Optimization of Impedance-Based Real-Time Assay in xCELLigence RTCA SP16 Device for the Analysis of Fully Differentiated Caco-2 Cells
Abstract
1. Introduction
1.1. Cell-Based Impedance Assays
1.2. TEER Method for Monitoring of Caco-2 Monolayer Integrity
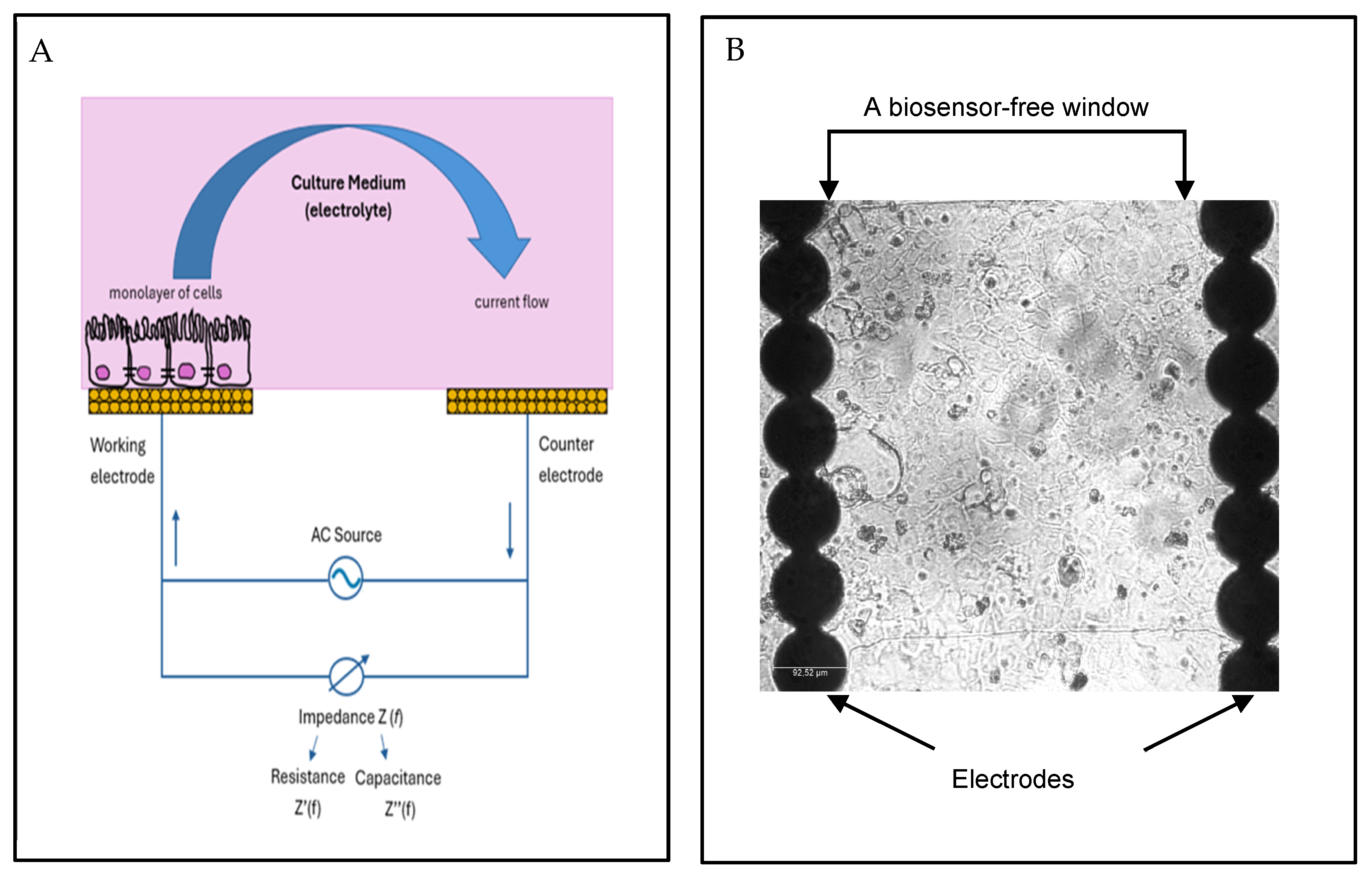
1.3. IBCA for Monitoring of Caco-2 Cells Membrane Integrity and Viability in Real-Time
2. Materials and Methods
2.1. Cell Culture General Procedure
2.2. xCELLigence System
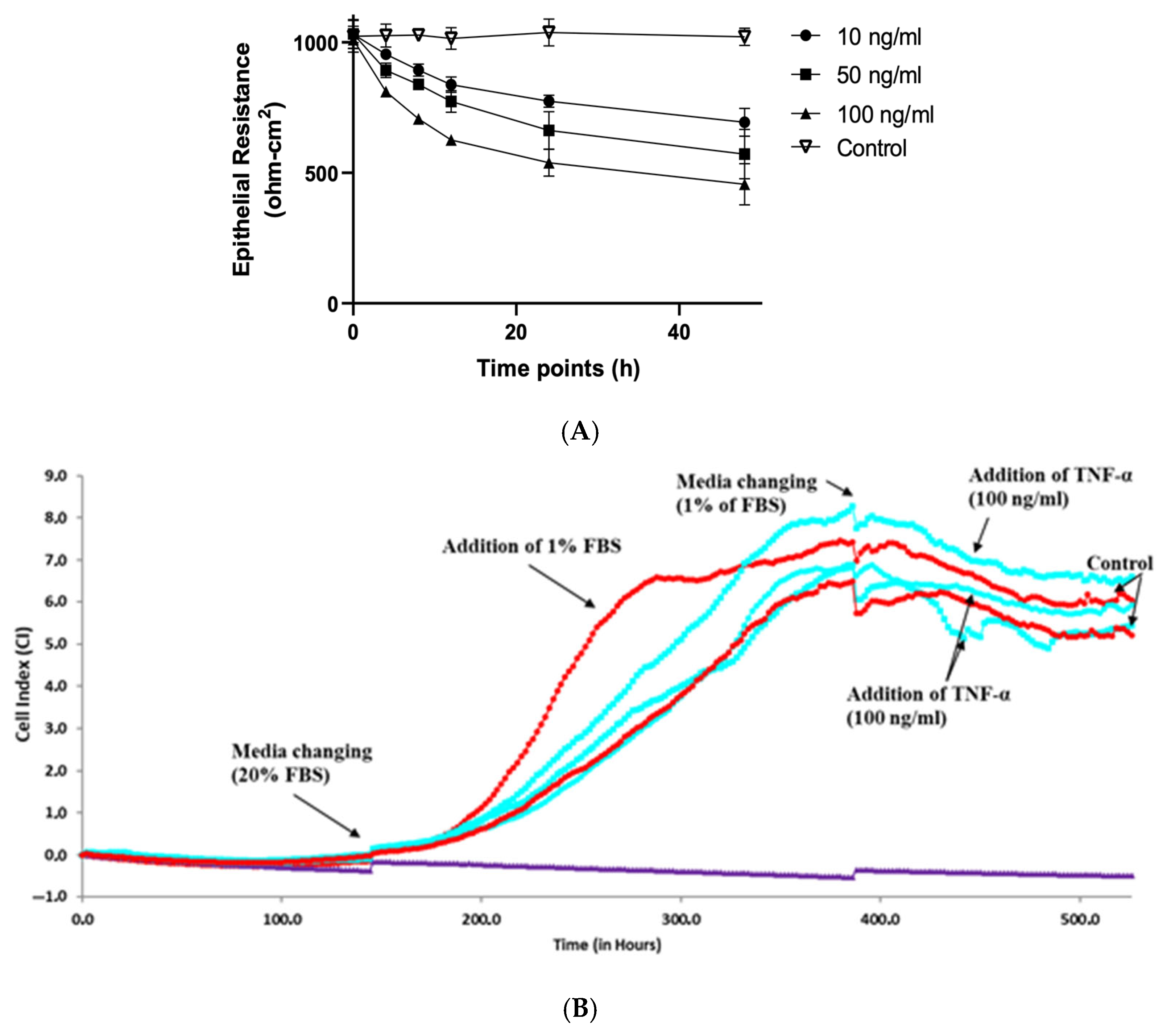
2.3. The Effect of TNF-α on Caco-2 Epithelial Barrier Integrity Monitored by TEER Method
2.4. Quantitative Assessment of Caco-2 Cell Kinetics in xCELLigence RTCA S16
2.4.1. Preparation of the Cells
2.4.2. Seeding of Cells onto xCELLigence E-Plate 16
2.4.3. Monitoring of Real-Time Caco-2 Cells Grow in E Plate 16
2.5. Optimization of Cultivation the Differentiated Caco-2 Cells in xCELLigence RTCA S16
2.5.1. Real-Time Monitoring of Caco-2 Cells Growth in Complete Media
2.5.2. Real-Time Monitoring of Caco-2 Cells Growth in Media Containing 1% of FBS
2.5.3. Staining Procedure of Caco-2 Cells for Occludin
2.6. The Effect of TNF-α on Caco-2 Epithelial Barrier Integrity Monitored by xCELLigence RTCA S16
2.7. The Effect of L-DOPA on Undifferentiated Caco-2 Cells Monitored by MTS Method
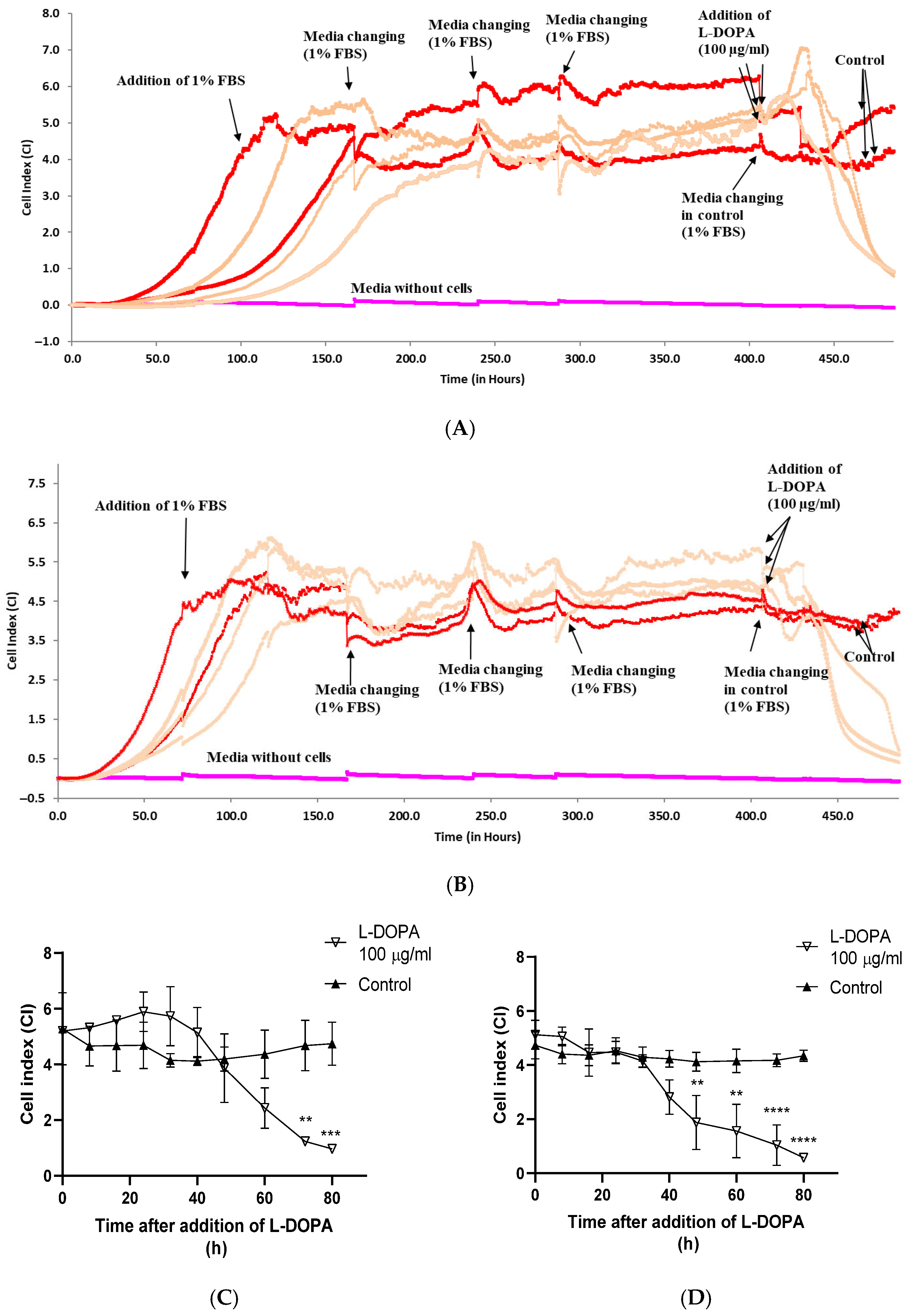
2.8. The Effect of L-DOPA on Differentiated Caco-2 Cells Monitored by xCELLigence RTCA S16
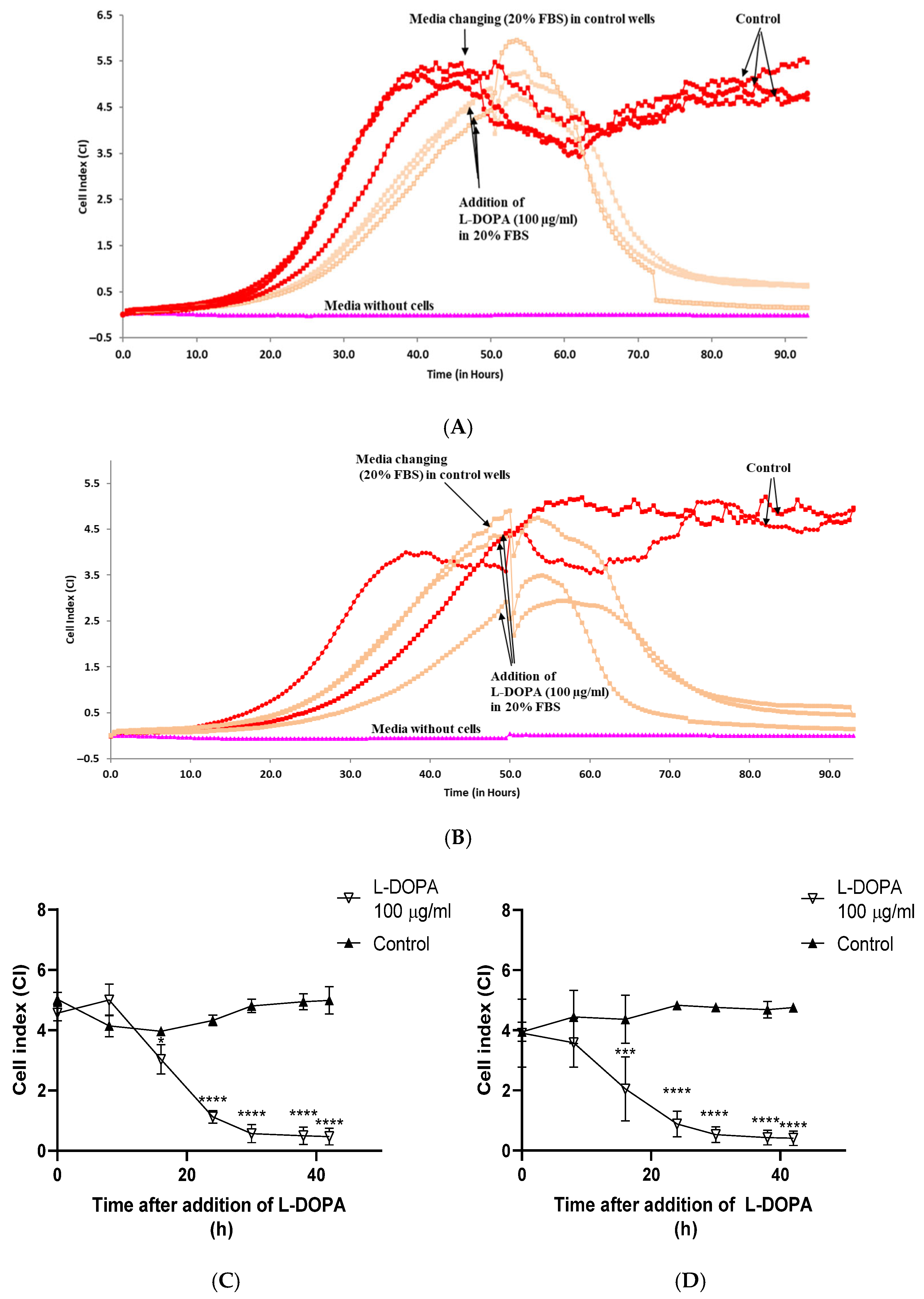
2.9. The Effect of L-DOPA on Undifferentiated Caco-2 Cells Monitored by xCELLigence RTCA S16
3. Results
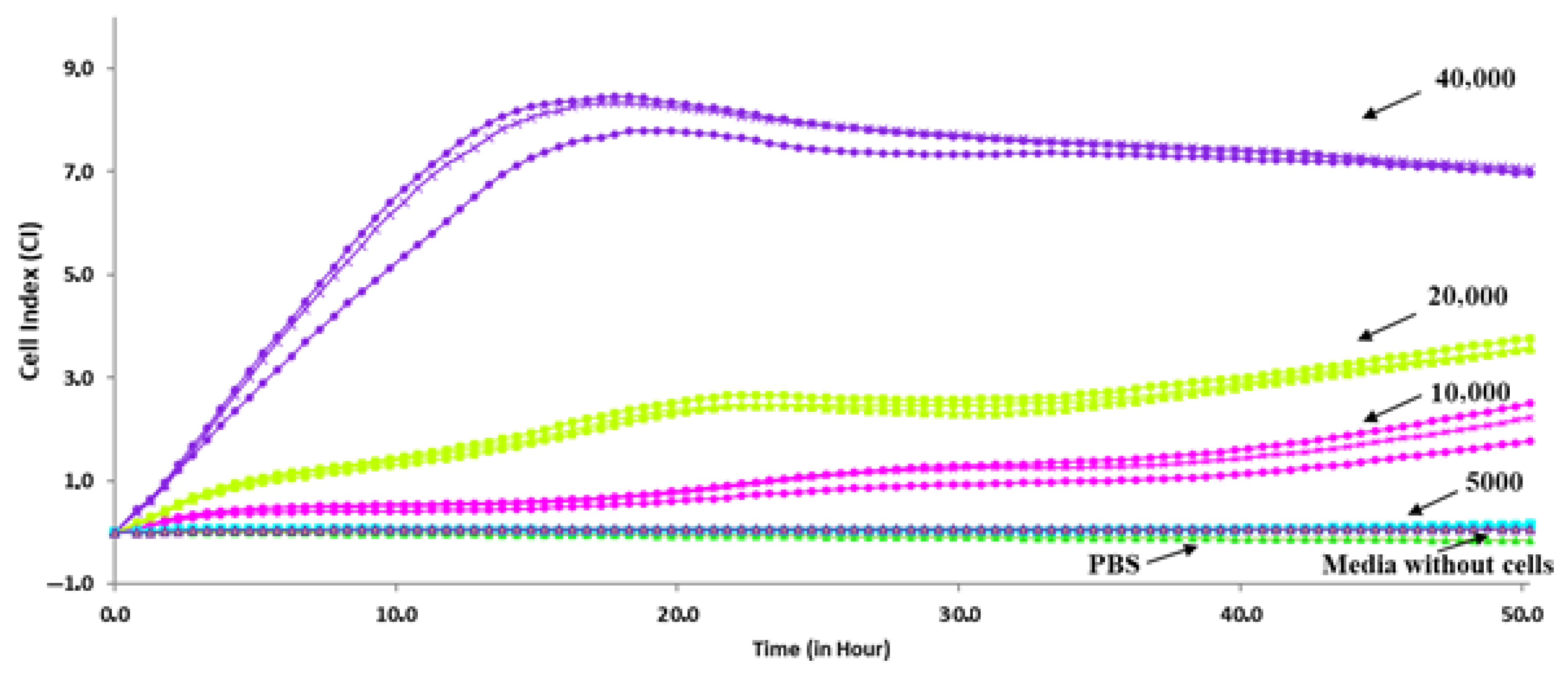
3.1. Determination of Optimal Density and FBS Concentrations for Caco-2 Cells Growth
3.2. The Assessment of TNF-α Effect on Caco-2 Epithelial Barrier Function by xCELLigence RTCA S16
3.3. The Effect of L-DOPA on Viability of Differentiated Caco-2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | cell (cellular) index |
| GIT | gastrointestinal tract |
| IBCA | impedance-based cellular assay |
| RTCA | real time cell analyzer |
| TJs | tight junctions |
| TEER | trans-epithelial electrical resistance |
References
- Shane, H.M.; Ung, B.; Bader, C.; Brooks, R.; Lazniewska, J.; Johnson, I.R.D.; Sorvina, A.; Logan, J.; Martini, C.; Moore, C.R.; et al. Fluorescence microscopy-an outline of hardware, biological handling, and fluorophore considerations. Cells 2022, 11, 35. [Google Scholar]
- Möller, C.; Slack, M. Impact of new technologies for cellular screening along the drug value chain. Drug. Discov. Today 2010, 15, 384–390. [Google Scholar] [CrossRef]
- Hamidi, H.; Lilja, J.; Ivaska, J. Using xCELLigence RTCA instrument to measure cell adhesion. Bio-Protocol 2017, 7, e2646. [Google Scholar] [CrossRef]
- Hillger, J.M.; Lieuw, W.L.; Heitman, L.H.; IJzerman, A.P. Label-free technology and patient cells: From early drug development to precision medicine. Drug Discov. Today. 2017, 22, 1808–1815. [Google Scholar] [CrossRef]
- McGuinness, R. Impedance-based cellular assay technologies: Recent advances, future promise. Curr. Opin. Pharmacol. 2007, 7, 535–540. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Staehelin, A.L. Three types of gap junctions interconnecting intestinal epithelial cells visualized by freeze-etching. Proc. Natl. Acad. Sci. USA 1972, 69, 1318–1321. [Google Scholar] [CrossRef]
- Förster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef]
- Harhaj, N.S.; Davis, A.A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escalera, S.; Wellejus, A. Evaluation of Caco-2 and human intestinal epithelial cells as in vitro models of colonic and small intestinal integrity. Biochem. Biophys. Rep. 2022, 31, 101314. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Berlin/Heidelberg, Germany, 2015; pp. 103–111. [Google Scholar]
- Benson, K.; Cramer, C.; Galla, H.J. Impedance-based cell monitoring: Barrier properties and beyond. Fluids Barriers CNS 2013, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Kho, D.; MacDonald, C.; Johnson, R.; Unsworth, C.P.; O’Carroll, S.J.; du Mez, E.; Angel, C.E.; Graham, E.S. Application of xcelligence RTCA biosensor technology for revealing the profile and window of drug responsiveness in real time. Biosensors 2015, 5, 199–222. [Google Scholar] [CrossRef]
- Morgan, K.; Gamal, W.; Samuel, K.; Morley, S.D.; Hayes, P.C.; Bagnaninchi, P.; Plevris, J.N. Application of impedance-based techniques in hepatology research. J. Clin. Med. 2020, 9, 50. [Google Scholar] [CrossRef]
- Sun, M.; Fu, H.; Cheng, H.; Cao, Q.; Zhao, Y.; Mou, X.; Zhang, X.; Liu, X.; Ke, Y. A dynamic real-time method for monitoring epithelial barrier function in vitro. Anal. Biochem. 2012, 425, 96–103. [Google Scholar] [CrossRef]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.J.; Fogh, S.A.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line Caco-2 in culture. Biol. Cell. 1983, 47, 323–330. [Google Scholar]
- Ferruzza, S.; Rossi, C.; Scarino, M.L.; Sambuy, Y. A protocol for differentiation of human intestinal Caco-2 cells in asymmetric serum-containing medium. Toxicol. In Vitro 2012, 26, 1252–1255. [Google Scholar] [CrossRef]
- Cui, W.; Li, L.X.; Sun, C.M.; Wen, Y.; Zhou, Y.; Dong, Y.L.; Liu, P. Tumor necrosis factor alpha increases epithelial barrier permeability by disrupting tight junctions in Caco-2 cells. Braz. J. Med. Biol. Res. 2010, 43, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Blasig, I.E.; Bellmann, C.; Cording, J.; Del Vecchio, G.; Zwanziger, D.; Huber, O.; Haseloff, R.F. Occludin protein family: Oxidative stress and reducing conditions. Antioxid. Redox Signal. 2011, 15, 1195–1219. [Google Scholar] [CrossRef]
- Balas, M.; Dumitrache, F.; Badea, M.A.; Fleaca, C.; Badoi, A.; Tanasa, E.; Dinischiotu, A. Coating dependent in vitro biocompatibility of new Fe-Si Nanoparticles. Nanomaterials 2018, 8, 495. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Adamska, A.; Bartoszewski, R.; Ochocka, J.R. Reuse of E-plate cell sensor arrays in the xCELLigence Real-Time Cell Analyzer. BioTechniques 2016, 61, 117–122. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 16, 2645465. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Iwamoto, G.K.; Hoa, N.T.; Akotia, V.; Pedram, A.; Boivin, M.A.; Said, H.M. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver. Physiol. 2004, 286, G367–G376. [Google Scholar] [CrossRef]
- Couto, M.R.; Andrade, N.; Magro, F.; Martel, F. Bile salts and proinflammatory cytokines inhibit MCT1-mediated cellular uptake of butyrate and interfere with its antiproliferative properties. Exp Cell Res. 2023, 429, 113670. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Mena, M.A.; Casarejos, M.J.; Paíno, C.L.; De Yébenes, J.G. Toxic effects of L-DOPA on mesencephalic cell cultures: Protection with antioxidants. Brain Res. 1995, 682, 133–143. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Soares-Da-Silva, P. Uptake and intracellular fate of L-DOPA in a human intestinal epithelial cell line: Caco-2. Am J Physiol. 1998, 275, C104–C112. [Google Scholar] [CrossRef]
- Jana, S.; Sinha, M.; Chanda, D.; Roy, T.; Banerjee, K.; Munshi, S.; Patro, B.S.; Chakrabarti, S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta. 2011, 1812, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Engle, M.J.; Goetz, G.S.; Alpers, D.H. Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J. Cell. Physiol. 1998, 174, 362–369. [Google Scholar] [CrossRef]
- Maubon, N.; Le Vee, M.; Fossati, L.; Audry, M.; Le Ferrec, E.; Bolze, S.; Fardel, O. Analysis of drug transporter expression in human intestinal Caco-2 cells by real-time PCR. Fundam. Clin. Pharmacol. 2007, 21, 659–663. [Google Scholar] [CrossRef]
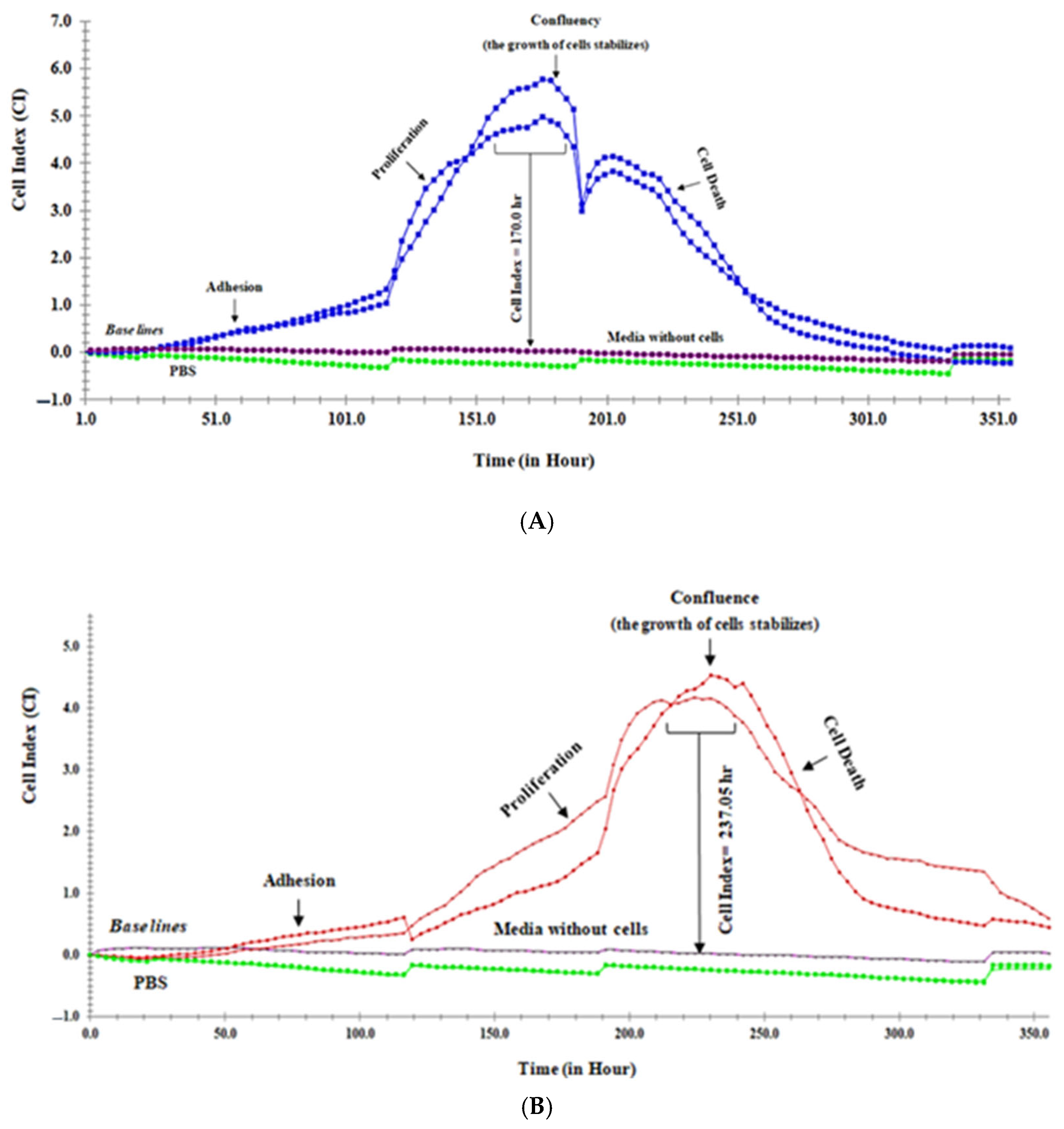

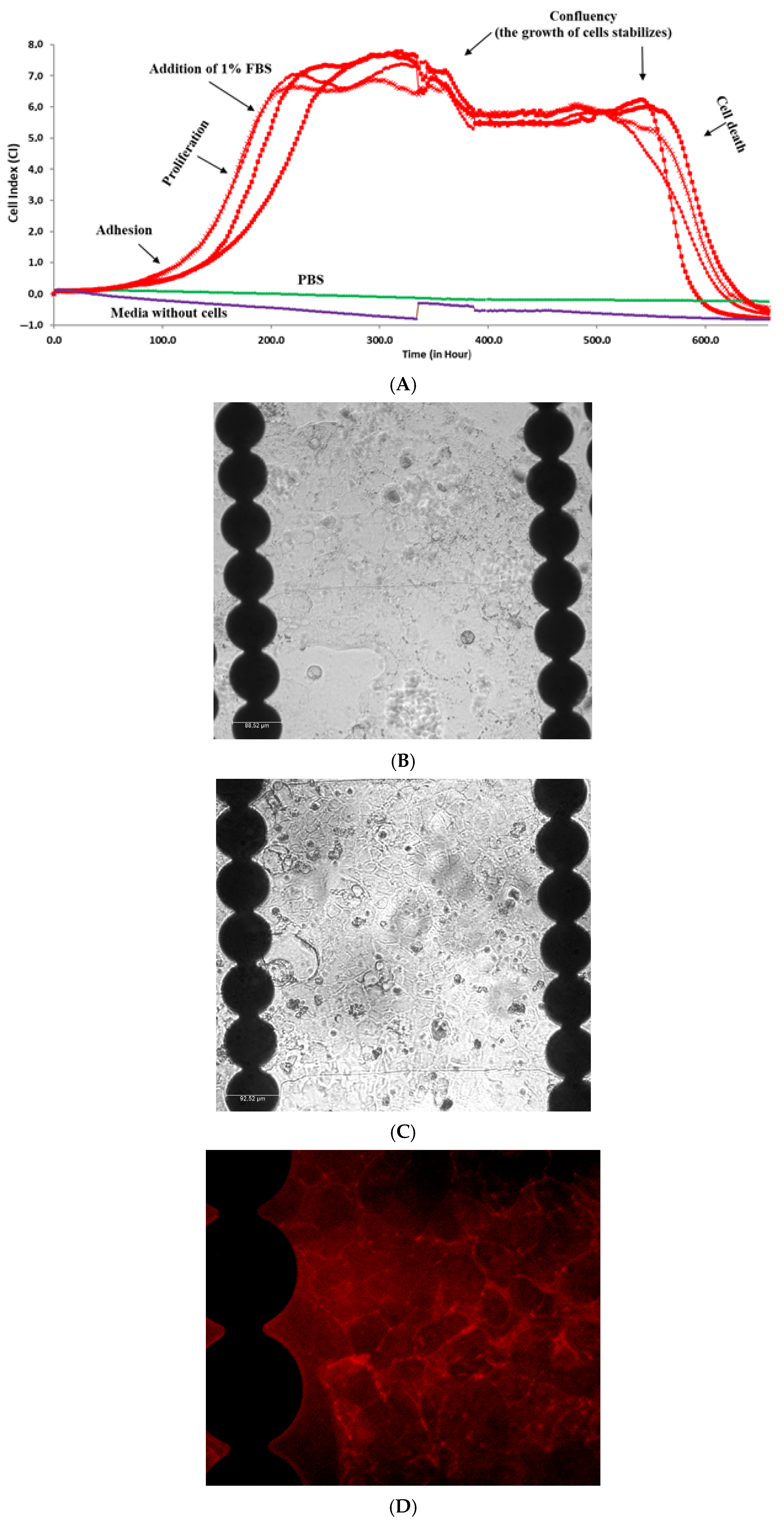

| Cell Growth Phase | Cell Adhesion | Differentiation | Confluency/ Plateau | Physiological Cell Death | Fluctuations upon Addition of Fresh Media | Fluctuations upon Addition of L-DOPA |
|---|---|---|---|---|---|---|
| Observed CI or changes in CI | Between 0–1.0 | Start: 0.5–1.0 End: 4.0–8.5 | Between 4.0 and 8.5 | Start: 4.0–8.5 End: 0.0–0.5 | max. ± 2.0 (± 40%) | max +2.0 (+40%) |
| TEER | xCELLigence RTCA S16 | |
|---|---|---|
| Method of measurement | Electrical resistance across a cell monolayer | Electrical impedance changes in a cell culture |
| Primary focus | Barrier integrity and function—tightness of cell junctions | Cell behavior such as viability, growth, proliferation, and differentiation |
| Secondary applications | Drug permeability and toxicity | Drug screening and cytotoxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Kurnik-Łucka, M.; Kudrycka, M.; Gil, K.; Latacz, G. Optimization of Impedance-Based Real-Time Assay in xCELLigence RTCA SP16 Device for the Analysis of Fully Differentiated Caco-2 Cells. Appl. Sci. 2025, 15, 8298. https://doi.org/10.3390/app15158298
Khan N, Kurnik-Łucka M, Kudrycka M, Gil K, Latacz G. Optimization of Impedance-Based Real-Time Assay in xCELLigence RTCA SP16 Device for the Analysis of Fully Differentiated Caco-2 Cells. Applied Sciences. 2025; 15(15):8298. https://doi.org/10.3390/app15158298
Chicago/Turabian StyleKhan, Nadia, Magdalena Kurnik-Łucka, Maja Kudrycka, Krzysztof Gil, and Gniewomir Latacz. 2025. "Optimization of Impedance-Based Real-Time Assay in xCELLigence RTCA SP16 Device for the Analysis of Fully Differentiated Caco-2 Cells" Applied Sciences 15, no. 15: 8298. https://doi.org/10.3390/app15158298
APA StyleKhan, N., Kurnik-Łucka, M., Kudrycka, M., Gil, K., & Latacz, G. (2025). Optimization of Impedance-Based Real-Time Assay in xCELLigence RTCA SP16 Device for the Analysis of Fully Differentiated Caco-2 Cells. Applied Sciences, 15(15), 8298. https://doi.org/10.3390/app15158298






