X-Ray Absorption and Emission Spectroscopy in Pharmaceutical Applications: From Local Atomic Structure Elucidation to Protein-Metal Complex Analysis—A Review
Abstract
1. Introduction
2. X-Ray Spectroscopy Techniques
2.1. X-Ray Absorption Spectroscopy
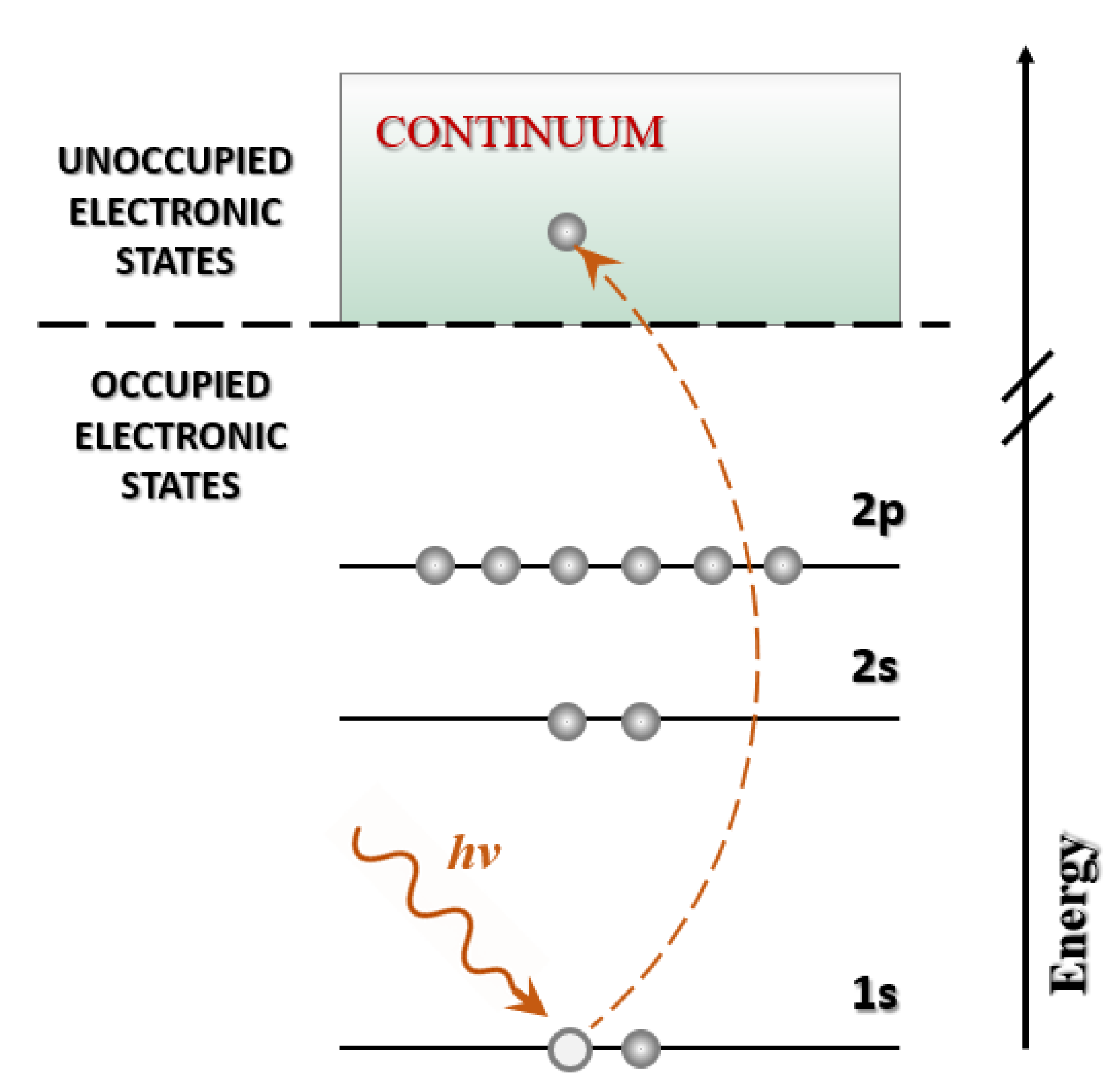
2.1.1. Fundamental Principles of XAS
2.1.2. X-Ray Absorption Edges and Interaction Mechanisms
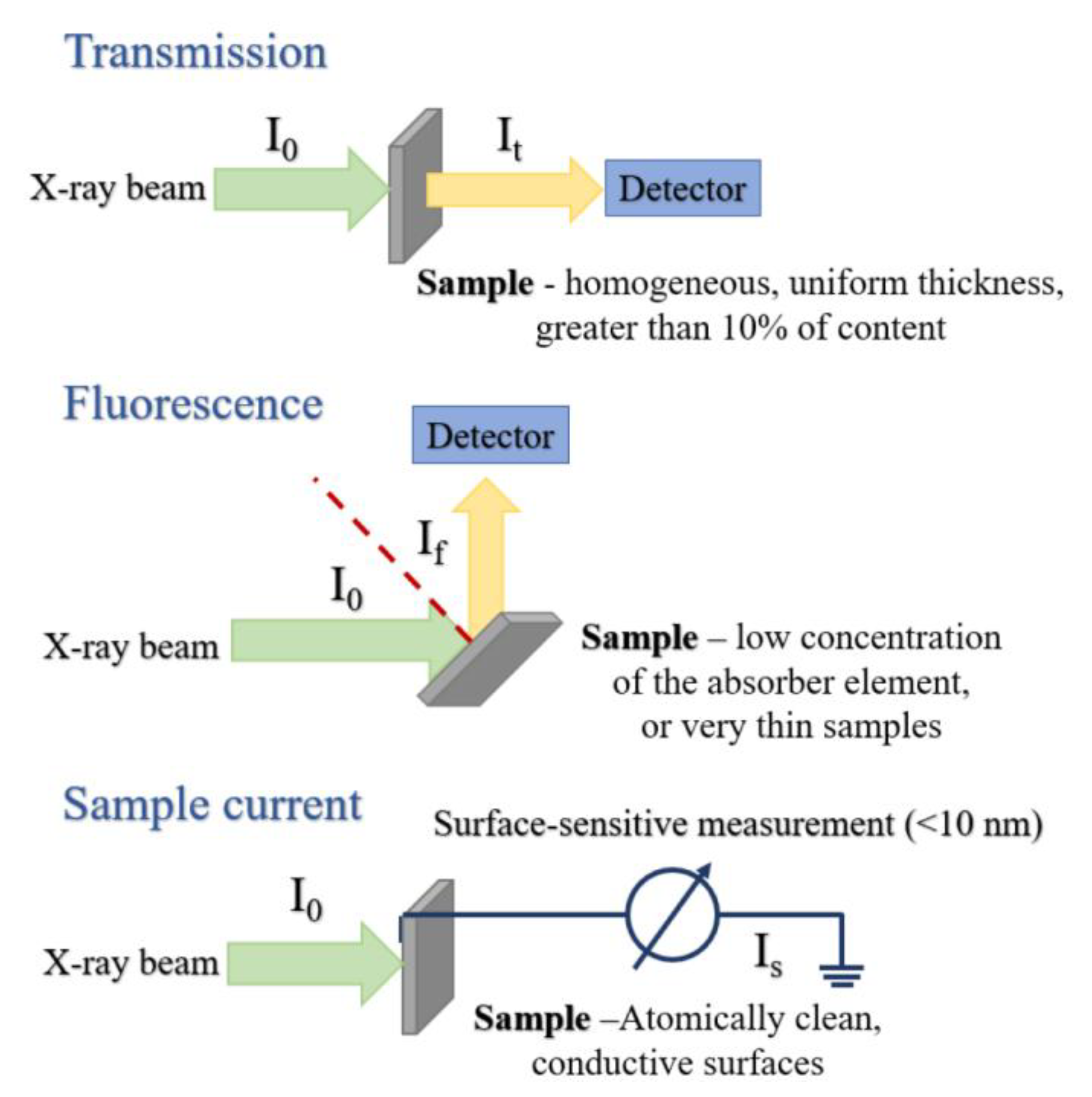
2.1.3. Measurement Modes: Transmission, Fluorescence, and Electron Yield
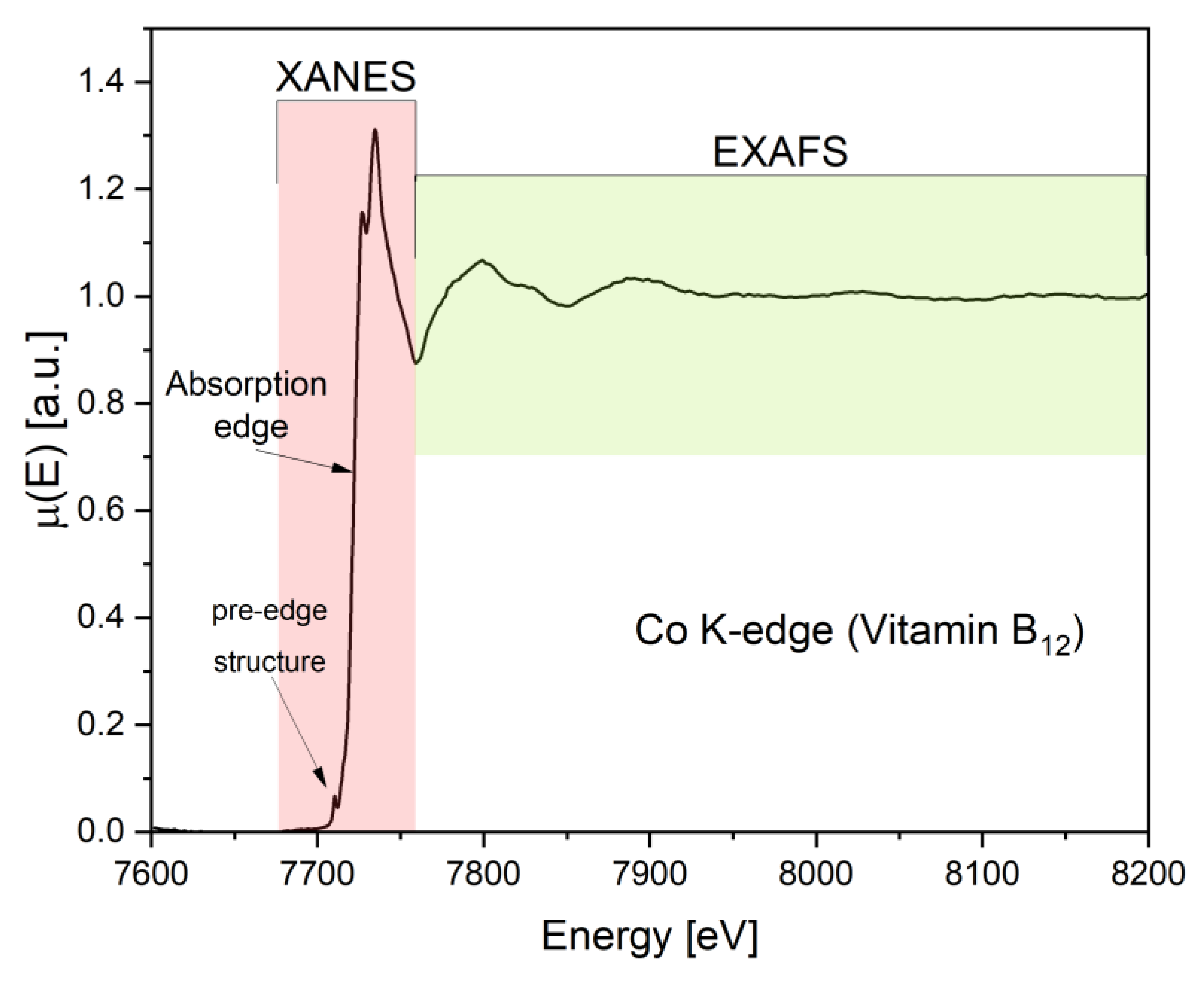
2.1.4. XAFS: Spectral Features and Structural Interpretation
2.2. X-Ray Emission Spectroscopy
2.2.1. Fundamental Principles and Measurement Setup
2.2.2. Emission Lines and Spectral Interpretation
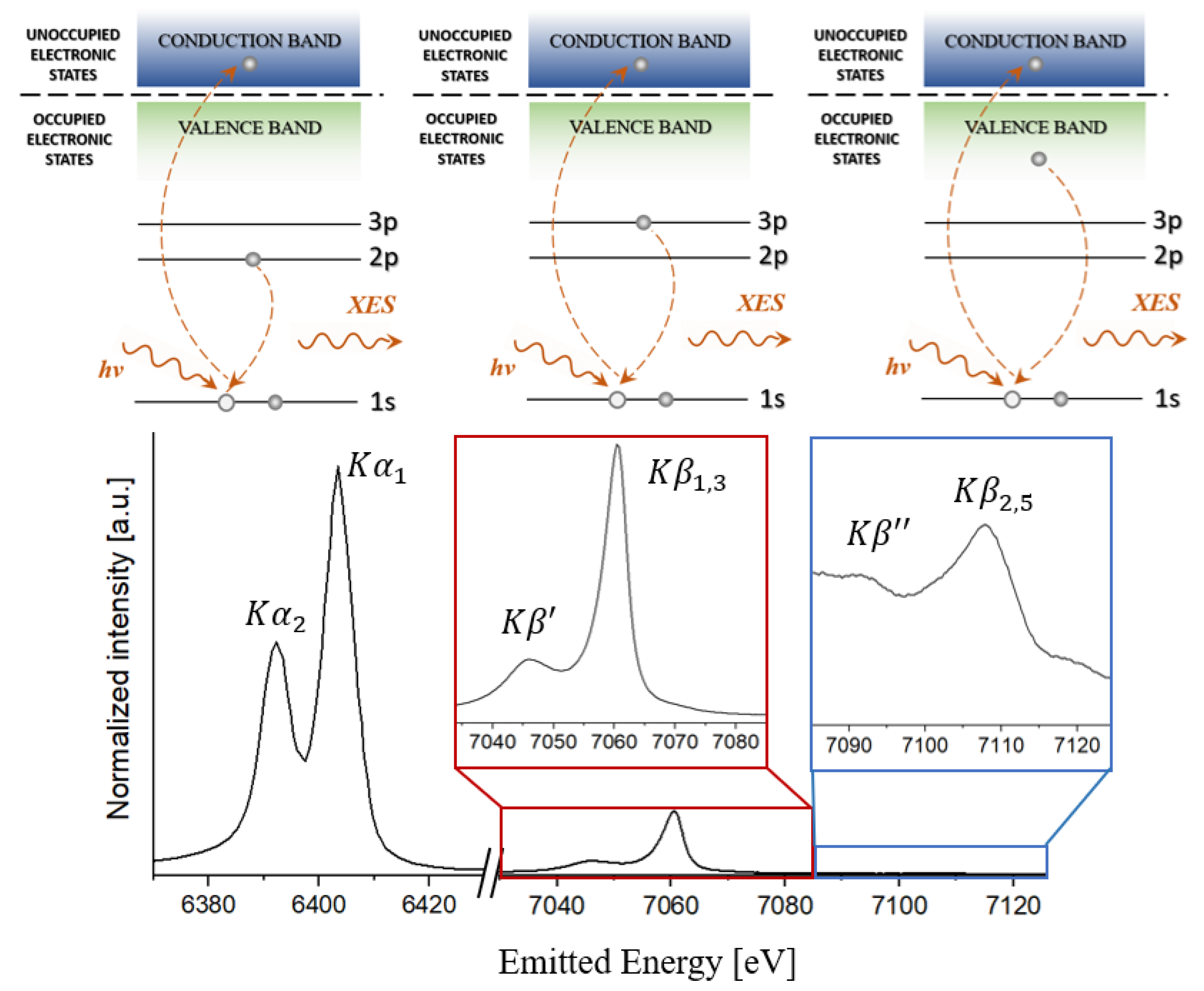
2.2.3. Applications XES in Pharmaceutical and Bioinorganic Systems
2.3. X-Ray Sources
2.4. How to Choose the Appropriate X-Ray Energy Regime
2.5. Research Objectives
3. Applications of X-Ray Spectroscopy in Pharmaceutical Sciences
3.1. Structural and Physicochemical Characterization of Drug
3.1.1. Crystalline Polymorphism and Solid-State Forms
3.1.2. Metal-Containing Pharmaceutical Compounds
3.2. Investigation of Differences in Drug Activity
3.2.1. Neurological Drugs (Cu-Targeting Chelators for Alzheimer’s Disease)
3.2.2. Anticancer Drugs (Rh and Ru Complexes)
3.3. Metal-Based Drug Interactions with Biomolecules
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| XAS | X-ray absorption spectroscopy |
| XES | X-ray emission spectroscopy |
| APIs | active pharmaceutical ingredients |
| RIXS | resonant inelastic X-ray scattering |
| RXES | resonant X-ray emission spectroscopy |
| LC-MS/MS | chromatography–mass spectrometry/mass spectrometry |
| NMR | nuclear magnetic resonance |
| NIR | near-infrared spectroscopy |
| XRD | X-ray diffraction |
| GC | gas chromatography |
| I0 | incident intensity |
| It | intensity of radiation after it passes through the sample (transmission) |
| If | intensity of the characteristic X-ray radiation (fluorescence) |
| Is | intensity of the sample current |
| TFY | total fluorescence yield |
| PFY | partial fluorescence yield |
| TEY | total electron yield |
| PEY | partial electron yield |
| UHV | ultra-high vacuum |
| XANES | X-ray absorption near-edge structure |
| EXAFS | Extended X-ray Absorption Fine Structure |
| XAFS | X-ray Absorption Fine Structure |
| RDF | pseudoradial distribution function |
| HERFD-XAS | high-energy resolution fluorescence-detected X-ray absorption spectroscopy |
| VtC | valence-to-core |
| LCF | linear combination fitting |
| E0 | absorption edge energy |
| BRH-HCl | bromhexine hydrochloride |
| FPC | ferric pyrophosphate citrate |
| SEP | soluble ferric pyrophosphate |
| APP | amyloid precursor protein |
| SSRL | Stanford Synchrotron Radiation Lightsource |
| BL | beamline |
| A549 | lung cancer cells |
| HR-XAS | high-resolution X-ray absorption spectroscopy |
| DOS | density of states |
References
- Zacharis, C.K.; Markopoulou, C.K. Recent Trends in Pharmaceutical Analytical Chemistry. Molecules 2020, 25, 3560. [Google Scholar] [CrossRef] [PubMed]
- Shinde, N.; Thakare, S.; Wagh, V.; Gurav, S.; Pawar, G.; Mali, S. Analytical Techniques in Pharmaceutical Analysis. Int. J. Pharm. Sci. 2024, 2, 1843–1853. [Google Scholar] [CrossRef]
- Wishart, D. NMR Spectroscopy and Protein Structure Determination: Applications to Drug Discovery and Development. Curr. Pharm. Biotechnol. 2005, 6, 105–120. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Yan, K.; Wei, Y.; Zhang, Q.; Lu, F.; Yan, Z. Real-time Quantitative Monitoring of Synthesis Process of Clevidipine Butyrate Using Raman Spectroscopy. Chin. J. Anal. Chem. 2017, 45, E1701–E1708. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2013, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Timoshenko, J.; Roldan Cuenya, B. In situ/Operando Electrocatalyst Charakterization by X-ray Absorpion Spectroscopy. Chem. Rev. 2021, 121, 882–961. [Google Scholar] [CrossRef]
- Czapla-Masztafiak, J.; Kwiatek, W.M.; Sá, J.; Szlachetko, J. X-Ray Spectroscopy on Biological Systems. In X-Ray Scattering; Ares, A.E., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Newville, M. Fundamental of XAFS. Rev. Mineral. Geochem. 2004, 78, 33–74. [Google Scholar] [CrossRef]
- Sá, J. High-Resolution XAS/XES: Analyzing Electronic Structuresof Catalysis; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Schnohr, C.S.; Ridgway, M.C. Introduction to X-Ray Absorption Spectroscopy. In X-Ray Absorption Spectroscopy of Semiconductors; Rhodes, W.T., Ed.; Springer Series in Optical Sciences; Springer: Atlanta, GA, USA, 2014; Volume 190, pp. 1–26. [Google Scholar]
- Takahara, A.; Higaki, Y.; Hirai, T.; Ishige, R. Application of Synchrotron Radiation X-ray Scattering and Spectroscopy to Soft Matter. Polymers 2020, 12, 1624. [Google Scholar] [CrossRef]
- Farges, F.; Wilke, M. Planetary, Geological and Environmental Sciences. In X-Ray Absorption and X-Ray Emission Spectroscopy Theory and Applications; Van Bokhoven, J.A., Lamberti, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Gianolio, D. How to Start an XAS Experiment. In X-Ray Absorption and X-Ray Emission Spectroscopy Theory and Applications; Van Bokhoven, J.A., Lamberti, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Błachucki, W.; Hoszowska, J.; Dousse, J.-C.; Kayser, Y.; Stachura, R.; Tyrała, K.; Wojtaszek, K.; Sá, J.; Szlachetko, J. High energy resolution off-resonant spectroscopy: A review. Spectrochim. Acta Part B At. Spectrosc. 2017, 136, 23–33. [Google Scholar] [CrossRef]
- Ravel, B. Understanding self-absorption in fluorescence XAS. In Proceedings of the Advanced EXAFS Data Analysis Workshop 2011, Ghent, Belgium, 12–14 January 2011. [Google Scholar]
- Penner-Hahn, J.E. X-Ray Absorption Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Carriere, M.; Pignol, D.; Arnoux, P. Co K Edge XAS Transmission and XAS Fluorescence of Organic and Inorganic Co(II) and Co(III) Reference Compounds for the Study of Co in Bacteria, Dataset/Spectral Data; SSHADE/FAME (OSUG Data Center): Grenoble, France, 2014; EXPERIMENT_MC_20141201_001. [CrossRef]
- Joly, J.; Grenier, S. Theory of X-Ray Absorption Near Edge Structure. In X-Ray Absorption and X-Ray Emission Spectroscopy Theory and Applications; Van Bokhoven, J.A., Lamberti, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 73–97. [Google Scholar] [CrossRef]
- Cotton, F.A. Chemical Applications of Group Teory, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1991. [Google Scholar]
- Szlachetko, J.; Sá, J. X-Ray Spectroscopy—The Driving Force to Understand and Develop Catalysis. In Advanced Catalytic Materials—Photocatalysis and Other Current Trends; Noreña, L., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- De Groot, F.; Kotani, A. Core level spectroscopy of solids. In Advances in Condensed Matter Science; Sarma, D.D., Kotliar, G., Tokura, Y., Eds.; CRC Press Taylor & Francis Groupe: Boca Raton, FL, USA, 2008; Volume 6. [Google Scholar] [CrossRef]
- Laskowski, R.; Blaha, P. Understanding the L2,3 x-ray absorption spectra of early 3d transition elements. Phys. Rev. B 2010, 82, 205104. [Google Scholar] [CrossRef]
- De Groot, F.M.F. X-ray absorption and dichroism of transition metals and their compounds. J. Electron. Spectrosc. Relat. Phenom. 1994, 67, 529–622. [Google Scholar] [CrossRef]
- Teo, B.K. EXAFS spectroscopy: Basic principles and data analysis. In Inorganic Chemistry Concepts, 1st ed.; Jorgensen, C.K., Lappert, M.F., Lippard, S.J., Margrave, J.L., Niedenzu, K., Noth, H., Parry, R.W., Yamatera, H., Eds.; Springer: New York, NY, USA, 1986; Volume 9. [Google Scholar]
- Koningsberger, D.C.; Mojet, B.L.; Van Dorssen, G.; Ramaker, D.E. XAFS Spectroscopy: Fundamental Principles and Data Analysis. Top. Catal. 2000, 10, 143–155. [Google Scholar] [CrossRef]
- Lee, P.A.; Citrin, P.H.; Eisenberger, P.; Kincaid, B. Extended x-ray absorption fine structure—Its strengths and limitations as a structural tool. Rev. Mod. Physiscs 1981, 53, 769. [Google Scholar] [CrossRef]
- Jeong, E.; Hwang, I.; Han, S. Quantitative analysis of EXAFS data sts using deep reinforcement learning. Sci. Rep. 2025, 15, 17417. [Google Scholar] [CrossRef]
- d’Acapito, F.; Rehman, M. Effectiveness of ab initio molecular dynamics in simulating EXAFS spectra from layered systems. J. Synchrotron Radiat. 2024, 31, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Ławniczak-Jabłońska, K.; Klepka, M.; Wolska, A.; Walczak, M.; Demchenko, I.N.; Zajdel, P.; Kisiel, A. Spektroskopia absorpcyjna promieniowania rentgenowskiego. In Promieniowanie Synchrotronowe w Fizyce i Chemii Ciała Stałego; Kowalski, B.J., Paszkowicz, W., Eds.; Wydownictwo Naukowe UAM: Poznań, Poland, 2024. [Google Scholar] [CrossRef]
- Koningsberger, D.C.; Prins, R. X-Ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS and XANES; John Wiley and Sons: New York, NY, USA, 1988. [Google Scholar]
- Glatzel, P.; Bergmann, U. High resolution 1s core hole X-ray spectroscopy in 3d transition metal complexes—Electronic and structural information. Coord. Chem. Rev. 2005, 249, 65–95. [Google Scholar] [CrossRef]
- Zimmermann, P.; Peredkov, S.; Abdala, P.M.; DeBeer, S.; Tromp, M.; Müller, C.; van Bokhoven, J.A. Modern X-ray spectroscopy: XAS and XES in the laboratory. Coord. Chem. Rev. 2020, 423, 213466. [Google Scholar] [CrossRef]
- Sá, J.; Czapla-Masztafiak, J.; Lipiec, E.; Kayser, Y.; Kwiatek, W.; Wood, B.; Deacon, G.B.; Berger, G.; Dufrasne, F.; Fernandes, D.L.; et al. The use of Resonant X-ray Emission Spectroscopy (RXES) for the electronic analysis of metal complexes and their interactions with biomolecules. Drug Discov. Today Technol. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Szlachetko, J.; Nachtegaal, M.; de Boni, E.; Willimann, M.; Safonova, O.; Sa, J.; Smolentsev, G.; Szlachetko, M.; van Bokhoven, J.A.; Dousse, J.-C.; et al. A von Hamos x-ray spectrometer based on a segmented-type diffraction crystal for single-shot x-ray emission spectroscopy and time-resolved resonant inelastic X-ray scattering studies. Rev. Sci. Instrum. 2012, 83, 103105. [Google Scholar] [CrossRef]
- Kvashnina, K.O.; Scheinost, A.C. A Johann-type X-ray emission spectrometer at the Rossendorf beamline. J. Synchrotron Radiat. 2016, 23, 836–841. [Google Scholar] [CrossRef]
- Kowalska, J.; DeBeer, S. The role of X-ray spectroscopy in understanding the geometric and electronic structure of nitrogenase. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2015, 1853, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.G.; Hahn, A.W.; Van Kuiken, B.E.; Henthorn, J.T.; McGale, J. DeBeer, S. Probing Physical Oxidation State by Resonant X-ray Emission Spectroscopy: Applications to Iron Model Complexes and Nitrogenase. Angew. Chem.Int. Ed. 2021, 60, 10112–10121. [Google Scholar] [CrossRef] [PubMed]
- Rogvall, J.; Singh, R.; Vacher, M.; Lundberg, M. Sensitivity of Kβ mainline X-ray emission to structural dynamics in iron photosensitizer. Phys. Chem. Chem. Phys. 2023, 25, 10447–10459. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, K.M.; Roemelt, M.; Ettenhuber, P.; Hu, Y.; Ribbe, M.W.; Neese, F.; Bergmann, U.; DeBeer, S. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor. Science 2011, 334, 974–977. [Google Scholar] [CrossRef]
- Rovezzi, M.; Glatzel, P. Hard x-ray emission spectroscopy: A powerful tool for the characterization of magnetic semiconductors. Semicond. Sci. Technol. 2014, 29, 023002. [Google Scholar] [CrossRef]
- Ould-Chikh, S.; Vollmer, I.; Aguilar Tapia, A. Fe K Edge XAS HERFD (Kbeta1,3) and XES of Synthetic Maghemite Gamma-Fe2O3 at Ambient Conditions, Dataset/Spectral Data; SSHADE/FAME (OSUG Data Center): Grenoble, France, 2018; EXPERIMENT_SOC_20181115_005. [CrossRef]
- Joe, Y.I.; O’neil, G.C.; Miaja-Avila, L.; Fowler, J.W.; Jimenez, R.; Silverman, K.L.; Swetz, D.S.; Ullom, J.N. Observation of iron spin-states using tabletop x-ray emission spectroscopy and microcalorimeter sensors. J. Phys. B At. Mol. Opt. Phys. 2016, 49, 024003. [Google Scholar] [CrossRef]
- Weinhardt, L.; Benkert, A.; Meyer, F.; Blum, M.; Hauschild, D.; Wilks, R.G.; Bär, M.; Yang, W.; Zharnikov, M.; Reinert, F.; et al. Local electronic structure of the peptide bond probed by resonant inelastic soft X-ray scattering. Phys. Chem. Chem. Phys. 2019, 21, 13216–13223. [Google Scholar] [CrossRef]
- Błachucki, W.; Kayser, Y.; Czapla-Masztafiak, J.; Guo, M.; Juranić, P.; Kavčič, M.; Källman, E.; Knopp, G.; Lundberg, M.; Milne, C.; et al. Inception of electronic damage of matter by photon-driven post-ionization mechanisms. Struct. Dyn. 2019, 6, 024901. [Google Scholar] [CrossRef]
- Bhargava, A.; Chen, C.Y.; Finkelstein, K.D.; Ward, M.J.; Robinson, R.D. X-ray emission spectroscopy: An effective route to extract site occupation of cations. Phys. Chem. Chem. Phys. 2018, 20, 28990–29000. [Google Scholar] [CrossRef]
- Ortega, R.; Carmona, A.; Llorens, I.; Solari, P.L. X-ray absorption spectroscopy of biological samples. A tutorial. J. Anal. At. Spectrom. 2012, 27, 2054–2065. [Google Scholar] [CrossRef]
- Porcaro, F.; Roudeau, S.; Carmona, A.; Ortega, R. Advances in element speciation analysis of biomedical samples using synchrotron-based techniques. TrAC Trends Anal. Chem. 2018, 104, 22–41. [Google Scholar] [CrossRef]
- Balerna, A.; Mobilio, S. Introduction to Synchrotron Radiation. In Synchrotron Radiation. Basics, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Pełka, J.B. Synchrotron Radiation in Biology and Medicine. Acta Phys. Pol. A 2008, 114, 309–329. [Google Scholar] [CrossRef]
- Kisiel, A. Synchrotron jako narzędzie: Zastosowania promieniowania synchrotronowego w spektroskopii ciała stałego. Synchrotron Radiat. Nat. Sci. 2006, 5. [Google Scholar]
- Bergmann, U.; Kern, J.; Schoenlein, R.; Wernet, P.; Yachandra, V.; Yano, J. Using X-ray free-electron lasers for spectroscopy of molecular catalysts and metalloenzymes. Nat. Rev. Phys. 2021, 3, 264–282. [Google Scholar] [CrossRef]
- Winick, H. Synchrotron Radiation Sources—Present Capabilities and Future Directions. J. Synchrotron Rad. 1998, 5, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gawelda, W.; Szlachetko, J.; Milne, C. X-Ray Spectroscopy at Free Electron Lasers. In X-Ray Absorption and X-Ray Emission Spectroscopy: Theory and Applications; Van Bokhoven, J.A., Lamberti, C., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Pełka, J.B. Synchrotron Radiation in Biology and Medicine. Acta Phys. Pol. A 2022, 141, 3–34. [Google Scholar] [CrossRef]
- Cao, M.; Dang, Z.; Wnag, Y. Multimodal techniques based on synchrotron radiation X-rays for revealing interactions of metallic nanoparticles with biological matrices. Fundam. Res. 2015. [Google Scholar] [CrossRef]
- Nango, E.; Kubo, M.; Tono, K.; Iwata, S. Pump-Probe Time-Resolved Serial Femtosecond Crystallography at SACLA: Current Status and Data Collection Strategies. Appl. Sci. 2019, 9, 5505. [Google Scholar] [CrossRef]
- Hitchcock, A.P.; Morin, C.; Zhang, X.; Araki, T.; Dynes, J.; Stöver, H.; Brash, J.; Lawrence, J.R.; Leppard, G.G. Soft X-ray spectromicroscopy of biological and synthetic polymer systems. J. Electron. Spectrosc. Relat. Phenom. 2005, 144–147, 259–269. [Google Scholar] [CrossRef]
- Sedlmair, J. Soft X-Ray Spectromicroscopy of Environmental and Biological Samples. In Göttingen Series in X-Ray Physics; Universitätsverlag Göttingen: Göttingen, Germany, 2011; Volume 7. [Google Scholar]
- Kathyola, T.; Chang, S.-Y.; Willneff, E.; Willis, C.; Cibin, G.; Wilson, P.; Kroner, A.; Shotton, E.; Dowding, P.; Schroeder, S. X-ray Absorption Spectroscopy as a Process Analytical Technology: Reaction Studies for the Manufacture of Sulfonate-Stabilized Calcium Carbonate Particles. Ind. Eng. Chem. Res. 2023, 62, 16198–16206. [Google Scholar] [CrossRef]
- Koziej, D.; DeBeer, S. Application of Modern X-ray Spectroscopy in Chemistry—Beyond Studying the Oxidation State. Chem. Mater. 2017, 29, 7051–7053. [Google Scholar] [CrossRef]
- Emamian, S. X-ray Emission Spectroscopy of Single Protein Crystals Yields Insights into HEME Enzyme Intermediates. J. Phys. Chem. Lett. 2023, 25, 41–48. [Google Scholar] [CrossRef]
- Hummer, A.; Rompel, A. X-Ray Absorption Spectroscopy: A Tool to Investigate the Local Structure of Metal-Based Anticancer Compounds In Vivo. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Maryland Heights, MO, USA, 2013; Volume 93, pp. 257–305. [Google Scholar] [CrossRef]
- Sherborne, G.; Nguyen, B. Recent XAS studies into Homogeneous metal catalyst in fine chemical and pharmaceutical syntheses. Chem. Cent. J. 2015, 9, 37. [Google Scholar] [CrossRef]
- Nicolis, I.; Deschamps, P.; Curis, E.; Corriol, O.; Acar, V.; Zerrouk, N.; Chaumeil, J.-C.; Guyone, F.; Bénazeth, S. XAS applied to pharmaceuticals: Drug administration and bioavailability. J. Synchrotron Rad. 2001, 8, 984–986. [Google Scholar] [CrossRef]
- Yang, Y.; Pushie, M.; Cooper, D. Structural Characterization of SmIII(EDTMP). Mol. Pharm. 2015, 12, 4108–4114. [Google Scholar] [CrossRef]
- Kuter, D.; Streltsov, V.; Davydova, N.; Venter, G.A.; Naidoo, K.J.; Egan, T.J. Solution structures of chloroquine–ferriheme complexes modeled using MD simulation and investigated by EXAFS spectroscopy. J. Inorg. Biochem. 2016, 154, 114–125. [Google Scholar] [CrossRef]
- Ito, M.; Shiba, R.; Suzuki, H.; Noguchi, S. Chlorine K-edge X-ray absorption near-edge structure analysis of clarithromycin hydrochloride metastable crystal. J. Pharm. Sci. 2020, 109, 2095–2099. [Google Scholar] [CrossRef]
- Huang, Z.; Suzuki, H.; Ito, M.; Noguchi, S. Direct detection of the crystal form of an active pharmaceutical ingredient in tablets by X-ray absorption fine structure spectroscopy. Int. J. Pharm. 2022, 625, 122057. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Ito, M.; Suzuki, H.; Noguchi, S. Characterization of Bisphosphonate Hydrate Crystals by Phosphorus K-Edge X-Ray Absorption Near-Edge Structure Spectroscopy. Chem. Pharm. Bull. 2024, 72, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Iwata, M.; Ito, M.; Noguchi, S. X-ray Absorption Near-Edge Spectroscopy Analysis of Indomethacin in Crystalline Forms and in Amorphous Solid Dispersions. Mol. Pharm. 2021, 18, 3475–3483. [Google Scholar] [CrossRef]
- Knapman, K. Polymorphic predictions: Understanding the nature of crystalline compounds can be critical in drug development and manufacture. Mod. Drug Discov. 2000, 3, 53–54. [Google Scholar]
- Lee, A.Y.; Erdemir, D.; Myerson, A.S. Crystal Polymorphism in Chemical Process Development. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 259–280. [Google Scholar] [CrossRef] [PubMed]
- Clavier, T. Impact of Polymorphism on Drug Formulation and Bioavailability. J. Chem. Pharm. Res. 2024, 16, 9–10. [Google Scholar] [CrossRef]
- Suzuki, H.; Tomita, A.; Ito, M.; Noguchi, S. Bromine K-edge X-ray absorption near-edge structure analysis on hydrobromide-salt crystals and the solid dispersion of active pharmaceutical ingredients. Chem. Pharm. Bull. 2022, 70, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Talaczynska, A.; Dzitko, J.; Cielecka-Piontek, J. Benefits and Limitations of Polymorphic and Amorphous Forms of Active Pharmaceutical Ingredients. Curr. Pharm. Des. 2016, 22, 4975–4980. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsushima, M.; Ito, M.; Noguchi, S. Analysis of Cimetidine Crystal Polymorphs by X-ray Absorption Near-Edge Spectroscopy. Mol. Pharm. 2023, 20, 1213–1221. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Joress, H.; Ravel, B.; Anber, E.; Hollenbach, J.; Sur, D.; Hattrick-Simpers, J.; Taheri, M.L.; DeCost, B. Why is EXAFS for complex concentrated alloys so hard? Challenges and opportunities for measuring ordering with X-ray absorption spectroscopy. Matter 2023, 6, 3763–3781. [Google Scholar] [CrossRef]
- Rojsatien, S.; Mannodi-Kanakkithodi, A.; Walker, T.; Nietzold, T.; Colegrove, E.; Lai, B.; Cai, Z.; Holt, M.; Chan, M.K.; Bertoni, M.I. Quantitative analysis of Cu XANES spectra using linear combination fitting of binary mixtures simulated by FEFF9. Radiat. Phys. Chem. 2023, 202, 110548. [Google Scholar] [CrossRef]
- Gupta, A.; Pratt, R.; Mishra, B. Physicochemical characterization of ferric pyrophosphate citrate. Biometals 2018, 31, 1091–1099. [Google Scholar] [CrossRef]
- Macdougall, I.; Tucker, B.; Thompson, J.; Tomson, C.R.; Baker, L.R.; Raine, A.E. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996, 50, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Roseman, G.; Schilling, K.M.; Dolgova, N.V.; Pushie, M.J.; Sokaras, D.; Kroll, T.; Harris, H.H.; Millhauser, G.L.; Pickering, I.J.; et al. Alzheimer’s Drug PBT2 Interacts with the Amyloid β 1–42 Peptide Differently than Other 8-Hydroxyquinoline Chelating Drugs. Inorg. Chem. 2022, 61, 14626–14640. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Tzeng, Y.; Chang, L.; Huang, H.; Lin, T.; Chyan, C.; Chen, Y. The correlation between neurotoxicity, aggregative ability and secondary structure studied by sequence truncated Aβ peptides. FEBS Lett. 2007, 581, 1161–1165. [Google Scholar] [CrossRef]
- De-Paula, V.J.; Radanović, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s disease. Subcell. Biochem. 2012, 65, 329–352. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci. 2018, 23, 446. [Google Scholar] [CrossRef]
- Proux, O.; Lahera, E.; Del Net, W.; Kieffer, I.; Rovezzi, M.; Testemale, D.; Irar, M.; Thomas, S.; Aguilar-Tapia, A.; Bazarkina, E.F.; et al. High-Energy Resolution Fluorescence Detected X-Ray Absorption Spectroscopy: A Powerful New Structural Tool in Environmental Biogeochemistry Sciences. J. Environ. Qual. 2017, 46, 1146–1157. [Google Scholar] [CrossRef]
- Liang, J.; Levina, A.; Jia, J.; Kappen, P.; Glover, C.; Johannessen, B. Reactivity and Transformation of Antimetastatic and Cytotoxic Rhodium(III)–Dimethyl Sulfoxide Complexes in Biological Fluids: An XAS Speciation Study. Inorg. Chem. 2019, 58, 4880–4893. [Google Scholar] [CrossRef]
- Mestroni, G.; Alessio, E.; Sessanta Santi, A.; Geremia, S.; Bergamo, A.; Sava, G.; Boccarelli, A.; Schettino, A.; Coluccia, M. Rhodium(III) Analogues of Antitumour-Active Ruthenium(III) Compounds: The Crystal Structure of [ImH] [trans-RhCl4(Im)2] (Im = imidazole). Inorg. Chem. 1998, 273, 62–71. [Google Scholar] [CrossRef]
- Levina, A.; Aitken, J.B.; Gwee, Y.Y.; Lim, Z.J.; Liu, M.; Singharay, A.M.; Wong, P.F.; Lay, P.A. Biotransformations of anticancer ruthenium(III) complexes: An X-ray absorption spectroscopic study. Chemistry 2013, 19, 3609–3619. [Google Scholar] [CrossRef]
- Lipiec, E.; Czapla, J.; Szlachetko, J.; Kayser, Y.; Kwiatek, W.; Wood, B.; Deacone, G.B.; Sá, J. Novel in situ methodology to observe the interactions of chemotherapeutical Pt drugs with DNA under physiological conditions. Dalton Trans. 2014, 4, 13839–13844. [Google Scholar] [CrossRef]
- Sá, J.; Czapla-Masztafiak, J.; Lipiec, E.; Kayser, Y.; Fernandes, D.L.A.; Szlachetko, J.J.; Dufrasnef, F.; Berger, G. Resonant X-ray emission spectroscopy of platinum(ii) anticancer complexes. Analyst 2016, 141, 1226–1232. [Google Scholar] [CrossRef]
- Diklić, N.; Clark, A.; Herranz, J.; Diercks, J.; Aegerter, D.; Nachtegaal, M.; Beard, A.; Schmidt, T. Potential Pitfalls in the Operando XAS Study of Oxygen Evolution Electrocatalysts. ACS Energy Lett. 2022, 7, 1735–1740. [Google Scholar] [CrossRef]
- Finzel, J.; Sanroman Gutierrez, K.; Hoffman, A.; Resasco, J.; Christopher, P.; Bare, S. Limits of Detection for EXAFS Characterization of Heterogeneous Single-Atom Catalysts. ACS Catal. 2023, 13, 6462–6473. [Google Scholar] [CrossRef]

| Drug/System | Spectroscopic Technique | Key Findings | Ref. |
|---|---|---|---|
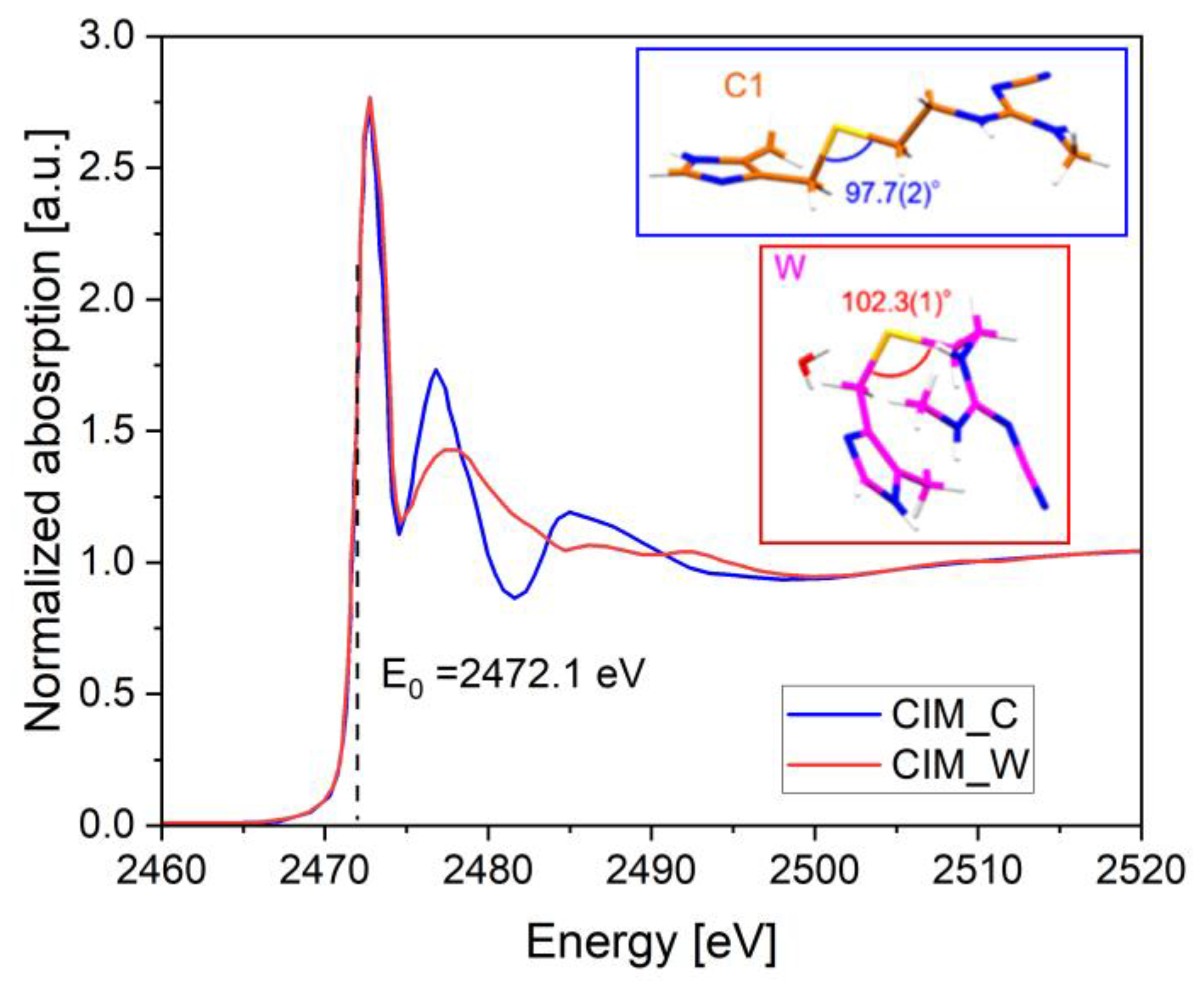
| Cimetidine (CIM) | S K-edge XANES, EXAFS | Differentiation of polymorphs (CIM_A, CIM_B, CIM_C, and CIM_W); quantification of mixtures via LCF (R2 = 0.9967) | [76] |
| Bromhexine hydrochloride (BRH-HCl) | Cl and Br K-edge XANES/EXAFS | Detection of polymorphic forms; identification of H-bonds, halogen–π, and halogen–halogen interactions; direct analysis of tablets in PTP | [68] |
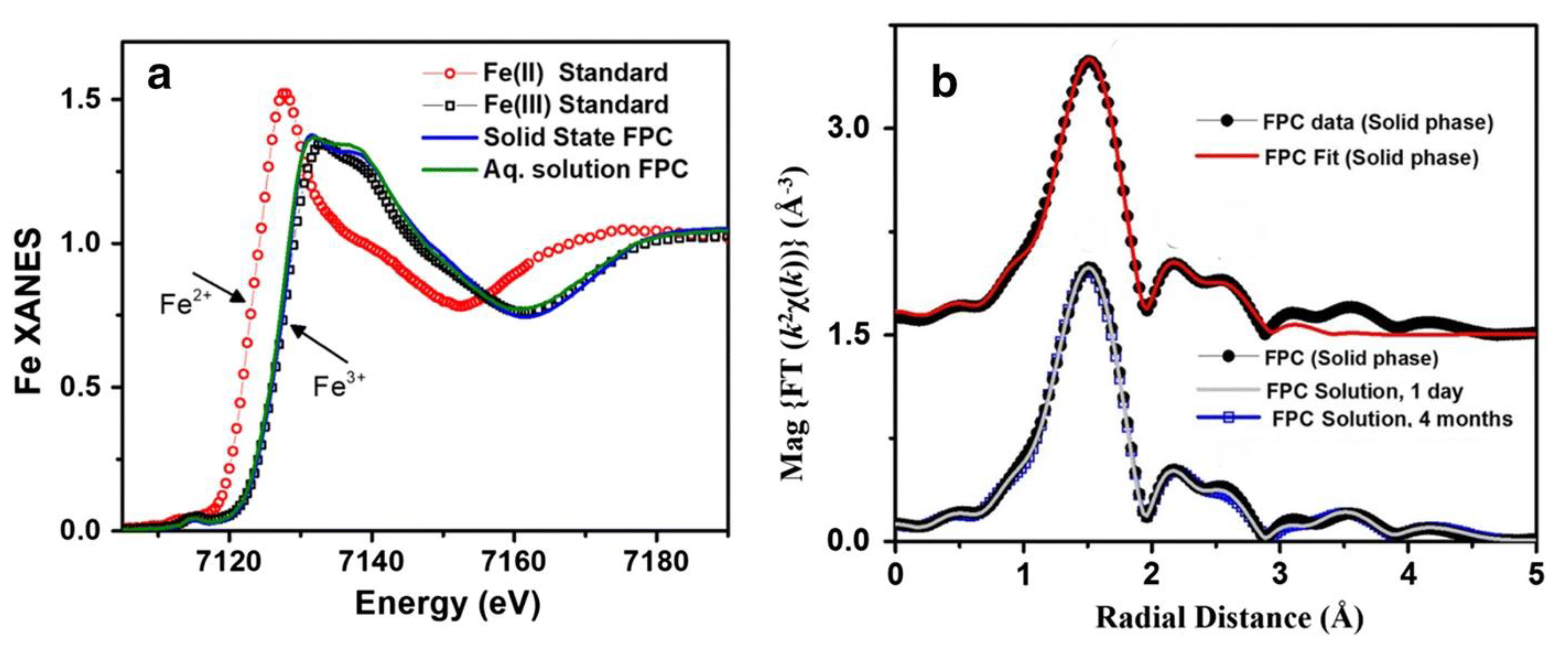
| Ferric pyrophosphate citrate (FPC) | Fe K-edge XANES, EXAFS | Confirmation of Fe3+; coordination by citrate and pyrophosphate; stability in solution; first clinical parenteral iron salt | [80] |
| Cu–Aβ peptide complexes (Alzheimer’s) | Cu K-edge HERFD-XAS | Chelators (PBT2, CQ, and B2Q) show distinct binding modes; differences explain therapeutic activity | [82] |
| Rhodium(III) complexes (A1, A2) | Rh K-edge XANES, EXAFS | Donor atom preferences correlate with cytotoxic vs. antimetastatic activity | [88] |
| Ruthenium(III) complexes (NAMI-A, KP1019) | Ru K-edge XAS | Aquation and protein binding; speciation explains distinct pharmacological profiles. | [90] |
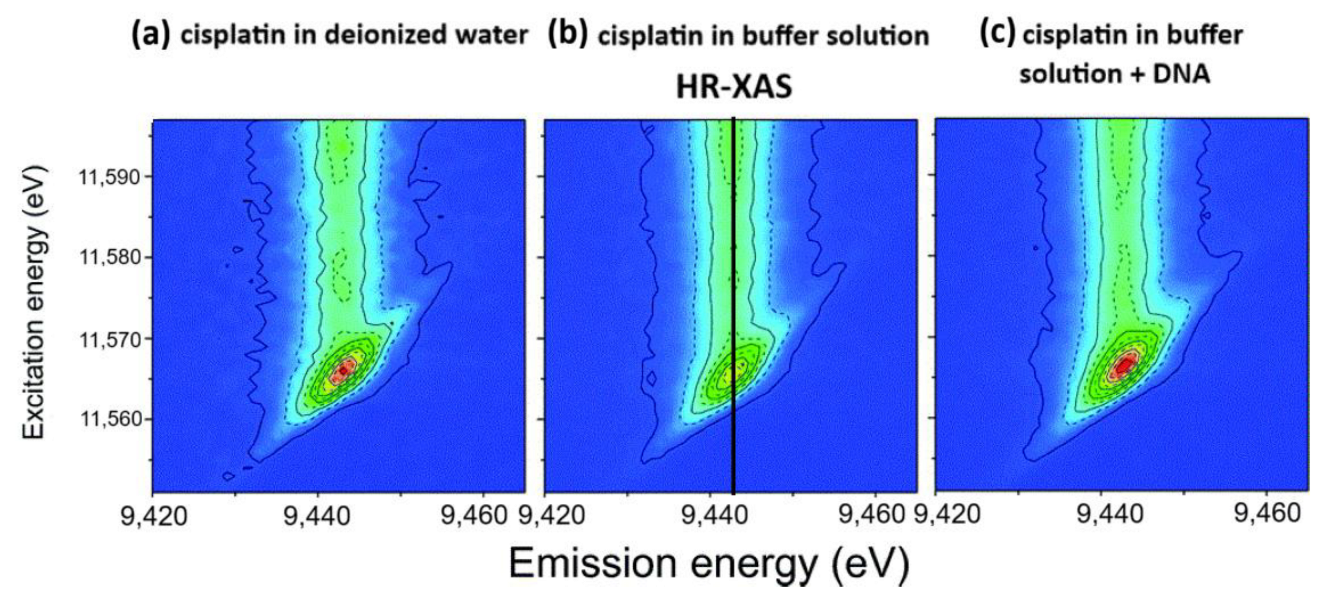
| Cisplatin–DNA | Pt L3-edge RIXS | In situ RIXS reveals hydration and DNA binding; formation of cis-Pt(NH3)2{d(GpG)-N7(1),-N7(2)} adduct | [91] |
| Chiral Pt complexes (cis/trans stereoisomers) | Pt L3-edge RXES, HR-XAS, VtC | Discrimination between isomers; subtle electronic differences correlate with ~50-fold difference in activity. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtaszek, K.; Tyrała, K.; Błońska-Sikora, E. X-Ray Absorption and Emission Spectroscopy in Pharmaceutical Applications: From Local Atomic Structure Elucidation to Protein-Metal Complex Analysis—A Review. Appl. Sci. 2025, 15, 10784. https://doi.org/10.3390/app151910784
Wojtaszek K, Tyrała K, Błońska-Sikora E. X-Ray Absorption and Emission Spectroscopy in Pharmaceutical Applications: From Local Atomic Structure Elucidation to Protein-Metal Complex Analysis—A Review. Applied Sciences. 2025; 15(19):10784. https://doi.org/10.3390/app151910784
Chicago/Turabian StyleWojtaszek, Klaudia, Krzysztof Tyrała, and Ewelina Błońska-Sikora. 2025. "X-Ray Absorption and Emission Spectroscopy in Pharmaceutical Applications: From Local Atomic Structure Elucidation to Protein-Metal Complex Analysis—A Review" Applied Sciences 15, no. 19: 10784. https://doi.org/10.3390/app151910784
APA StyleWojtaszek, K., Tyrała, K., & Błońska-Sikora, E. (2025). X-Ray Absorption and Emission Spectroscopy in Pharmaceutical Applications: From Local Atomic Structure Elucidation to Protein-Metal Complex Analysis—A Review. Applied Sciences, 15(19), 10784. https://doi.org/10.3390/app151910784





