 2 | 50 | - | 10 |
| P4 | 4 | 50 | - | - |
| P4_BC5 | 4 | 50 | 5 | - |
| P4_BC10 | 4 | 50 | 10 | - |
| P4_BB5 | 4 | 50 | - | 5 |
| P4_BB10 | 4 | 50 | - | 10 |
| Sample | L* | a* | b* |
|---|---|---|---|
| P2 | 21.50 ± 0.03 k | −0.11 ± 0.07 a | 7.80 ± 0.05 k |
| P2_BC5 | 12.10 ± 0.05 h | 16.30 ±0.11 i | 4.70 ± 0.12 g |
| P2_BC10 | 8.65 ± 0.07 f | 17.90 ±0.20 j | 5.50 ± 0.10 h |
| P2_BB5 | 6.98 ± 0.06 e | 11.20 ± 0.18 f | 2.39 ± 0.13 d |
| P2_BB10 | 4.84 ± 0.07 c | 8.26 ± 0.20 e | 2.01 ± 0.10 c |
| P4 | 25.80 ± 0.03 l | 1.44 ± 0.06 c | 12.20 ± 0.11 l |
| P4_BC5 | 11.40 ± 0.05 g | 18.20 ± 0.05 k | 6.82 ± 0.08 i |
| P4_BC10 | 6.40 ± 0.10 d | 20.70 ± 0.13 l | 7.40 ± 0.16 j |
| P4_BB5 | 15.90 ± 0.07 j | 13.90 ± 0.15 h | 3.01 ± 0.15 e |
| P4_BB10 | 12.40 ± 0.05 i | 11.70 ± 0.19 g | 3.19 ± 0.14 f |
| Blackcurrant juice (BC) | 2.70 ± 0.06 b | 4.70 ± 0.22 d | 0.86 ± 0.11 b |
| Bilberry juice (BB) | 1.31 ± 0.05 a | 0.36 ±0.16 b | 0.26 ± 0.07 a |
| Film | L* | a* | b* | ΔE | O (a.u./mm) |
|---|---|---|---|---|---|
| P2 | 92.20 ± 1.39 f | 0.02 ± 0.37 a | 11.90 ± 3.23 ef | - | 6.93 ± 1.25 b |
| P2_BC5 | 79.60 ± 3.46 e | 16.40 ± 2.87 c | 10.90 ± 0.83 def | 20.69 ± 4.05 b | 4.56 ± 1.09 a |
| P2_BC10 | 69.80 ± 4.71 d | 32.10 ± 3.56 de | 8.66 ± 1.39 cd | 39.24 ± 5.67 a | 5.48 ± 1.31 ab |
| P2_BB5 | 61.50 ± 5.20 c | 34.50 ± 2.94 e | 2.95 ± 1.33 a | 47.07 ± 5.44 a | 6.17 ± 1.62 b |
| P2_BB10 | 49.60 ± 1.77 a | 41.60 ± 2.49 f | 9.33 ± 1.60 cde | 59.59 ± 2.09 b | 12.90 ± 1.54 d |
| P4 | 91.80 ± 0.70 f | −0.41 ± 0.08 a | 12.9 ± 1.97 f | - | 6.69 ± 1.36 b |
| P4_BC5 | 89.30 ± 1.37 f | 5.49 ± 1.06 b | 7.48 ± 0.95 bc | 8.41 ± 1.44 a | 3.92 ± 0.48 a |
| P4_BC10 | 81.50 ± 2.34 e | 16.40 ± 2.34 c | 9.02 ± 2.16 cde | 20.09 ± 3.37 b | 5.78 ± 1.36 b |
| P4_BB5 | 70.90 ± 2.93 d | 28.30 ± 2.74 d | 4.58 ± 0.38 ab | 36.60 ± 4.28 c | 4.08 ± 1.10 a |
| P4_BB10 | 55.60 ± 2.05 b | 41.60 ± 0.62 f | 8.37 ± 0.63 cd | 55.70 ± 1.91 d | 9.78 ± 1.31 c |
| Film | Tensile Strength (MPa) | Elongation at Break (%) | Modul Younga (MPa) |
|---|---|---|---|
| P2 | 1.97 ± 1.02 ab | 12.87 ± 1.92 ab | 71.23 ± 7.94 bc |
| P2_BC5 | 2.07 ± 1.40 ab | 11.90 ± 0.83 a | 62.84 ± 9.54 a |
| P2_BC10 | 2.90 ± 0.53 ab | 12.59 ± 0.67 ab | 67.69 ± 6.28 ab |
| P2_BB5 | 1.10 ± 0.91 a | 12.92 ± 0.39 ab | 64.59 ± 6.63 a |
| P2_BB10 | 1.22 ± 0.41 ab | 12.92 ± 1.17 ab | 67.38 ± 2.99 ab |
| P4 | 7.28 ± 0.82 d | 17.0 ± 2.67 b | 88.43 ± 13.88 c |
| P4_BC5 | 5.34 ± 0.44 cd | 15.40 ± 0.85 ab | 81.77 ± 3.45 bc |
| P4_BC10 | 3.87 ± 0.86 ab | 15.41 ± 2.09 ab | 71.59 ± 8.73 bc |
| P4_BB5 | 2.30 ± 1.25 ab | 16.74 ± 0.56 b | 69.23 ± 3.87 bc |
| P4_BB10 | 2.44 ± 1.04 ab | 16.40 ± 0.51 b | 69.06 ± 5.93 bc |
| Film | WVP (·10−11 g/m·s·Pa) |
|---|---|
| P2 | 7.43 ± 0.54 a |
| P2_BC5 | 20.54 ± 0.32 abc |
| P2_BC10 | 23.39 ± 5.48 bc |
| P2_BB5 | 21.37 ± 2.53 bc |
| P2_BB10 | 27.98 ± 3.76 c |
| P4 | 11.51 ± 0.32 ab |
| P4_BC5 | 23.09 ± 8.65 bc |
| P4_BC10 | 31.21 ± 6.86 c |
| P4_BB5 | 21.37 ± 2.53 bc |
| P4_BB10 | 23.64 ± 7.28 bc |
| Film | 1st Stage 0–100 °C | 2nd Stage 100–200 °C | 3th Stage 200–280 °C | 4th Stage 280–600 °C | ||||
|---|---|---|---|---|---|---|---|---|
| T (°C) | WL (%) | T (°C) | WL (%) | T (°C) | WL (%) | T (°C) | WL (%) | |
| P2 | 67.97 | 0.93 | 147.16 | 6.60 | 227.58 | 15.04 | 318.34 | 14.99 |
| P2_BC5 | 84.53 | 4.47 | 162.88 | 11.51 | 229.74 | 9.93 | 337.55 | 17.22 |
| P2_BC10 | 64.19 | 1.62 | 170.66 | 17.01 | 229.22 | 5.70 | 335.85 | 15.67 |
| P2_BB5 | 62.62 | 2.47 | 171.02 | 13.37 | 228.42 | 12.12 | 321.51 | 15.17 |
| P2_BB10 | 64.25 | 1.73 | 171.89 | 16.15 | 230.34 | 8.45 | 316.30 | 17.05 |
| P4 | 80.38 | 2.99 | 143.57 | 3.20 | 227.59 | 15.91 | 321.47 | 15.64 |
| P4_BC5 | 64.17 | 1.66 | 171.66 | 17.19 | 227.91 | 10.11 | 335.68 | 14.35 |
| P4_BC10 | 78.32 | 1.68 | 165.04 | 12.56 | 230.66 | 10.61 | 332.23 | 16.29 |
| P4_BB5 | 79.42 | 3.67 | 168.27 | 10.85 | 222.64 | 12.03 | 337.62 | 14.41 |
| P4_BB10 | 79.81 | 1.52 | 170.07 | 15.28 | 227.74 | 10.30 | 321.49 | 17.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakulska, A.; Mikus, M.; Karwacka, M.; Galus, S. The Potential of Bilberry and Blackcurrant Juices as a Source of Colorants in Intelligent Pectin Films. Appl. Sci. 2025, 15, 10789. https://doi.org/10.3390/app151910789
Pakulska A, Mikus M, Karwacka M, Galus S. The Potential of Bilberry and Blackcurrant Juices as a Source of Colorants in Intelligent Pectin Films. Applied Sciences. 2025; 15(19):10789. https://doi.org/10.3390/app151910789
Chicago/Turabian StylePakulska, Anna, Magdalena Mikus, Magdalena Karwacka, and Sabina Galus. 2025. "The Potential of Bilberry and Blackcurrant Juices as a Source of Colorants in Intelligent Pectin Films" Applied Sciences 15, no. 19: 10789. https://doi.org/10.3390/app151910789
APA StylePakulska, A., Mikus, M., Karwacka, M., & Galus, S. (2025). The Potential of Bilberry and Blackcurrant Juices as a Source of Colorants in Intelligent Pectin Films. Applied Sciences, 15(19), 10789. https://doi.org/10.3390/app151910789








