Is Bioelectrical Impedance Vector Analysis (BIVA) a Useful Exploratory Tool to Assess Exercise-Induced Metabolic and Mechanical Responses in Endurance-Trained Male Trail Runners?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Procedures
2.4. Statistical Analysis
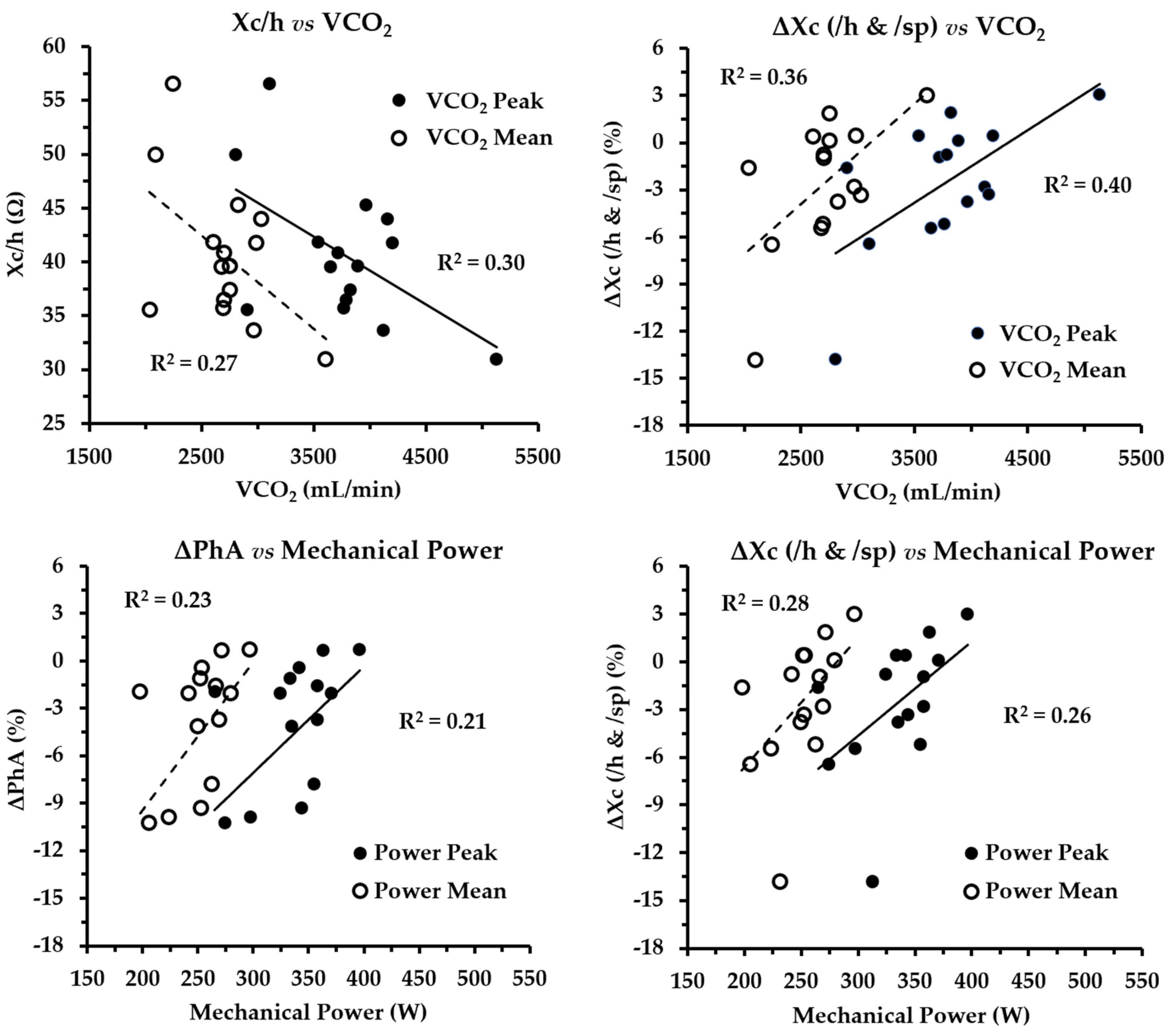
3. Results
4. Discussion
Practical Applications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIA | Bioelectrical impedance analysis |
| BIVA | Bioelectrical impedance vector analysis |
| R | Resistance |
| Xc | Reactance |
| Z | Impedance modulus |
| PhA | Phase angle |
| R/h, Xc/h, Z/h | Height-normalized resistance, reactance, impedance |
| Rsp, Xcsp, Zsp | Specific BIVA indices adjusted for segment cross-sectional areas |
| RXc | Resistance–reactance graph |
| O2 | Oxygen uptake |
| CO2 | Carbon dioxide production |
| O2max | Maximal oxygen uptake |
| RQ | Respiratory quotient |
| HR | Heart rate |
| EEM | Energy expenditure per minute |
| PO | Power output |
| POmean, POpeak | Mean, peak power output |
| SD | Standard deviation |
| CI | Confidence interval |
| FDR | False discovery rate |
| BH | Benjamini–Hochberg (procedure) |
| %Δ | Change (pre- to post-exercise) |
| EIM | Electrical impedance myography (muscle-localized bioimpedance) |
References
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of body composition in athletes: A narrative review of available methods with special reference to quantitative and qualitative bioimpedance analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Campa, F.; Buffa, R.; Stagi, S.; Matias, C.N.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin. Nutr. 2020, 39, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Talluri, A. Phase angle as an index of physiological status: Validating bioelectrical assessments of hydration and cell mass in health and disease. Rev. Endocr. Metab. Disord. 2023, 24, 371–379. [Google Scholar] [CrossRef]
- Castizo-Olier, J.; Irurtia, A.; Jemni, M.; Carrasco-Marginet, M.; Fernández-García, R.; Rodríguez, F.A. Bioelectrical impedance vector analysis (BIVA) in sport and exercise: Systematic review and future perspectives. PLoS ONE 2018, 13, e0197957. [Google Scholar] [CrossRef]
- Ward, L.C.; Brantlov, S. Bioimpedance basics and phase angle fundamentals. Rev. Endocr. Metab. Disord. 2023, 24, 381–391. [Google Scholar] [CrossRef]
- Cebrián-Ponce, Á.; Irurtia, A.; Castizo-Olier, J.; Garnacho-Castaño, M.V.; Espasa-Labrador, J.; Noriega, Z.; Carrasco-Marginet, M. Bioelectrical, anthropometric, and hematological analysis to assess body fluids and muscle changes in elite cyclists during the Giro d’Italia. Biology 2023, 12, 450, Erratum in Biology 2023, 12, 863. [Google Scholar] [CrossRef]
- Short, T.; Yamada, P.M. Exploring the mechanistic trail connecting cellular function, health, and athletic performance with phase angle: A review on the physiology of phase angle and exercise-based interventions. Top. Exerc. Sci. Kinesiol. 2024, 5, 7. [Google Scholar]
- Annunziata, G.; Paoli, A.; Frias-Toral, E.; Marra, S.; Campa, F.; Verde, L.; Colao, A.; Lukaski, H.; Simancas-Racines, D.; Muscogiuri, G.; et al. Use of phase angle as an indicator of overtraining in sport and physical training. J. Transl. Med. 2024, 22, 1084. [Google Scholar] [CrossRef]
- Tortu, E.; Deliceoglu, G.; Nefes, A.; Kaya, S. Body composition and regional phase angle as indicators of VO2max in elite male and female combat athletes. Int. J. Sport Cult. Sci. 2024, 12, 1–11. [Google Scholar] [CrossRef]
- Matias, C.N.; Campa, F.; Cerullo, G.; D’antona, G.; Giro, R.; Faleiro, J.; Reis, J.F.; Monteiro, C.P.; Valamatos, M.J.; Teixeira, F.J. Bioelectrical impedance vector analysis discriminates aerobic power in futsal players: The role of body composition. Biology 2022, 11, 505. [Google Scholar] [CrossRef]
- Silvino, V.O.; Barros, K.R.B.; Brito, F.M.; Magalhães, F.M.D.; Carioca, A.A.F.; Loureiro, A.C.C.; Veras-Silva, A.S.; Drummond, M.D.M.; dos Santos, M.A.P. Phase angle as an indicator of body composition and physical performance in handball players. BMC Sports Sci. Med. Rehabil. 2024, 16, 114. [Google Scholar]
- Campa, F.; Matias, C.N.; Marini, E.; Heymsfield, S.B.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Identifying athlete body fluid changes during a competitive season with bioelectrical impedance vector analysis. Int. J. Sports Physiol. Perform. 2020, 15, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Castizo-Olier, J.; Carrasco-Marginet, M.; Roy, A.; Chaverri, D.; Iglesias, X.; Pérez-Chirinos, C.; Rodríguez, F.; Irurtia, A. Bioelectrical impedance vector analysis (BIVA) and body-mass changes in an ultra-endurance triathlon event. J. Sports Sci Med. 2018, 17, 571–579. [Google Scholar] [PubMed]
- Cebrián-Ponce, Á.; Marini, E.; Stagi, S.; Castizo-Olier, J.; Carrasco-Marginet, M.; Garnacho-Castaño, M.V.; Noriega, Z.; Espasa-Labrador, J.; Irurtia, A. Body fluids and muscle changes in trail runners of various distances. PeerJ 2023, 11, e16563. [Google Scholar] [CrossRef] [PubMed]
- Gravina-Cognetti, F.; Chaverri, D.; Planas, A.; Montraveta, J.; Carrasco-Marginet, M.; Puigarnau, S.; Espasa-Labrador, J.; Iglesias, X. Mechanical running power and energy expenditure in uphill and downhill running. Sports 2025, 13, 294. [Google Scholar] [CrossRef]
- Björklund, G.; Swarén, M.; Born, D.P.; Stöggl, T. Biomechanical adaptations and performance indicators in short trail running. Front. Physiol. 2019, 10, 506. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Williamson, C.M.; Nickerson, B.S.; Bechke, E.E.; McLester, C.N.; Kliszczewicz, B.M. Influence of acute consumption of caffeine vs. placebo over Bia-derived measurements of body composition: A randomized, double-blind, crossover design. J. Int. Soc. Sports Nutr. 2018, 15, 7. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Cerezuela-Espejo, V.; Hernández-Belmonte, A.; Courel-Ibáñez, J.; Conesa-Ros, E.; Mora-Rodríguez, R.; Pallarés, J.G. Are we ready to measure running power? repeatability and concurrent validity of five commercial technologies. Eur. J. Sport Sci. 2021, 21, 341–350. [Google Scholar] [CrossRef]
- Campa, F.; Gatterer, H.; Lukaski, H.; Toselli, S. Stabilizing bioimpedance-vector-analysis measures with a 10-min cold shower after running exercise to enable assessment of body hydration. Int. J. Sports Physiol. Perform. 2019, 14, 1006–1009. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Giorgi, A.; Vicini, M.; Pollastri, L.; Lombardi, E.; Magni, E.; Andreazzoli, A.; Gatterer, H. Bioimpedance patterns and bioelectrical impedance vector analysis (BIVA) of road cyclists. J. Sports Sci. 2018, 36, 2608–2613. [Google Scholar] [CrossRef]
- Abdelnour, M.; Berkachy, R.; Nasreddine, L.; Fares, E.J. Bioelectrical impedance vector analysis (BIVA) for assessment of hydration status: A comparison between endurance and strength university athletes. Sensors 2024, 24, 6024. [Google Scholar] [CrossRef]
- Genton, L.; Mareschal, J.; Norman, K.; Karsegard, V.L.; Delsoglio, M.; Pichard, C.; Graf, C.; Herrmann, F.R. Association of phase angle and running performance. Clin. Nutr. ESPEN 2020, 37, 65–68. [Google Scholar] [CrossRef]
- Sardinha, L.B.; Rosa, G.B. Phase angle, muscle tissue, and resistance training. Rev. Endocr. Metab. Disord. 2023, 24, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Marginet, M.; Castizo-Olier, J.; Rodríguez-Zamora, L.; Iglesias, X.; Rodríguez, F.A.; Chaverri, D.; Brotons, D.; Irurtia, A. Bioelectrical impedance vector analysis (BIVA) for measuring the hydration status in young elite synchronized swimmers. PLoS ONE 2017, 12, e0178819. [Google Scholar] [CrossRef] [PubMed]
- Nescolarde, L.; Roca, E.; Bogónez-Franco, P.; Hernández-Hermoso, J.; Bayes-Genis, A.; Ara, J. Relationship between bioimpedance vector displacement and renal function after a marathon in non-elite runners. Front. Physiol. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Costa Pereira, J.P.D.; Rebouças, A.S.; Prado, C.M.; Gonzalez, M.C.; Cabral, P.C.; Diniz, A.D.S.; Trussardi Fayh, A.P.; Silva, F.M. Phase angle as a marker of muscle quality: A systematic review and meta-analysis. Clin. Nutr. 2024, 43, 308–326. [Google Scholar] [CrossRef]
- Bellido, D.; García-García, C.; Talluri, A.; Lukaski, H.C.; García-Almeida, J.M. Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 2023, 24, 563–583. [Google Scholar] [CrossRef]
- Short, T.; Yamada, P.M. Maximal running speed and critical speed are positively related to phase angle in healthy young adults. Int. J. Exerc. Sci. 2024, 17, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Marra, M.; Di Vincenzo, O.; Cioffi, I.; Sammarco, R.; Morlino, D.; Scalfi, L. Resting energy expenditure in elite athletes: Development of new predictive equations based on anthropometric variables and bioelectrical impedance analysis derived phase angle. J. Int. Soc. Sports Nutr. 2021, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Nescolarde, L.; Yanguas, J.; Hernández-Hermoso, J.A. Localized hamstring bioimpedance in marathon runners is related to muscle high-energy enzyme serum levels and predicts race time. Front. Physiol. 2024, 15, 1413949. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Hirata, K.; Iida, N.; Kanda, A.; Shoji, M.; Yoshida, T.; Myachi, M.; Akagi, R. Membrane capacitance and characteristic frequency are associated with contractile properties of skeletal muscle. Med. Eng. Phys. 2022, 106, 103832. [Google Scholar] [CrossRef]
- Renaud, J.M.; Ørtenblad, N.; McKenna, M.J.; Overgaard, K. Exercise and fatigue: Integrating the role of K+, Na+ and Cl− in the regulation of sarcolemmal excitability of skeletal muscle. Eur. J. Appl. Physiol. 2023, 123, 2345–2378. [Google Scholar] [CrossRef]
- McKenna, M.J.; Renaud, J.M.; Ørtenblad, N.; Overgaard, K. A century of exercise physiology: Effects of muscle contraction and exercise on skeletal muscle Na+, K+-ATPase, Na+ and K+ ions, and on plasma K+ concentration-historical developments. Eur. J. Appl. Physiol. 2024, 124, 681–751. [Google Scholar] [CrossRef]
- Bishop, D.J.; Granata, C.; Eynon, N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 1266–1275. [Google Scholar] [CrossRef]
- Cooper, S.T.; McNeil, P.L. Membrane repair: Mechanisms and pathophysiology. Physiol. Rev. 2015, 95, 1205–1240. [Google Scholar] [CrossRef]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef]
- Cebrián-Ponce, Á.; Irurtia, A.; Carrasco-Marginet, M.; Saco-Ledo, G.; Girabent-Farrés, M.; Castizo-Olier, J. Electrical impedance myography in health and physical exercise: A systematic review and future perspectives. Front. Physiol. 2021, 12, 740877. [Google Scholar] [CrossRef]
- da Silva, B.R.; Gonzalez, M.C.; Cereda, E.; Prado, C.M. Exploring the potential role of phase angle as a marker of oxidative stress: A narrative review. Nutrition 2022, 93, 111493. [Google Scholar] [CrossRef]
| Mean ± SD (n = 15) | Range (Min–Max) | |
|---|---|---|
| Age (years) | 37.27 ± 6.55 | 27.32–48.25 |
| Body mass (kg) | 70.89 ± 7.05 | 61.80–81.80 |
| Stature (cm) | 176.06 ± 5.96 | 167.60–187.00 |
| BMI (kg/m2) | 22.85 ± 1.63 | 20.19–25.03 |
| Mean ± SD | Range (Min–Max) | |
|---|---|---|
| VO2max (mL·min−1·kg−1) | 61.04 ± 6.91 | 51.01–76.42 |
| VO2mean (mL·min−1) | 3387.89 ± 532.43 | 2258.04–4114.46 |
| VO2peak (mL·min−1) | 4401.4 ± 700.18 | 2919.10–5294.99 |
| VCO2mean (mL·min−1) | 2712.37 ± 390.08 | 2037.35–3608.51 |
| VCO2peak (mL·min−1) | 3772.23 ± 569.95 | 2806.70–5130.74 |
| RQmean | 0.80 ± 0.08 | 0.67–0.92 |
| RQpeak | 0.87 ± 0.11 | 0.71–1.10 |
| HRmean (bpm) | 138.26 ± 9.50 | 115.00–157.99 |
| HRpeak (bpm) | 158.61 ± 9.33 | 138.00–176.44 |
| EEMmean (kcal·min−1) | 16.2 ± 2.41 | 11.05–19.31 |
| EEMpeak (kcal·min−1) | 21.27 ± 3.14 | 14.59–25.00 |
| POmean (W) | 250.21 ± 26.95 | 197.74–296.90 |
| POpeak (W) | 335.21 ± 35.75 | 265.00–396.17 |
| Pre-Test | Post-Test | Δ% | Statistical Differences | |
|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | (Delta Value Differences) | ||
| Impedance (Ω) | 512.03 ± 47.87 (432.01–593.31) | 525.71 ± 46.78 (435.98–602.27) | 2.73 ± 2.11 | t = −5.03; p = 0.001; CI 95% = −19.52, −7.85 |
| Resistance (Ω) | 506.97 ± 48.06 (428.29–588.50) | 521.05 ± 46.81 (432.49–597.5) | 2.84 ± 2.20 | t = −5.02; p = 0.001; CI 95% = −20.09, −08.06 |
| Reactance (Ω) | 71.08 ± 9.58 (56.4–96.1) | 69.32 ± 8.87 (55–89.9) | −2.36 ± 3.48 | t = 2.70; p = 0.02; CI 95% = 0.36, 3.16 |
| Impedance (Ω/h) | 291.30 ± 27.21 (256.84–344.38) | 298.76 ± 26.80 (259.2–345.55) | 2.60 ± 1.96 | t = −5.18; p = 0.001; CI 95% = −10.55, −4.37 |
| Resistance (Ω/h) | 288.41 ± 27.23 (254.63–341.38) | 298.43 ± 25.88 (257.13–342.79) | 2.71 ± 2.05 | t = −5.16; p = 0.001; CI 95% = −10.89, −4.50 |
| Reactance (Ω/h) | 40.50 ± 5.97 (30.97–56.53) | 39.43 ± 5.38 (31.91–52.88) | −2.46 ± 3.74 | t = 2.66; p = 0.02; CI 95% = 0.21, 1.94 |
| Impedance (Ω/sp) | 350.07 ± 26.76 (295.33–397.09) | 358.49 ± 26.50 (307.37–405.91) | 2.44 ± 1.92 | t = −5.04; p = 0.001; CI 95% = −12.00, −4.84 |
| Resistance (Ω/sp) | 346.62 ± 27.09 (289.13–393.83) | 355.31 ± 26.59 (302.16–402.71) | 2.55 ± 2.01 | t = −5.07; p = 0.001; CI 95% = −12.36, −5.02 |
| Reactance (Ω/sp) | 48.56 ± 5.60 (36.78–60.21) | 47.29 ± 5.58 (36.19–56.61) | −2.60 ± 4.13 | t = 2.63; p = 0.02; CI 95% = 0.24, 2.32 |
| Phase angle (°) | 8.02 ± 1.22 (6.88–11.76) | 7.57 ± 0.95 (6.19–10.56) | −4.91 ± 4.78 | W = 115; p = 0.01; CI 95% = 0.13, 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gravina-Cognetti, F.; Espasa-Labrador, J.; Cebrián-Ponce, Á.; Carrasco-Marginet, M.; Puigarnau, S.; Chaverri, D.; Iglesias, X.; Irurtia, A. Is Bioelectrical Impedance Vector Analysis (BIVA) a Useful Exploratory Tool to Assess Exercise-Induced Metabolic and Mechanical Responses in Endurance-Trained Male Trail Runners? Appl. Sci. 2025, 15, 10768. https://doi.org/10.3390/app151910768
Gravina-Cognetti F, Espasa-Labrador J, Cebrián-Ponce Á, Carrasco-Marginet M, Puigarnau S, Chaverri D, Iglesias X, Irurtia A. Is Bioelectrical Impedance Vector Analysis (BIVA) a Useful Exploratory Tool to Assess Exercise-Induced Metabolic and Mechanical Responses in Endurance-Trained Male Trail Runners? Applied Sciences. 2025; 15(19):10768. https://doi.org/10.3390/app151910768
Chicago/Turabian StyleGravina-Cognetti, Fabrizio, Javier Espasa-Labrador, Álex Cebrián-Ponce, Marta Carrasco-Marginet, Silvia Puigarnau, Diego Chaverri, Xavier Iglesias, and Alfredo Irurtia. 2025. "Is Bioelectrical Impedance Vector Analysis (BIVA) a Useful Exploratory Tool to Assess Exercise-Induced Metabolic and Mechanical Responses in Endurance-Trained Male Trail Runners?" Applied Sciences 15, no. 19: 10768. https://doi.org/10.3390/app151910768
APA StyleGravina-Cognetti, F., Espasa-Labrador, J., Cebrián-Ponce, Á., Carrasco-Marginet, M., Puigarnau, S., Chaverri, D., Iglesias, X., & Irurtia, A. (2025). Is Bioelectrical Impedance Vector Analysis (BIVA) a Useful Exploratory Tool to Assess Exercise-Induced Metabolic and Mechanical Responses in Endurance-Trained Male Trail Runners? Applied Sciences, 15(19), 10768. https://doi.org/10.3390/app151910768








