Effect of Denture Adhesives on the Surface Roughness and Hardness of Denture Base Resins—A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Resin Specimens for Denture Base
2.2. Dental Adhesives
2.3. Experimental Protocol
2.3.1. Surface Roughness
2.3.2. Shore D Hardness
2.4. Statistical Analysis
3. Results
3.1. Baseline Evaluation (T0)—Comparison Between Materials
3.2. Effect of Adhesive Solutions (T28)—Comparison Between Subgroups
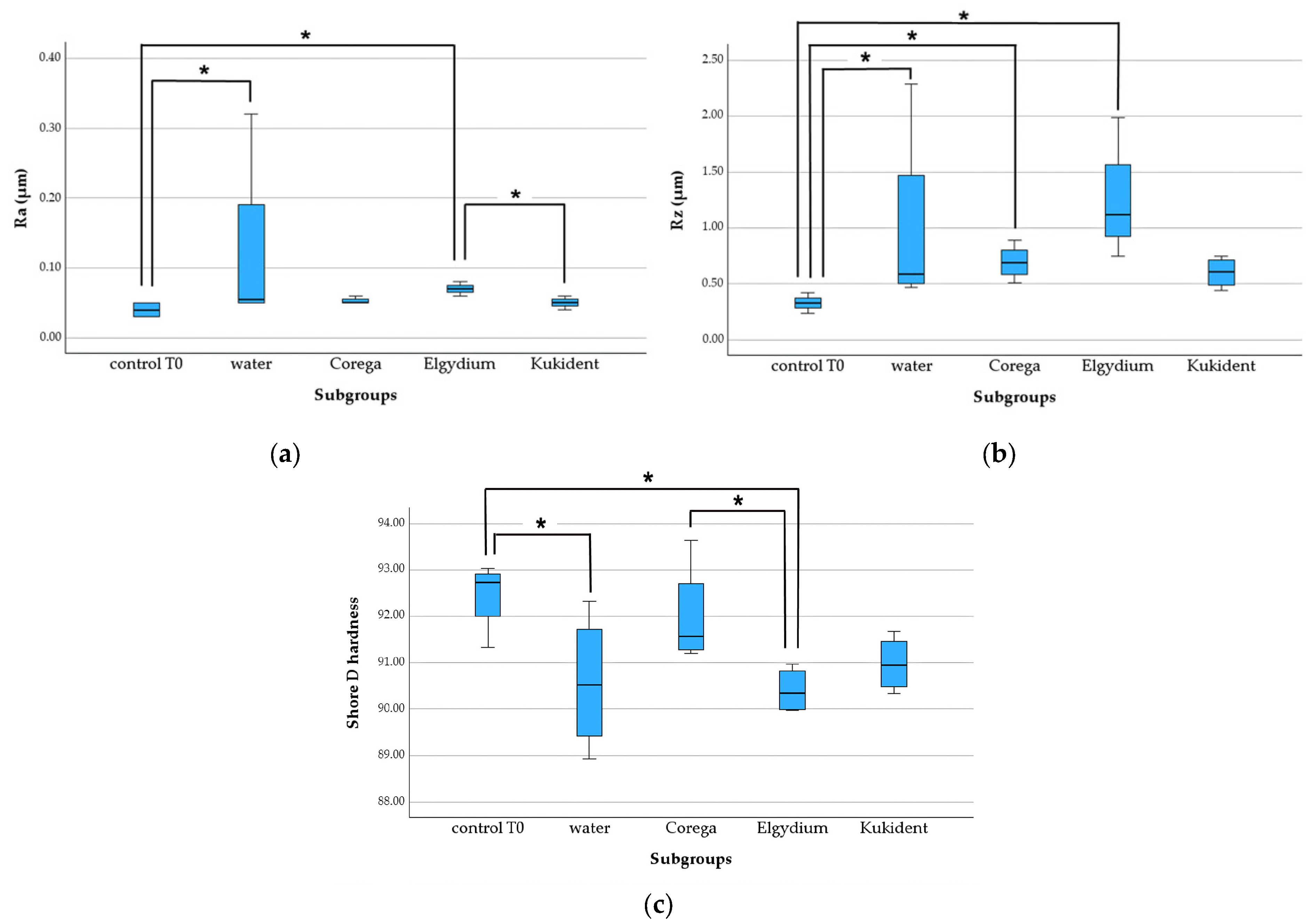
3.2.1. Heat-Cured Group
- Ra: control—water (p = 0.047), control—Elgydium (p = 0.02), and Elgydium—Kukident (p = 0.028);
- Rz: control—water (p = 0.042), control—Corega (p = 0.027), and control—Elgydium (p < 0.001);
- Shore D hardness: control—water (p = 0.023), control—Elgydium (p = 0.009), and Corega—Elgydium (p = 0.031).
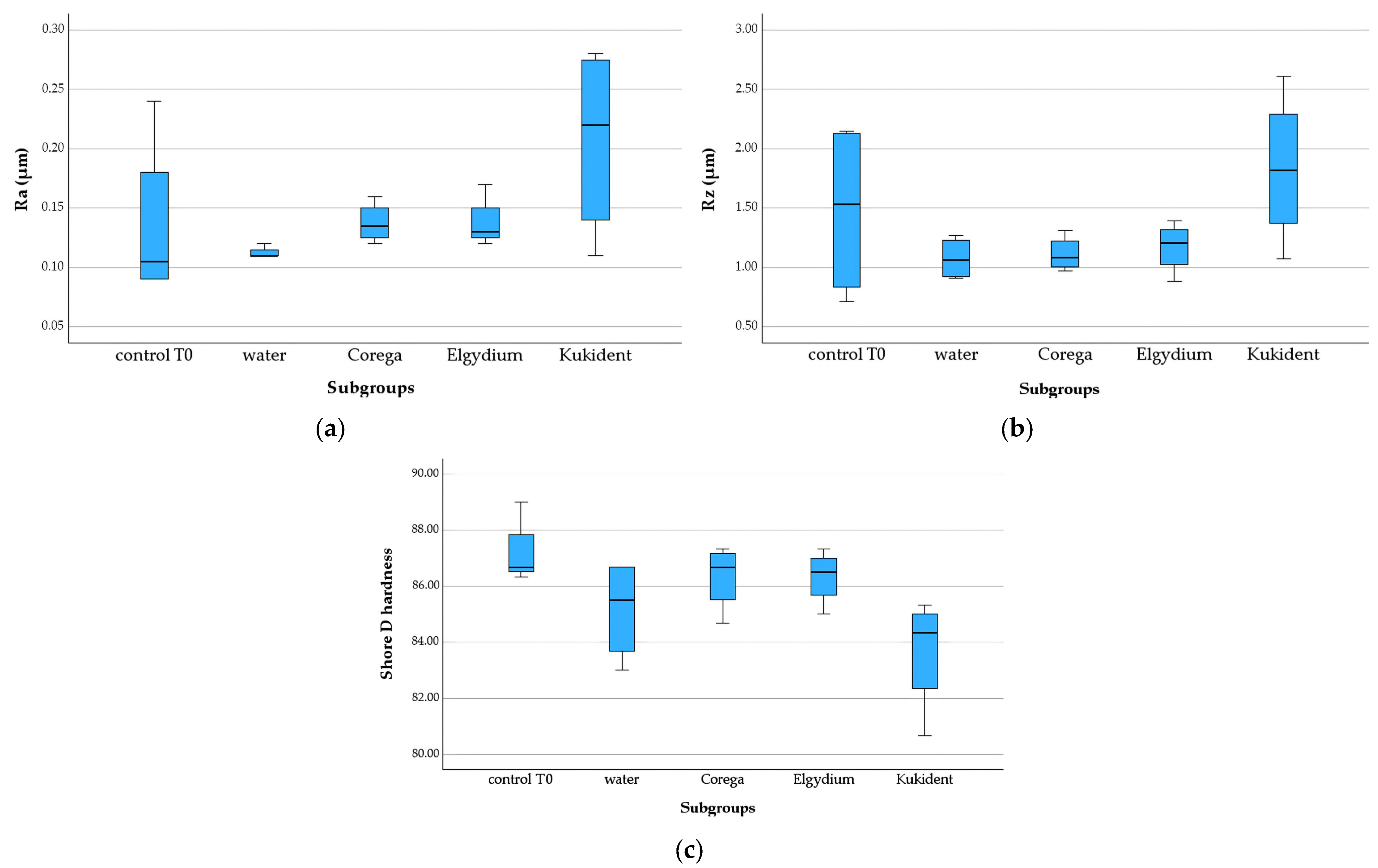
3.2.2. 3D-Printed Group
3.2.3. Milled Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grasso, J.E. Denture adhesives: Changing attitudes. J. Am. Dent. Assoc. 1996, 127, 90–96. [Google Scholar] [CrossRef]
- Lemos, C.A.A.; da Fonte Porto Carreiro, A.; Rosa, C.D.D.R.D.; Luna Gomes, J.M.; de Oliveira Limirio, J.P.J.; Mendonça, G.; Pellizzer, E.P. Does the use of an adhesive improve conventional complete dentures? A systematic review of randomized controlled trials. J. Prosthet. Dent. 2022, 128, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Florêncio Costa, R.T.; Leite Vila-Nova, T.E.; Barbosa de França, A.J.; Gustavo da Silva Casado, B.; de Souza Leão, R.; Dantas de Moraes, S.L. Masticatory performance of denture wearers with the use of denture adhesives: A systematic review. J. Prosthet. Dent. 2022, 127, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Almusallam, M.O.; Almarar, F.H.; Al khaldi, H.O.; Aldossary, A.K.; Almutairi, W.M.; Alghamdi, N.A.; Alssaialiy, Y.S.; Alghamdi, N.I.; Nazir, M.A. Denture Adhesive Utilization and Associated Factors among Dental Practitioners in the Eastern Province, Saudi Arabia. Medicina 2023, 59, 974. [Google Scholar] [CrossRef]
- Felton, D.; Cooper, L.; Duqum, I.; Minsley, G.; Guckes, A.; Haug, S.; Meredith, P.; Solie, C.; Avery, D.; Deal Chandler, N. Evidence-based guidelines for the care and maintenance of complete dentures: A publication of the American College of Prosthodontists. J. Prosthodont. 2011, 20, S1–S12. [Google Scholar] [CrossRef]
- Ereifej, N.S.; Oweis, Y.G.; Abu-Awwad, M. The effect of using denture adhesives on patient satisfaction with complete dentures; a randomized clinical trial. BMC Oral Health 2023, 23, 1027. [Google Scholar] [CrossRef]
- Kelsey, C.C.; Lang, B.R.; Wang, R.F. Examining patients’ responses about the effectiveness of five denture adhesive pastes. J. Am. Dent. Assoc. 1997, 128, 1532–1538. [Google Scholar] [CrossRef]
- Pradíes, G.; Sanz, I.; Evans, O.; Martínez, F.; Sanz, M. Clinical study comparing the efficacy of two denture adhesives in complete denture patients. Int. J. Prosthodont. 2009, 22, 361–367. [Google Scholar]
- Pinto, J.R.; Bonifácio, C.C.; Campos, J.C. Avaliação das propriedades físico-químicas de adesivos para próteses totais. Rev. Odontol. UNESP 2020, 49, e20200056. [Google Scholar]
- Pisani, M.X.; Malheiros-Segundo, P.A.; Paranhos, H.F. Uso de adesivos para prótese total: Revisão e recomendações clínicas. Rev. Odontol. Bras. Cent. 2015, 24, 34–39. [Google Scholar]
- Shay, K. Denture adhesives: Choosing wisely. Compend. Contin. Educ. Dent. 2003, 24, 18–26. [Google Scholar]
- Polyzois, G.L.; Lagouvardos, P.E.; Vahidi, F. Effects of zinc-containing denture adhesives on oral and systemic health: A review. J. Prosthodont. 2011, 20, 294. [Google Scholar]
- Osman, R.B.; Khoder, G.; Fayed, B.; Kedia, R.A.; Elkareimi, Y.; Alharbi, N. Influence of Fabrication Technique on Adhesion and Biofilm Formation of Candida albicans to Conventional, Milled, and 3D-Printed Denture Base Resin Materials: A Comparative In Vitro Study. Polymers 2023, 15, 1836. [Google Scholar] [CrossRef] [PubMed]
- Radford, D.R.; Sweet, S.P.; Challacombe, S.J.; Walter, J.D. Adherence of Candida albicans to denture-base materials with different surface finishes. J. Dent. 1998, 26, 577–583. [Google Scholar] [CrossRef]
- Arzani, S.; Khorasani, E.; Mokhlesi, A.; Azadian, S.; Ghodsi, S.; Mosaddad, S.A. Do 3D-Printed and Milled Denture Bases Differ in Microbial Activity and Adhesion? A Systematic Review and Meta-Analysis. Int. Dent. J. 2025, 75, 100857. [Google Scholar] [CrossRef]
- Perić, M.; Petrović, S.; Čairović, A.; Vlajić Tovilović, T.; Racić, A.; Panajotović, R.; Živković, R.; Miličić, B.; Radunović, M. Influence of ageing on surface properties and biofilm adhesion on denture base resin material fabricated by different manufacturing techniques. Clin. Oral Investig. 2025, 29, 423. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.P.; Harty, D.W.S.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef]
- Figueiral, M.H.; Azul, A.; Pinto, E.; Fonseca, P.A.; Branco, F.M.; Scully, C. Denture-related stomatitis: Identification of aetiological and predisposing factors—A large cohort. J. Oral Rehabil. 2007, 34, 448–455. [Google Scholar] [CrossRef]
- Sanitá, P.V.; Pavarina, A.C.; Giampaolo, E.T.; Silva, M.M.; Mima, E.G.; Ribeiro, D.G.; Vergani, C.E. Candida spp. prevalence in well-controlled type 2 diabetic patients with denture stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 726–733. [Google Scholar] [CrossRef]
- Ramage, G.; Martínez, J.P.; López-Ribot, J.L. Candida biofilms on implanted biomaterials: A clinically significant problem. FEMS Yeast Res. 2006, 6, 979–986. [Google Scholar] [CrossRef]
- Hanno, K.I.; Metwally, N.A. The wettability of complete denture base materials constructed by conventional versus digital techniques: An in-vitro study. BMC Oral Health 2024, 24, 1081. [Google Scholar] [CrossRef] [PubMed]
- Sri, H.; Maiti, S.; Rajaraman, V.; Ganapathy, D. Comparative evaluation of Wettability of Conventional vs CAD/CAM denture base resins. J. Coast. Life Med. 2022, 10, 270–279. [Google Scholar]
- Srinivasan, M.; Kalberer, N.; Kamnoedboon, P.; Mekki, M.; Durual, S.; Özcan, M.; Müller, F. CAD-CAM complete denture resins: An evaluation of biocompatibility, mechanical properties, and surface characteristics. J. Dent. 2021, 114, 103785. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Çakmak, G.; Fonseca, M.; Schimmel, M.; Yilmaz, B. Effect of thermocycling on the surface properties of CAD-CAM denture base materials after different surface treatments. J. Mech. Behav. Biomed. Mater. 2021, 121, 104646. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef]
- Shahal, Y.; Steinberg, D.; Hirschfeld, Z.; Bronshteyn, M.; Kopolovic, K. In vitro bacterial adherence onto pellicle-coated aesthetic restorative materials. J. Oral Rehabil. 1998, 25, 52–58. [Google Scholar] [CrossRef]
- Verran, J.; Maryan, C.J. Retention of Candida albicans on acrylic resin and silicone of different surface topography. J. Prosthet. Dent. 1997, 77, 535–539. [Google Scholar] [CrossRef]
- Gomes, A.S.; Sampaio-Maia, B.; Vasconcelos, M.; Fonseca, P.A.; Figueiral, H. In situ evaluation of the microbial adhesion on a hard acrylic resin and a soft liner used in removable prostheses. Int. J. Prosthodont. 2015, 28, 65–71. [Google Scholar] [CrossRef][Green Version]
- Poker, B.C.; Oliveira, V.C.; Macedo, A.P.; Gonçalves, M.; Ramos, A.P.; Silva-Lovato, C.H. Evaluation of surface roughness, wettability and adhesion of multispecies biofilm on 3D-printed resins for the base and teeth of complete dentures. J. Appl. Oral Sci. 2024, 32, e20230326. [Google Scholar] [CrossRef]
- Singh, B.; Jain, S.; Bhasin, N.; Kaur, J.; Borse, P.; Longkumer, P. Comparative Evaluation of Surface Roughness, Wettability, and Hardness of Conventional, Heat-Polymerized, Computer-Aided Designed and Milled, and Three-Dimensionally Printed Polymethyl Methacrylate Denture Base Resins: An In Vitro Study. Cureus 2025, 17, e85008. [Google Scholar] [CrossRef]
- Ghahramanzadeh, S.; Givshad, F.G.; Allahbakhshi, H. Effect of accelerated aging on surface roughness and hardness of conventional and CAD-CAM denture base materials. J. Prosthet. Dent. 2022, 127, 138–143. [Google Scholar]
- Gultekin, P.; Akin, H.; Akin, G.E. Effect of denture adhesives on the surface hardness of denture base materials. Eur. Oral Res. 2021, 55, 128–135. [Google Scholar]
- Sharma, P.; Arora, A.; Madan, R.; Singhal, S. Effect of denture adhesive on surface roughness and bond strength of denture base materials: An in vitro study. J. Indian Prosthodont. Soc. 2017, 17, 379–386. [Google Scholar]
- Gad, M.M.; Fouda, S.M.; Abualsaud, R.; Alshahrani, F.A.; Al-Thobity, A.M.; Khan, S.Q.; Akhtar, S.; Ateeq, I.S.; Helal, M.A.; Al-Harbi, F.A. Strength and Surface Properties of a 3D-Printed Denture Base Polymer. J. Prosthodont. 2022, 31, 412–418. [Google Scholar] [CrossRef]
- Darwish, M.; Nassani, M.Z. Evaluation of the effect of denture adhesives on surface roughness of two chemically different denture base resins. Eur. J. Dent. 2016, 10, 321–326. [Google Scholar] [CrossRef][Green Version]
- Ibraheem, E.M.A.; Hammad, H.G.H. Effect of commercially available denture adhesives on microhardness of a flexible denture base material. Open Access Maced. J. Med. Sci. 2019, 7, 862–868. [Google Scholar] [CrossRef]
- Haleon. Corega Power Max. Available online: https://www.haleonhealthpartner.com/pt-br/oral-health/brands/corega/overview/ (accessed on 16 May 2025).
- Pierre Fabre Oral Care. Elgydium Fix. Available online: https://www.pierrefabre-oralcare.com/pt-pt (accessed on 16 May 2025).
- Procter & Gamble. Kukident Pro Ultimate. Available online: https://kukident.es/es-es (accessed on 16 May 2025).
- ISO 868:2003(E); Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). International Organization for Standardization: Geneva, Switzerland, 2003.
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A comparison of the surface properties of CAD/CAM and conventional denture base materials. J. Prosthodont. 2019, 28, 452–457. [Google Scholar] [CrossRef]
- Fujimoto, K.; Minami, N.; Goto, T.; Ishida, Y.; Watanabe, M.; Nagao, K.; Ichikawa, T. Hardness, cohesiveness, and adhesiveness of oral moisturizers and denture adhesives: Selection criteria for denture wearers. Dent. J. 2016, 4, 34. [Google Scholar] [CrossRef]
- Ahmed, K.E.; McCord, J.F. Assessment of Shore D hardness of acrylic resin denture base materials in dry and wet conditions. J. Prosthodont. 2017, 26, 65–71. [Google Scholar]
- Kattadiyil, M.T.; AlHelal, A. An update on computer-engineered complete dentures: A systematic review on clinical outcomes. J. Prosthet. Dent. 2017, 117, 478–485. [Google Scholar] [CrossRef]
- Hata, K.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. Dental Poly(methyl methacrylate)-Based Resin Containing a Nanoporous Silica Filler. J. Funct. Biomater. 2022, 13, 32. [Google Scholar] [CrossRef]
- Arslan, E.; Akay, C.; Erdönmez, D.; Avukat, E.N. Evaluation of the effect of new generation denture base materials aged in artificial saliva at different pH levels on surface roughness and Candida albicans adhesion. BMC Oral Health 2025, 25, 356. [Google Scholar] [CrossRef]
- Garcia, R.C.M.R.; Souza, J.A.J., Jr.; Rached, R.N.; Del Bel Cury, A.A. Effect of denture cleansers on the surface roughness and hardness of a microwave-cured acrylic resin and dental alloys. J. Prosthodont. 2004, 13, 173–178. [Google Scholar] [CrossRef]
- Davi, L.R.; Felipucci, D.N.B.; Souza, R.F.; Bezzon, O.L.; Silva, C.H.L. Effect of denture cleansers on metal ion release and surface roughness of denture base materials. Braz. Dent. J. 2012, 23, 387–393. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Oliveira, V.M.; Vieira, A.C.; Rambob, I. In vivo assessment of the effect of an adhesive for complete dentures on colonisation of Candida species. Gerodontology 2010, 27, 303–307. [Google Scholar] [CrossRef]



| Denture Adhesive | Composition |
|---|---|
| Corega Power Max® Haleon [38] | Sodium PVM/MA copolymer, petrolatum, cellulose gum, liquid paraffin. |
| Elgydium Fix® Pierre Fabre [39] | Calcium/sodium copolymer PVM/MA, petrolatum, cellulose gum, liquid paraffin, PVP, isopropyl palmitate; isopropyl myristate. |
| Kukident Pro Ultimate® Procter & Gamble [40] | Calcium/zinc copolymer PVM/MA (35%), petrolatum, cellulose gum (20%), liquid paraffin, hydrated silica, BHT, tocopherol, CI 45410, CI 15985 (colorants). |
| Denture Adhesive/Denture Base Material | Lot Number | Expiry Date |
|---|---|---|
| Corega PowerMax® | A 58F | 05/2027 |
| Elgydium Fix® | T 72 | 02/2028 |
| Kukident Pro Ultimate® | 410028890 | 03/2026 |
| ProBase Hot®, IVOCLAR | 20190524 | NA * |
| NextDent Denture 3D+® | WU495N03 | 07/12/2025 |
| Exaktus® AROSE 30 | 20190524 | NA * |
| Material Groups | T0 | T28 | T28 | T28 | T28 |
|---|---|---|---|---|---|
| Control | Water | Corega | Elgydium | Kukident | |
| Heat-cured resin | 0.04 [0.03–0.05] | 0.06 [0.05–0.25] | 0.05 [0.05–0.06] | 0.07 [0.06–0.08] | 0.05 [0.04–0.06] |
| 3D-printed resin | 0.11 [0.09–0.21] | 0.11 [0.11–0.12] | 0.14 [0.12–0.16] | 0.13 [0.12–0.16] | 0.22 [0.13–0.28] |
| Milled resin | 0.04 [0.03–0.09] | 0.06 [0.04–0.07] | 0.07 [0.45–0.08] | 0.04 [0.03–0.05] | 0.06 [0.05–0.11] |
| Material Groups | T0 | T28 | T28 | T28 | T28 |
|---|---|---|---|---|---|
| Control | Water | Corega | Elgydium | Kukident | |
| Heat-cured resin | 0.33 [0.25–0.40] | 0.59 [0.49–1.88] | 0.69 [0.55–0.85] | 1.12 [0.84–1.78] | 0.61 [0.47–0.73] |
| 3D-printed resin | 1.53 [0.77–2.14] | 1.07 [0.92–1.25] | 1.09 [0.99–1.27] | 1.21 [0.95–1.35] | 1.88 [1.22–2.45] |
| Milled resin | 0.45 [0.27–0.66] | 0.49 [0.44–0.72] | 0.82 [0.44–0.97] | 0.40 [0.29–0.76] | 0.49 [0.43–0.84] |
| Material Groups | T0 | T28 | T28 | T28 | T28 |
|---|---|---|---|---|---|
| Control | Water | Corega | Elgydium | Kukident | |
| Heat-cured resin | 92.74 [91.67–92.97] | 90.52 [89.17–92.03] | 91.57 [91.24–93.17] | 90.34 [89.98–90.90] | 90.95 [90.41–91.57] |
| 3D-printed resin | 86.67 [86.42–88.42] | 85.50 [83.33–86.67] | 86.67 [85.09–87.25] | 86.55 [85.33–87.17] | 84.34 [81.50–85.17] |
| Milled resin | 92.77 [92.28–93.51] | 91.17 [90.75–91.59] | 91.50 [90.58–92.77] | 92.37 [92.33–92.90] | 92.94 [91.47–93.25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, G.B.; Sampaio-Fernandes, M.M.; Fernandes, C.; Góis, F.; Graça, B.; Bonfante, E.; Figueiral, M.H. Effect of Denture Adhesives on the Surface Roughness and Hardness of Denture Base Resins—A Preliminary Study. Appl. Sci. 2025, 15, 10749. https://doi.org/10.3390/app151910749
Alves GB, Sampaio-Fernandes MM, Fernandes C, Góis F, Graça B, Bonfante E, Figueiral MH. Effect of Denture Adhesives on the Surface Roughness and Hardness of Denture Base Resins—A Preliminary Study. Applied Sciences. 2025; 15(19):10749. https://doi.org/10.3390/app151910749
Chicago/Turabian StyleAlves, Guilherme Bezerra, Maria Margarida Sampaio-Fernandes, Carlos Fernandes, Francisco Góis, Bruno Graça, Estevam Bonfante, and Maria Helena Figueiral. 2025. "Effect of Denture Adhesives on the Surface Roughness and Hardness of Denture Base Resins—A Preliminary Study" Applied Sciences 15, no. 19: 10749. https://doi.org/10.3390/app151910749
APA StyleAlves, G. B., Sampaio-Fernandes, M. M., Fernandes, C., Góis, F., Graça, B., Bonfante, E., & Figueiral, M. H. (2025). Effect of Denture Adhesives on the Surface Roughness and Hardness of Denture Base Resins—A Preliminary Study. Applied Sciences, 15(19), 10749. https://doi.org/10.3390/app151910749







