Anthocyanins Separated from Degrained Purple-Corn Cobs with Aqueous Biphasic Systems as Food Pigments
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of the Binodal Diagram
2.3. Separation of Bioactive Compounds
2.4. Pigmenting Potential
2.5. Evaluation of Variables
2.6. Data Analysis
3. Results and Discussion
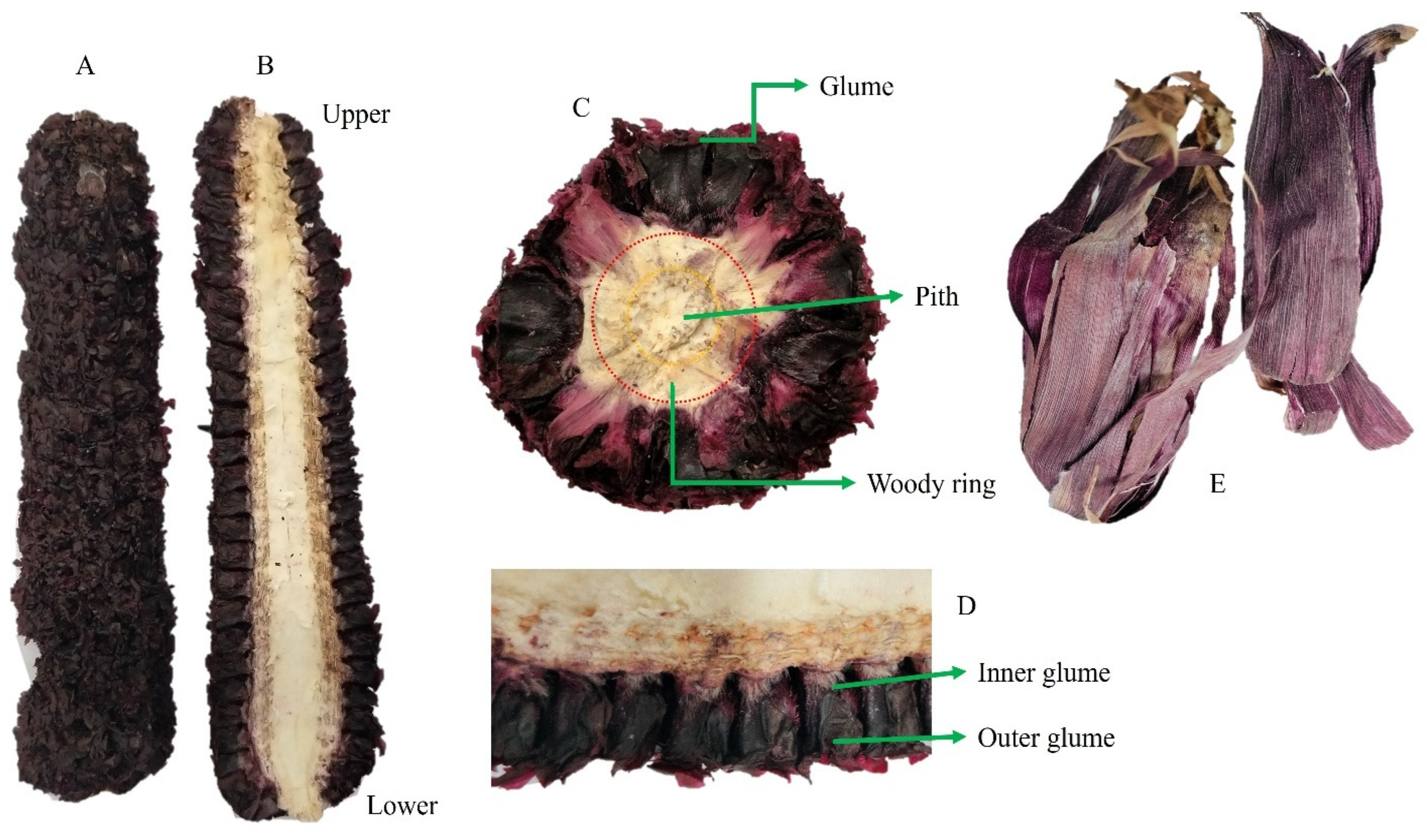
3.1. Visual and Compositional Characteristics of Degrained Corn Cob Flours
3.2. Binodal Phase Diagram
3.3. Bioactive Compounds

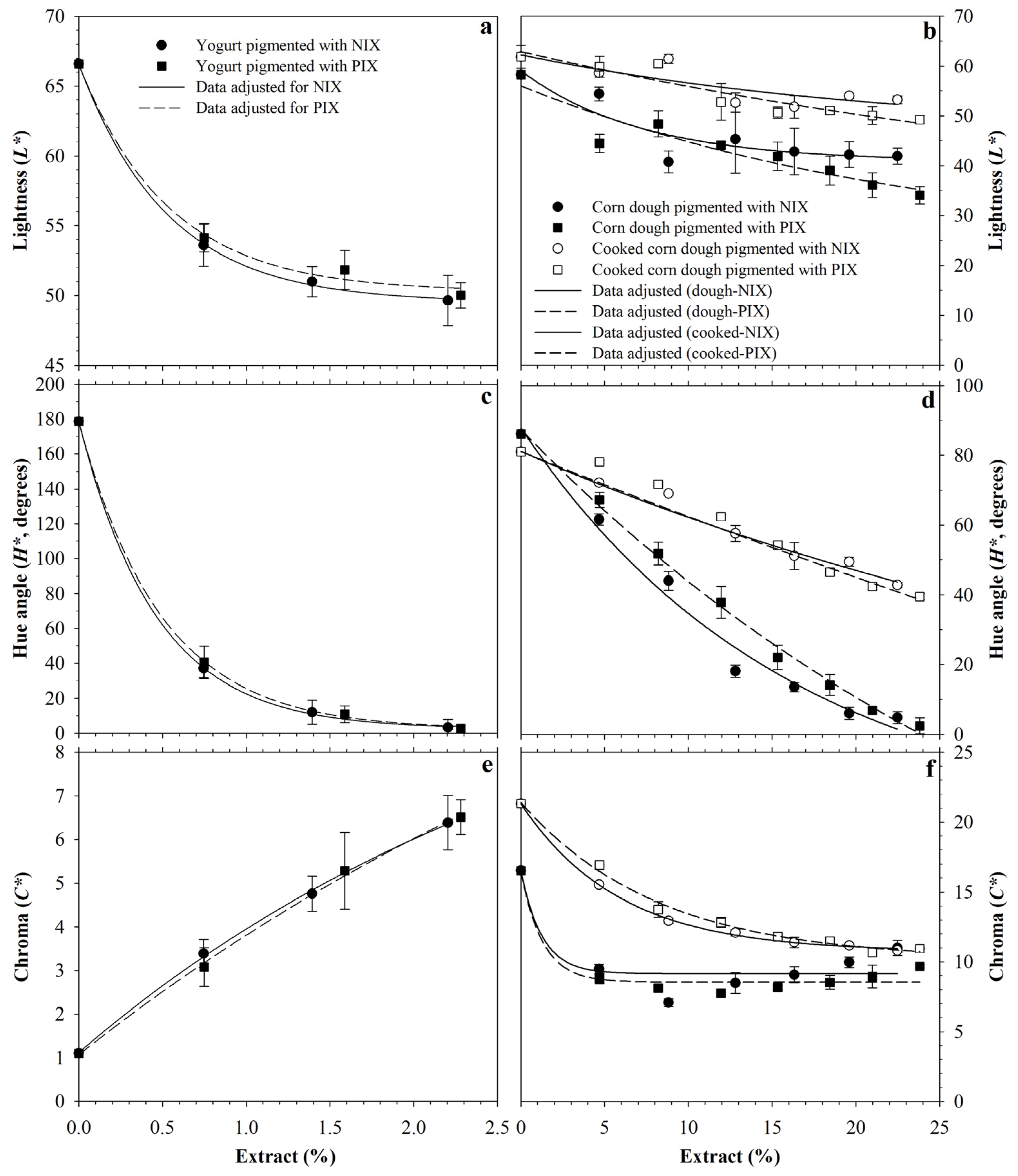
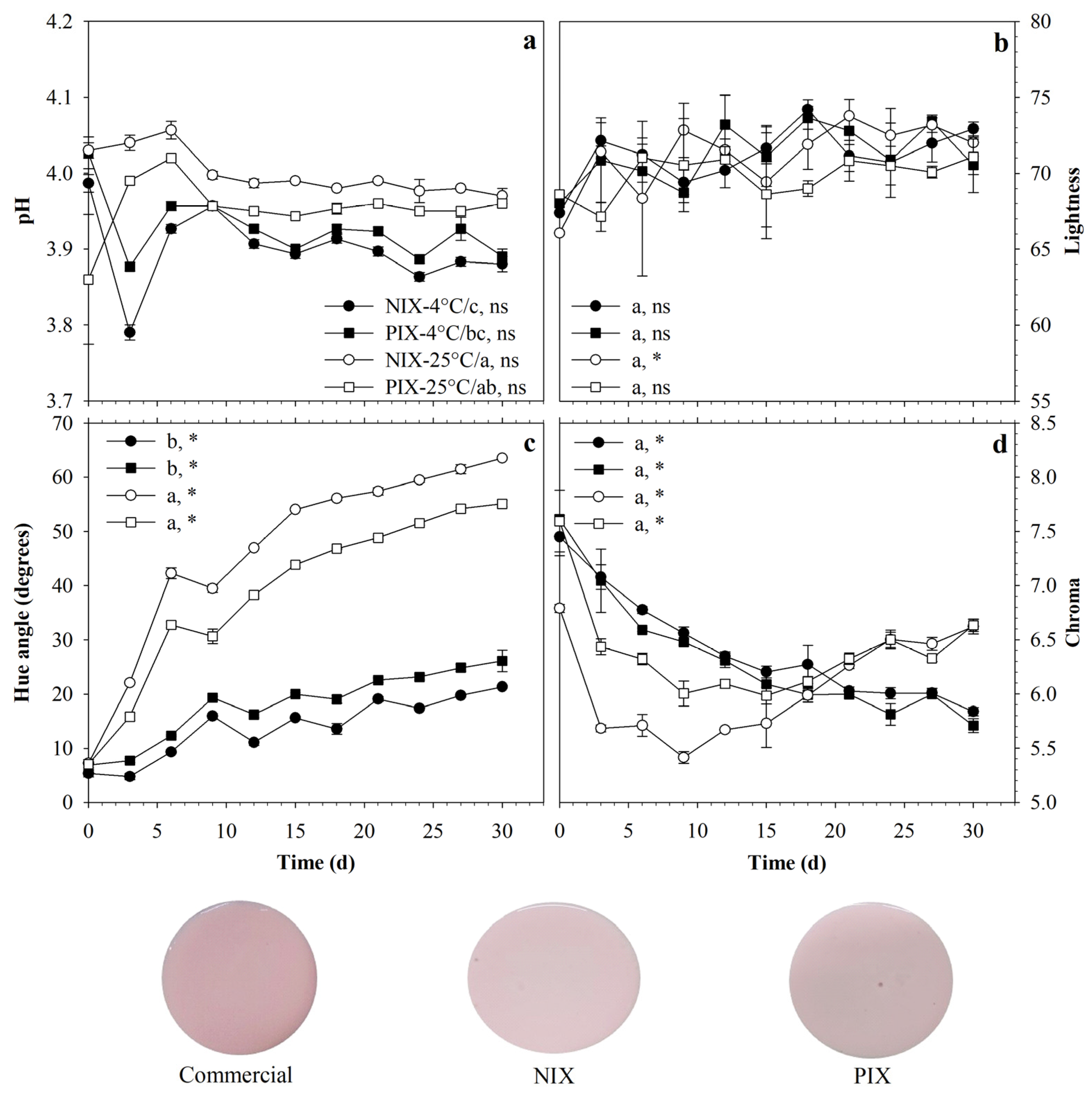
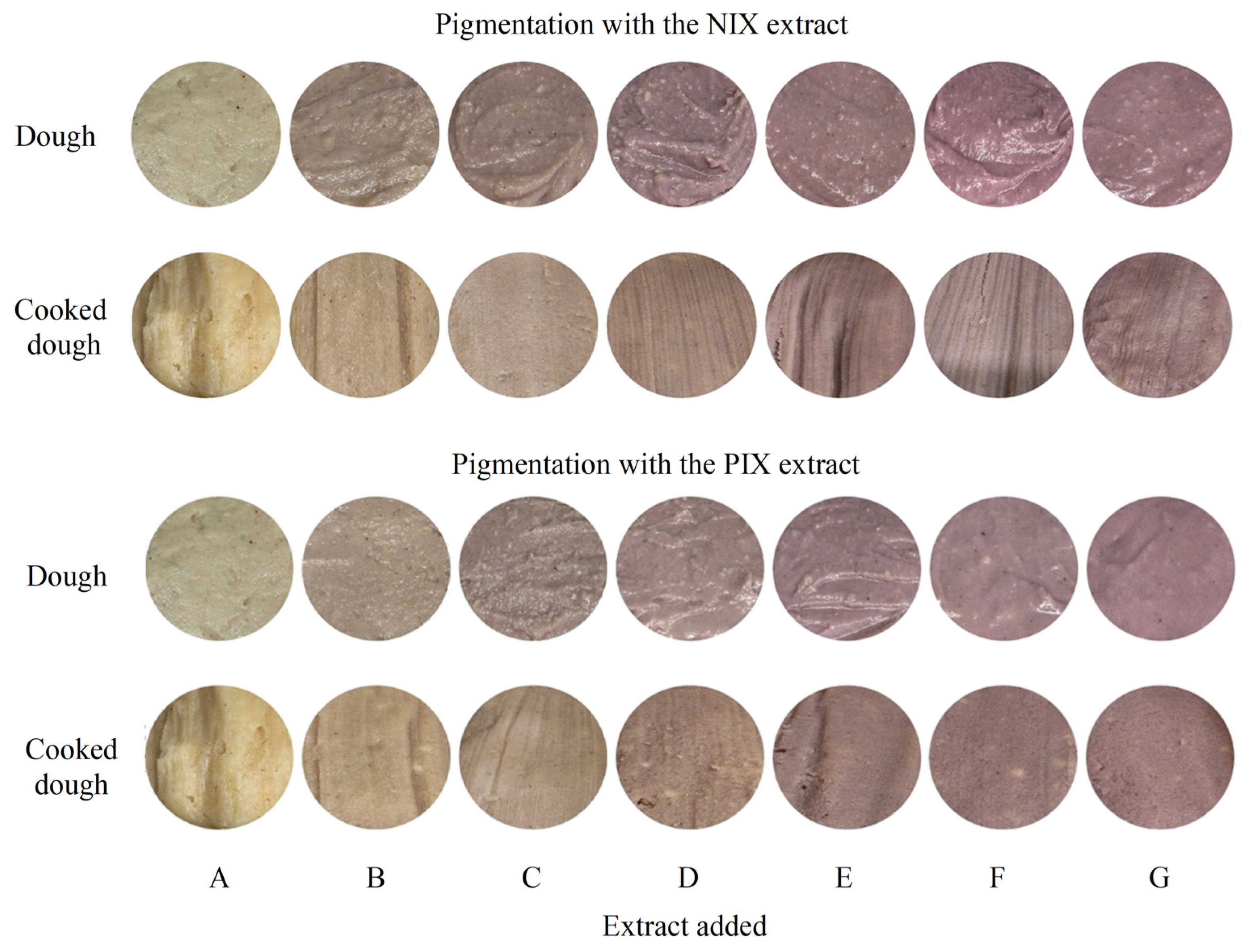
3.4. Pigmentation Potential of Foods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chroma | |
| Hue angle (degrees) | |
| Lightness | |
| Ant | Anthocyanin content (mg CyEs/100 g) |
| CA | Citric acid (3-carboxy-3-hydroxypentanedioic acid) |
| CyE | Cyanidin equivalent |
| EtOH | Ethanol (CH3-CH2-OH) grade 96 |
| FAE | Ferulic acid equivalent |
| NIX | Negro de Ixtenco purple corn |
| pH | Potential of hydrogen |
| PIX | Negro de Ixtenco x Negro de Perú purple corn |
| SC | Trisodium citrate (Na3C3H5O(COO)3) |
| TSP | Total soluble phenol content (mg FAEs/100 g) |
References
- POWO Plants of the World Online. Royal Botanic Gardens, Kew. 2025. Available online: https://powo.science.kew.org/ (accessed on 8 June 2025).
- Hernández, E.d.l.C.; Andueza-Noh, R.H.; Latournerie-Moreno, L.; Ruiz-Sanchez, E.; Gordillo Ruiz, M.C.; Pérez, G.R. Diversity and Genetic Structure of Maize Landraces Cultivated in the Zoque Region from Chiapas, Mexico. Diversity 2025, 17, 159. [Google Scholar] [CrossRef]
- Pérez Ruiz, R.V.; Aguilar Toalá, J.E.; Cruz Monterrosa, R.G.; Rayas Amor, A.A.; Rodríguez, M.H.; Villasana, Y.C.; Pérez, J.H. Mexican Native Maize: Origin, Races and Impact on Food and Gastronomy. Int. J. Gastron. Food Sci. 2024, 37, 100978. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, C.G.; Mendoza-Castillo, M.D.C.; Delgado-Alvarado, A.; Sánchez-Ramírez, F.J.; Kato-Yamakake, T.Á. Anthocyanins Content in the Kernel and Corncob of Mexican Purple Corn Populations. Maydica 2020, 65, 1–10. [Google Scholar]
- Rojas, M.L.; Ramirez, K.; Linares, G. Biocompounds Recovery from Purple Corn Cob By-Product: Extraction Kinetics, Thermal and Physicochemical Stability of Liquid and Powdered Anthocyanin-Rich Extract. Food Bioprod. Process. 2025, 149, 25–35. [Google Scholar] [CrossRef]
- Trezzi, S.; Scaccabarozzi, G.; Nossa, R.; Piazza, C.; Bianchi, A.R.; Rosi, E.; Tizzoni, F.; Mauri, M.; Camillo, L.; Baragetti, A.; et al. Behavioural, Cognitive, and Neurophysiological Effects of a Synbiotic Supplementation Enriched with Pigmented Corn Extract or Cornstarch in Drug-Naïve Children with Attention-Deficit Hyperactivity Disorder: A Randomised, Double-Blind, Comparison-Controlle. Clin. Nutr. ESPEN 2025, 65, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Castorina, G.; Cappa, C.; Negrini, N.; Criscuoli, F.; Casiraghi, M.C.; Marti, A.; Rollini, M.; Consonni, G.; Erba, D. Characterization and Nutritional Valorization of Agricultural Waste Corncobs from Italian Maize Landraces through the Growth of Medicinal Mushrooms. Sci. Rep. 2023, 13, 21148. [Google Scholar] [CrossRef]
- Mandache, M.; Topală, C.M.; Vijan, L.E.; Cosmulescu, S. The Characterization of Peach Pomace and the Influence of Its Incorporation on the Chemical Composition of Biscuits. Appl. Sci. 2025, 15, 6983. [Google Scholar] [CrossRef]
- Colombo, R.; Ferron, L.; Papetti, A. Colored Corn: An up-Date on Metabolites Extraction, Health Implication, and Potential Use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef]
- Pérez-Orozco, J.P.; Sánchez-Herrera, L.M.; Barrios-Salgado, E.; Sumaya-Martínez, M.T. Kinetics of Solid-Liquid Extraction of Anthocyanins Obtained from Hibiscus Rosa-Sinensis. Rev. Mex. Ing. Química 2020, 19, 813–826. [Google Scholar] [CrossRef]
- Tena, N.; Asuero, A.G. Up-to-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Singh, B.; Bhatt, I.D. NADES-Based Extraction Optimization and Enrichment of Cyanidin-3-O-Galactoside from Rhododendron Arboreum Sm.: Kinetics and Thermodynamics Insights. Food Chem. 2024, 455, 139793. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, N.I.M.; Khushairi, N.A.A.A.; Ijod, G.; Azman, E.M. Extraction of Anthocyanins and Other Phenolics from Dried Blackcurrant (Ribes nigrum L.) Pomace via Ultrasonication. Sustain. Chem. Environ. 2025, 9, 100208. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Peng, J.; Wang, Z.; Zhao, T.; Cao, J.; Liu, Y.; Cheng, G.; Brennan, C. Chemical Composition and Functional Properties of Protein-Polyphenol Complexes from Purple Rice: Effects of Alkaline and Enzymatic Extraction. Food Chem. 2025, 489, 145010. [Google Scholar] [CrossRef]
- Maciel-Silva, F.W.; Castro, L.E.N.; Sganzerla, W.G.; Costa, J.M.; Rostagno, M.A.; Forster-Carneiro, T. Valorization of Black Grape Pomace by Pressurized Hot Water Extraction and Hydrolysis: Real-Time Online and in-Line Monitoring. J. Environ. Chem. Eng. 2024, 12, 111641. [Google Scholar] [CrossRef]
- Santos, R.O.; Keller, L.M.; Mohammad, S.S.; Barbosa Júnior, J.L.; Barbosa, M.I.M.J. Adsorption and Desorption Process for High Recovery and Purification of Anthocyanins from (Clitoria ternatea L.) Using Macroporous Resins. J. Dispers. Sci. Technol. 2024, 1–11. [Google Scholar] [CrossRef]
- Pereda-Miranda, R.; Castañeda-Gómez, J.F.; Fragoso-Serrano, M. Recycling Preparative Liquid Chromatography, the Overlooked Methodology for the Purification of Natural Products. Rev. Bras. Farmacogn. 2024, 34, 927–947. [Google Scholar] [CrossRef]
- Carranza-Gomez, M.; Valle-Guadarrama, S.; Domínguez-Puerto, R.; Sandoval-Castilla, O.; Guerra-Ramírez, D. Bioactive Compounds and Pigmenting Potential of Vaccinium corymbosum Extracts Separated with Aqueous Biphasic Systems Aided by Centrifugation. Processes 2025, 13, 1072. [Google Scholar] [CrossRef]
- García-Cruz, L.; Valle-Guadarrama, S.; Soto-Hernández, R.M.; Guerra-Ramírez, D.; Zuleta-Prada, H.; Martínez-Damián, M.T.; Ramírez-Valencia, Y.D. Separation of Pitaya (Stenocereus pruinosus) Betaxanthins, Betacyanins, and Soluble Phenols through Multistage Aqueous Two-Phase Systems. Food Bioprocess Technol. 2021, 14, 1791–1804. [Google Scholar] [CrossRef]
- Didion, P.Y.; Gijsbert Tjalsma, T.; Su, Z.; Malankowska, M.; Pinelo, M. What Is next? The Greener Future of Solid Liquid Extraction of Biobased Compounds: Novel Techniques and Solvents Overpower Traditional Ones. Sep. Purif. Technol. 2023, 320, 124147. [Google Scholar] [CrossRef]
- Gomes, J.; Ferreira, S.; Reinert, O.; Gandol, R.; Ayra, L.; Santos, V.; Veloso, C.M.; Ilh, C.; Cristina, R.; Bonomo, F. Evaluation of Salting-out Effect in the Liquid e Liquid Equilibrium of Aqueous Two-Phase Systems Composed of 2-Propanol and Na2SO4/MgSO4 at Different Temperatures. Fluid Phase Equilib. 2017, 450, 184–193. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, G.; Valle-Guadarrama, S.; Guerra-Ramírez, D.; Domínguez-Puerto, R.; López-Cruz, I.L. Anthocyanins of Ardisia spp. Fruits Separated with pH Driven-Aqueous Biphasic Systems. Sep. Sci. Technol. 2025, 60, 426–437. [Google Scholar] [CrossRef]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J. Optimisation of Aqueous Two-Phase Extraction of Anthocyanins from Purple Sweet Potatoes by Response Surface Methodology. Food Chem. 2013, 141, 3034–3041. [Google Scholar] [CrossRef]
- Feitosa, B.F.; Decker, B.L.A.; de Brito, E.S.; Marques, M.C.; Rodrigues, S.; Mariutti, L.R.B. Anthocyanins Stability Theory—Evidence Summary on the Effects of Microencapsulation. Food Bioprod. Process. 2025, 153, 77–86. [Google Scholar] [CrossRef]
- SPSS, Inc. Sigma Plot 2000 User’s Guide; SPSS Inc.: Chicago, IL, USA, 2000; ISBN 1-56827-232-4. [Google Scholar]
- Merchuk, J.C.; Andrews, B.A.; Asenjo, J.A. Aqueous Two-Phase Systems for Protein Separation Studies on Phase Inversion. J. Chromatogr. B 1998, 711, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Seader, J.D.; Henley, E.J.; Roper, D.K. Separation Process Principles: Chemical and Biochemical Operations, 3rd ed.; John Wiley & Sons, Inc.: Danvers, MA, USA, 2011; ISBN 978-0-470-48183-7. [Google Scholar]
- Williamson, J.C. Liquid–Liquid Demonstrations: Phase Equilibria and the Lever Rule. J. Chem. Educ. 2021, 98, 2356–2363. [Google Scholar] [CrossRef]
- Marchel, M.; João, K.G.; Marrucho, I.M. On the Use of Ionic Liquids as Adjuvants in PEG-(NH4)2SO4 Aqueous Biphasic Systems: Phase Diagrams Behavior and the Effect of IL Concentration on Myoglobin Partition. Sep. Purif. Technol. 2019, 210, 710–718. [Google Scholar] [CrossRef]
- The Mathworks Inc. Optimization ToolboxTM User’s Guide, R2008a; The Mathworks Inc.: Natick, MA, USA, 2008. [Google Scholar]
- Beltran, J.G.; Bruusgaard, H.; Servio, P. Gas Hydrate Phase Equilibria Measurement Techniques and Phase Rule Considerations. J. Chem. Thermodyn. 2012, 44, 1–4. [Google Scholar] [CrossRef]
- Pilcher, J.M. Que Vivan Los Tamales!: Food and the Making of Mexican Identity; University of New Mexico Press: Albuquerqe, NM, USA, 1998; ISBN 978-0-8263-1873-2. [Google Scholar]
- AOAC. Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Sant’Anna, V.; Gurak, P.D.; Ferreira, M.L.D.; Tessaro, I.C. Tracking Bioactive Compounds with Colour Changes in Foods—A Review. Dye. Pigment. 2013, 98, 601–608. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Madadi, B.; Pazuki, G.; Nasernejad, B. Partitioning of Cefazolin in Biocompatible Aqueous Biphasic Systems Based on Surfactant. J. Chem. Eng. Data 2013, 58, 2785–2792. [Google Scholar] [CrossRef]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, Nutritional and Nutraceutical Properties of Pigmented Native Maize (Zea mays L.) Genotypes from the Northeast of Mexico. Arab. J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Lee, C.M.; Gan, Y.L.; Chan, Y.L.; Yap, K.L.; Tang, T.K.; Tan, C.P.; Lai, O.M. Physicochemical and Sensory Analyses of High Fibre Bread Incorporated with Corncob Powder. Int. Food Res. J. 2019, 26, 1609–1616. [Google Scholar]
- Njideka, B.E.; Chijioke, M.B.; Chioma, O.N.; Nnennaya, A.N.; Iheduzaju, P.I.A. Maize Cob as Dietary Fiber Source for High-Fiber Biscuit Primary Tabs. GSC Biol. Pharm. Sci. 2020, 12, 138–144. [Google Scholar] [CrossRef]
- do Nascimento, G.K.S.; dos Santos, T.M.; Andressa, I.; Benassi, V.M.; de Andrade Neves, N.; Schmiele, M. Exploring the Use of Purple Corn Cob Flour as a New Fiber Source in Pan Bread Improved by Commercial Hemicellulases. Recent Prog. Nutr. 2024, 4, 1–17. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LC–MS Analysis of Anthocyanins from Purple Corn Cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Yang, Z.; Zhai, W. Identification and Antioxidant Activity of Anthocyanins Extracted from the Seed and Cob of Purple Corn (Zea mays L.). Innov. Food Sci. Emerg. Technol. 2010, 11, 169–176. [Google Scholar] [CrossRef]
- Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Ketthaisong, D.; Scott, M.P.; Lertrat, K.; Suriharn, B. Genotypic Variation in Anthocyanins, Phenolic Compounds, and Antioxidant Activity in Cob and Husk of Purple Field Corn. Agronomy 2018, 8, 271. [Google Scholar] [CrossRef]
- Aguilar-Hernández, Á.D.; Salinas Moreno, Y.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I.; Bautista-Ramírez, E.; Flores-López, H.E. Antocianinas y Color en Grano y Olote de Maíz Morado Peruano Cultivado En Jalisco, México. Rev. Mex. Ciencias Agrícolas 2019, 10, 1071–1082. [Google Scholar] [CrossRef]
- Hao, R.; Zhang, H.; Feng, Y.; Yang, D.; Zhao, Z.; Zhao, S. The Effect of Different Solvents and Acidifying Reagents on the Anthocyanin Profiles and Antioxidant Capacity of Purple Corn. Chem. Pap. 2022, 76, 4691–4704. [Google Scholar] [CrossRef]
- Medina-Hoyos, A.; Narro-León, L.; Chávez-Cabrera, A. Purple Corn (Zea mays L.) Crop in the Peruvian Highlands: Adaptation and Identification of High-Yield and High Anthocyanin Content Cultivars. Sci. Agropecu. 2020, 11, 291–299. [Google Scholar] [CrossRef]
- González-Amado, M.; Rodil, E.; Arce, A.; Soto, A.; Rodríguez, O. Fluid Phase Equilibria The Effect of Temperature on Polyethylene Glycol (4000 or 8000)—(Sodium or Ammonium) Sulfate Aqueous Two Phase Systems. Fluid Phase Equilib. 2016, 428, 95–101. [Google Scholar] [CrossRef]
- Sandate-Flores, L.; Rodríguez-Rodríguez, J.; Velázquez, G.; Mayolo-Deloisa, K.; Rito-Palomares, M.; Torres, J.A.; Parra-Saldívar, R. Low-Sugar Content Betaxanthins Extracts from Yellow Pitaya (Stenocereus pruinosus). Food Bioprod. Process. 2020, 121, 178–185. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W.H. Freeman and Company: New York, NY, USA, 2008. [Google Scholar]
- Gullón, P.; Eibes, G.; Lorenzo, J.M.; Pérez-Rodríguez, N.; Lú-Chau, T.A.; Gullón, B. Green Sustainable Process to Revalorize Purple Corn Cobs within a Biorefinery Frame: Co-Production of Bioactive Extracts. Sci. Total Environ. 2020, 709, 136236. [Google Scholar] [CrossRef]
- Li, C.-Y.; Kim, H.-W.; Won, S.; Min, H.-K.; Park, K.-J.; Park, J.-Y.; Ahn, M.-S.; Rhee, H.-I. Corn Husk as a Potential Source of Anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416. [Google Scholar] [CrossRef]
- De Nisi, P.; Borlini, G.; Parizad, P.A.; Scarafoni, A.; Sandroni, P.; Cassani, E.; Adani, F.; Pilu, R. Biorefinery Approach Applied to the Valorization of Purple Corn Cobs. ACS Sustain. Chem. Eng. 2021, 9, 3781–3791. [Google Scholar] [CrossRef]
- Benjaruk, V.; Varaporn, L. Antioxidant Properties and Color Stability of Anthocyanin Purified Extracts from Thai Waxy Purple Corn Cob. J. Food Nutr. Res. 2015, 3, 629–636. [Google Scholar]
- Vázquez-Carrillo, M.G.; Palacios-Rojas, N.; Mier-Sainz Trapaga, R.; García-Cruz, L.; Rosas-Zamora, G.; Rosales-Nolasco, A.; Santillano-Gómez, L.C. Commercial Limes (Calcium Hydroxide) in Corn Tortilla Production: Changes in PH, Color, Sensory Characteristics, and Shelf Life. Qual. Assur. Saf. Crop. Foods 2025, 17, 173–185. [Google Scholar] [CrossRef]
- Félix-Sámano, A.L.; Félix-Medina, J.V.; Quintero-Soto, M.F.; Diaz-Peña, I.; Gutiérrez-Dorado, R. Nixtamalization and Extrusion Processes: Effects on Physicochemical, Nutritional, and Nutraceutical Properties in the Processing of Corn into Tortilla. Int. J. Food Sci. 2025, 2025, 1–26. [Google Scholar] [CrossRef]
- Vernon-Carter, E.J.; Alvarez-Ramirez, J.; Bello-Perez, L.A.; Gonzalez, M.; Reyes, I.; Alvarez-Poblano, L. Supplementing White Maize Masa with Anthocyanins: Effects on Masa Rheology and on the in Vitro Digestibility and Hardness of Tortillas. J. Cereal Sci. 2020, 91, 102883. [Google Scholar] [CrossRef]
- Xie, J.; Sameen, D.E.; Xiao, Z.; Zhu, H.; Lai, Y.; Tang, T.; Rong, X.; Fu, F.; Qin, W.; Chen, M.; et al. Protecting Anthocyanins of Postharvest Blueberries through Konjac Glucomannan/Low-Acyl Gellan Gum Coatings Contained Thymol Microcapsule during Low-Temperature Storage. Food Chem. 2025, 463, 141347. [Google Scholar] [CrossRef]
- Ma, Z.; Jing, P. Stabilization of Black Rice Anthocyanins by Self-Assembled Silk Fibroin Nanofibrils: Morphology, Spectroscopy and Thermal Protection. Int. J. Biol. Macromol. 2020, 146, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, N.; Kimura, Y.; Ohno, T. Examination of Molecular Mechanism for the Enhanced Thermal Stability of Anthocyanins by Metal Cations and Polysaccharides. Food Chem. 2014, 143, 452–458. [Google Scholar] [CrossRef]
- da Rosa, R.G.; Ferreira, V.C.; Barroso, T.L.C.T.; Castro, L.E.N.; Forster-Carneiro, T. Corn Biomass as a Feedstock for Biorefinery: A Bibliometric Analysis of Research on Bioenergy, Biofuel and Value-added Products. Biofuels Bioprod. Biorefin. 2025, 19, 206–230. [Google Scholar] [CrossRef]
- García-Cordero, A.L.; Jimenez-Alvarado, R.; Bautista, M.; Diaz-Sánchez, F.; Ibarra, I.S.; Sánchez-Ortega, I.; Santos, E.M. Improvement of Corn Extruded Snacks Properties by Incorporation of Pulses. Rev. Mex. Ing. Química 2024, 23, 1–28. [Google Scholar] [CrossRef]
- Sánchez-Nuño, Y.A.; Nuño, K.; Martínez-Preciado, A.H.; Silva-Jara, J.M.; Velázquez-Carriles, C.A.; Gomez-Aldapa, C.A.; Villarruel-López, A. Design and Optimization of a Second-Generation Extruded Snack Using Carrot Waste, Blue Corn Flour, and Ellagic Acid as Functional Ingredients. Foods 2025, 14, 1657. [Google Scholar] [CrossRef]
- Sharma, N.; Bhardwaj, A.; Esua, O.J.; Pojić, M.; Tiwari, B.K. Cereal Processing By-Products and Wastewater for Sustainable Protein Extraction. Waste Manag. 2025, 201, 114790. [Google Scholar] [CrossRef] [PubMed]
- Asiri, A.S.; Matar, A.; Mahmoud Ismail, A. Chemical Composition, Physical Properties, and Sensory Evaluation of Wheat-Based Cookies Enriched with Different Proportions of Corn Silk. Ital. J. Food Sci. 2024, 36, 317–330. [Google Scholar] [CrossRef]
| Matlab® Code |
|---|
| function F = system (vars) W_t = vars (1); W_b = vars (2); |
| Z_t = k1*exp(k2*sqrt(W_t) − k3*W_top^3); Z_b = k1*exp(k2*sqrt(W_b) − k3*W_b^3); |
| F (1) = β*W_t + (1 − β)*W_b-Wm; F (2) = β*Z_t + (1 − β)*Z_b-Zm; |
| end |
| W_t_initial =; W_b_initial =; |
| initial_solution = [W_t_initial, W_b_initial]; options = optimoptions (‘fsolve’,‘Display’, ‘iter’); [solution, fval] = fsolve (@system, initial_solution, options); |
| W_t = solution (1); W_b = solution (2); |
| Variable | Flour | |
|---|---|---|
| NIX | PIX | |
| Proximal analysis | ||
| Moisture (%) | 7.13 (±0.03) a | 6.99 (±0.02) b |
| Crude protein (%) | 3.91 (±0.05) a | 3.44 (±0.06) a |
| Ash (%) | 4.70 (±0.00) a | 4.25 (±0.04) b |
| Ethereal extract (%) | 0.72 (±0.09) a | 0.79 (±0.22) a |
| Crude fiber (%) | 36.59 (±2.82) b | 38.87 (±0.33) a |
| Carbohydrates (%) | 46.95 (±2.56) a | 45.66 (±0.32) a |
| Bioactive compounds | ||
| TSP | 4678.1 (±149.0) a | 4740.1 (±170.8) a |
| Ant | 1678.2 (±6.1) a | 1651.8 (±11.7) a |
| Color | ||
| 26.57 (±2.64) a | 28.24 (±2.52) a | |
| 17.04 (±4.07) a | 21.75 (±4.06) a | |
| 10.01 (±0.12) a | 10.97 (±0.39) a | |
| Lightness () | Hue Angle () | Chroma () | |||||||||
| Pigmentation of yogurt with extract of NIX | |||||||||||
| 66.6 | −17.1 | 1.8858 | 0.999 | 178.7 | −176.3 | 2.1656 | 0.999 | 1.1 | 10.3 | 0.3210 | 0.999 |
| Pigmentation of yogurt with extract of PIX | |||||||||||
| 66.6 | −16.3 | 1.8500 | 0.995 | 178.7 | −176.5 | 2.0205 | 0.999 | 1.1 | 14.1 | 0.2170 | 0.998 |
| Pigmentation of corn dough with extract of NIX | |||||||||||
| 59.1 | −18.3 | 0.1401 | 0.831 | 87.8 | −101.3 | 0.0844 | 0.985 | 16.5 | −7.4 | 0.7933 | 0.838 |
| Pigmentation of corn dough with extract of PIX | |||||||||||
| 56.0 | −33.4 | 0.0411 | 0.887 | 87.4 | −127.8 | 0.0736 | 0.996 | 16.5 | −8.0 | 0.7742 | 0.958 |
| Pigmentation of cooked corn dough with extract of NIX | |||||||||||
| 62.3 | −15.8 | 0.0444 | 0.696 | 81.2 | −88.9 | 0.0289 | 0.995 | 21.3 | −10.6 | 0.1700 | 0.999 |
| Pigmentation of cooked corn dough with extract of PIX | |||||||||||
| 62.9 | −35.3 | 0.0221 | 0.889 | 81.1 | −133.6 | 0.0185 | 0.996 | 21.4 | −11.3 | 0.1225 | 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Herrera, A.; Ortega-Paczka, R.A.d.S.C.; Sandoval-Castilla, O.; García-Cruz, L.; Valle-Guadarrama, S. Anthocyanins Separated from Degrained Purple-Corn Cobs with Aqueous Biphasic Systems as Food Pigments. Appl. Sci. 2025, 15, 10730. https://doi.org/10.3390/app151910730
López-Herrera A, Ortega-Paczka RAdSC, Sandoval-Castilla O, García-Cruz L, Valle-Guadarrama S. Anthocyanins Separated from Degrained Purple-Corn Cobs with Aqueous Biphasic Systems as Food Pigments. Applied Sciences. 2025; 15(19):10730. https://doi.org/10.3390/app151910730
Chicago/Turabian StyleLópez-Herrera, Abigail, Rafael Angel del Sagrado Corazón Ortega-Paczka, Ofelia Sandoval-Castilla, Leticia García-Cruz, and Salvador Valle-Guadarrama. 2025. "Anthocyanins Separated from Degrained Purple-Corn Cobs with Aqueous Biphasic Systems as Food Pigments" Applied Sciences 15, no. 19: 10730. https://doi.org/10.3390/app151910730
APA StyleLópez-Herrera, A., Ortega-Paczka, R. A. d. S. C., Sandoval-Castilla, O., García-Cruz, L., & Valle-Guadarrama, S. (2025). Anthocyanins Separated from Degrained Purple-Corn Cobs with Aqueous Biphasic Systems as Food Pigments. Applied Sciences, 15(19), 10730. https://doi.org/10.3390/app151910730






