1. Introduction
During the last decades, a healthier lifestyle in terms of physical activity and balanced nutrition has been embraced by consumers [
1]. This shift has fed demand for ‘functional foods’, i.e., products defined as natural or processed foods showing known and unknown biologically active molecules that are clinically proven and documented for health-beneficial effects [
2]. Among them, probiotics-enriched foods represent a major food category with a variety of types, dominated by dairy-based products [
3]. However, concerns related to the consumption of dairy products, such as high cholesterol levels, potential allergenicity, and other health-related issues, have contributed to a growing interest in non-dairy probiotic products. As a result, researchers have explored the use of fruits and vegetables as carriers for probiotic delivery [
4]. To confer health benefits, probiotic products should have at least 10
6 CFU/mL of live microorganisms, ensuring a daily intake of 10
8–10
9 viable cells [
5]. Probiotics are thought to enhance health by modulating the native intestinal microbiota through various mechanisms, including immunomodulation, direct antagonism, and competitive exclusion. Among the most widely used species are
Lactobacillus spp. and
Bifidobacterium spp. and are granted ‘
Generally Recognized As Safe’ (GRAS) by several regulatory bodies. Frequently applied strains include
Lactobacillus acidophilus,
Lacticaseibacillus casei,
Lactiplantibacillus plantarum,
Lacticaseibacillus rhamnosus, and
Bifidobacterium lactis to be incorporated into functional food products, enhancing their health-promoting characteristics [
6]. Nevertheless, ensuring survival, viability, and stability of probiotics in non-dairy matrices throughout food processing, storage, and consumption can be more challenging compared to dairy-based products [
7].
To overcome this issue, a range of microencapsulation methods has been developed to enhance the survival of probiotic bacteria under harsh environmental and gastrointestinal conditions. Microencapsulation systems should preserve probiotic stability during processing and/or storage, protect the cells in the upper GIT, ensure targeted release in the colon, and enhance their capability to adhere to mucosal surfaces [
8]. In recent years, natural polymer-based carriers have been widely employed due to their biodegradability and food-grade status. Biopolymers such as alginate, pectin, and chitosan have been employed to improve probiotic stability and viability. Additionally, protein-based carriers including gelatin, collagen, casein, albumin, and whey proteins have also gained attention for the delivery of bioactive compounds. Whey protein concentrate (WPC) and isolate (WPI) have been often examined as an encapsulation vehicle due to their gel-forming and emulsion-stabilizing properties [
9]. Among encapsulation methods, complex coacervation offers high loading capacity and controlled release in response to mechanical stress, temperature, or pH [
9].
As mentioned above, fruit juices are being investigated as probiotic vehicles due to their nutrient content that supports probiotic survival and their broad consumer acceptance [
10]. However, the removal of the protective skin of fruits during processing can contaminate the juices with pathogens and spoilage microorganisms [
11]. Among foodborne pathogens,
Salmonella is a major concern representing one of the greatest threats to public health worldwide [
12]. According to EFSA,
Salmonella Enteritidis continues to be the most identified pathogen in reported foodborne illnesses and outbreaks [
13]. In 2023, it was responsible for the highest number of outbreaks and cases and ranked second in hospitalizations. Furthermore,
Salmonella accounted for the majority of multi-country outbreaks in the EU in the same year [
14]. Infection begins with adhesion and invasion of intestinal epithelial cells, particularly in the ileum. Studies have shown that lactic acid bacteria can adhere to intestinal mucosa, preventing pathogenic bacteria from attaching and exerting cytotoxic effects [
15,
16]. Increasing clinical evidence indicates that probiotics influence the composition and function of gut microbiota by inhabiting the gastrointestinal tract, where they contribute to maintaining microbial equilibrium and suppress the growth of harmful pathogens such as
Salmonella [
17].
Given the importance of developing functional probiotic fruit juices in the food industry and the need to provide scientific evidence for their safety, the aims of the present study were as follows: (i) to evaluate the survival of encapsulated probiotics (two commercial strains as co-culture) in orange juice during storage at 4 °C and 12 °C for 5 days and their subsequent survival in simulated gastrointestinal tract (GIT) conditions after each day of consumption, and (ii) to study the effect of probiotics on the survival of co-inoculated S. enterica strains in the above conditions.
2. Materials and Methods
2.1. Experimental Design
A multifactorial experiment was carried out to assess the survival of probiotics (both free and encapsulated cells) in orange juice during storage at two temperatures, while also monitoring their viability under simulated gastrointestinal tract (GIT) conditions. Additionally, the ability of probiotics to suppress the probability of
Salmonella growth under the same conditions was evaluated. Juices were stored at two temperatures, 4 and 12 °C: 4 °C, representing recommended refrigeration, and 12 °C, representing mild temperature abuse conditions frequently encountered in the cold chain. The latter was selected to mimic possible deviations from optimal refrigeration during transportation and domestic storage. A schematic representation of this approach is shown in
Figure 1, and the various experimental conditions are detailed in the following explanatory subparagraphs.
- A.
Evaluation of the survival of free and encapsulated probiotic strains in orange juice, stored at 4 °C and 12 °C for 5 days and subsequent survival in simulated gastrointestinal tract (GIT) conditions after each day of consumption.
For this study, two different cases of orange juice were produced: (a) orange juice with a cocktail of free probiotic strains
Lcb. casei Shirota and
Lcb. rhamnosus GG (9.0 log CFU/mL) (FC), and (b) orange juice with a cocktail of encapsulated probiotic strains
Lcb. casei Shirota and
Lcb. rhamnosus GG (9.0 log CFU/mL) (EC). The selection of these well-established commercial probiotics was based upon their ability to maintain high viable counts above 6 log CFU/mL after encapsulation (based on preliminary experiments), which is a prerequisite for substantiating health claims and for a product to be classified as functional; they are accredited with
GRAS status and are well documented for their resistance to low pH (acidic conditions) and bile salts hydrolysis, ensuring survival through gastrointestinal transit. In addition, since the orange juices produced in this study (without the pathogen) were also intended for human consumption [
18], it was necessary to use commercial strains with a history of safe human use and official approval for inclusion in food applications.
Specifically, two batches of samples with four technical replicates in each batch were stored at 4 °C and 12 °C for five days. Every day during the five-day period, samples were withdrawn from both temperatures (4 °C and 12 °C) for microbiological analysis in order to examine the effect of storage in the population of probiotic strains both as free and encapsulated forms. The days of storage (5 days) were selected in alignment with our previous clinical study [
18], in which the same orange juice formulations containing encapsulated probiotic strains (
Lacticaseibacillus casei Shirota and
Lacticaseibacillus rhamnosus GG) were administered to volunteers. In parallel, every 24 h, orange juice samples stored at 4 °C and 12 °C were collected to undergo the in vitro static model of GIT. Specifically, samples were examined daily for the survival of probiotic strains in both free and encapsulated form, during and after exposure in simulated GIT conditions (gastric phase after 1 h, gastric phase after 2 h, intestinal phase after 1 h, intestinal phase after 2 h).
Sensory analysis was performed daily for FC and EC, assessing the attributes of aroma, taste, color, and overall acceptance.
- B.
Evaluation of the effect of free and encapsulated probiotic strains on the survival of Salmonella enterica in orange juice, stored at 4 °C and 12 °C for 5 days and in simulated GIT conditions after each day of consumption.
In this section, three cases were prepared: (a) orange juice with free probiotic strains Lcb. casei Shirota and Lcb. rhamnosus GG (9.0 log CFU/mL) co-cultured with Salmonella enterica (FCS) (1.7 log CFU/mL), (b) orange juice with encapsulated probiotic strains Lcb. casei Shirota and Lcb. rhamnosus GG (9.0 log CFU/mL) co-cultured with S. enterica (1.7 log CFU/mL) (ECS), and (c) orange juice only with S. enterica (1.7 log CFU/mL) (S) to serve as control case.
Two batches of samples with four technical replicates in each batch were stored at 4 °C and 12 °C for five days. Every day, samples were withdrawn from both temperatures (4 °C and 12 °C) for microbiological analysis to examine the effect of probiotic strains both as free and encapsulated forms on co-cultured S. enterica in orange juice. In parallel, samples were examined daily for the survival of S. enterica during and after exposure to simulated GIT conditions. In detail, the population of S. enterica with probiotic strains were examined in the gastric phase after 1 h, gastric phase after 2 h, intestinal phase after 1 h, and intestinal phase after 2 h.
2.2. Bacterial Strains and Inoculum Preparation
Probiotic strains: The probiotic microorganisms Lcb. casei Shirota ACA-DC 6002 and Lcb. rhamnosus GG ATCC 53103 were used. Stock cultures were preserved at −80 °C in Den Man, Rogosa, and Sharpe (MRS) broth (MRS broth, 4017292, Biolife, Milano, Italy) supplemented with glycerol (APPLICHEM, Darmstadt, Germany) (70:30). The monocultures of the two strains were incubated at 30 °C for 24 h in 10 mL MRS broth, and then a second culture of each strain was grown under the same conditions. Then, the fresh monocultures were harvested by centrifugation (6000× g, 10 min, 4 °C) and washed with ddH2O with adjusted pH at 4.0 to acquire better cell adaptation. The supernatant was discarded, and pellets of the two strains were mixed in equal volumes in Gum Arabic (3% w/w) solution, reaching a final concentration of 10 log CFU/mL of both probiotics’ strains.
Salmonella strains: A 3-strain cocktail of
S. enterica (
S. enterica DSM 554 ser. Typhimurium,
S. enterica DT 193 ser. Typhimurium, and
S. enterica ATCC 13076 ser. Enteritidis) was used. Selection of the strains was based on the EFSA report [
13], which identified serovars Enteritidis and Typhimurium as responsible for approximately 80% of human Salmonellosis cases. The monocultures of the strains were grown at 37 °C for 18 ± 2 h in Tryptic Soy Broth (TSB, Neogen NCM0019A, Lansing, MI, USA), and then a second culture of each strain was grown under the same conditions. Monocultures of the strains from 18 h culture (37 °C) were harvested by centrifugation (6000×
g, 10 min, at 4 °C), washed twice in ¼ strength Ringer’s solution (Neogen, NCM0191K, MO, USA), and finally resuspended in 10 mL ¼ strength Ringer’s solution. The
Salmonella strains were mixed at equal volumes to achieve a cocktail culture with a final population of 1.7 log CFU/mL (approximately 50 cells of the pathogen) in orange juice. The initial inoculum level was selected to reflect realistic contamination scenarios in line with EFSA risk assessment principles [
19].
2.3. Encapsulation Process in Whey Protein Isolate–Gum Arabic (WPI:GA)
The encapsulation process of the two probiotic strains (in cocktail) was performed as previously described by Bosnea et al. (2014) with slight modifications [
20]. Powdered WPI Bipro Tm (92.08%
w/
w protein, 1.08%
w/
w fat, 4.08%
w/
w lactose) was purchased from Davisco Foods International, Inc. (Le Sueur, MN, USA). Gum Arabic (GA) was purchased from Sigma Chemicals (Gillingham, UK). In detail, aqueous solutions of powdered Whey Protein Isolate (3%
w/
w) (Davisco Foods International Inc) and Gum Arabic (3%
w/
w) (Sigma Chemicals) were prepared using ddH
2O. The solutions were placed under gentle stirring (4 h at 210 rpm) (Orbital and Linear Digital shaker, RS Lab/ RSLAB-7) at room temperature (18–20 °C). The solutions were stored at a cold temperature (4 °C) for 24 h for complete solubilization and hydration. To prepare the WPI:GA coacervate, the aqueous solutions of WPI and GA were mixed at a ratio of 2:1. Consequently, this final solution was inoculated with the cocktail of the probiotic strains under continuous agitation. The coacervates were produced using a mixing method, which involves first preparing each biopolymer dispersion separately and then mixing them, adjusting their pH to the optimal interaction level to facilitate rapid electrostatic interactions between the biopolymers. The optimal pH for maximum WPI complex coacervates is 4.0, achieved by adding 10% (
w/
v) food-grade citric acid to adjust the final solution. To ensure completion of the formation of complex coacervate and avoid stressing the LAB cells during their purification steps, ddH
2O with adjusted pH at 4.0 was used to promote LAB cell adaptation and enhance resistance to this pH level. This step was necessary because the pH of the WPI:GA solution was subsequently adjusted to 4 for capsule formation. Then, 10 mL of this solution was added to sterilized 50 mL centrifuge tubes and the mixture was left at room temperature for phase separation. After 1 h, the supernatant was discarded, while the resulting precipitate constituted the WPI:GA microencapsulation system containing the two probiotic microorganisms (2-strain cocktail) in a final population of 9.0 log CFU/mL. All treatments were performed in a laminar flow cabinet to ensure the aseptic conditions of the samples.
2.4. Orange Juice Preparation and Inoculation
Pasteurized orange juice (Hellenic Juice Industry C. Dedes ASPIS S.A., Argos, Greece) was used. In brief, tubes (50 mL) were filled with 10 mL of orange juice and then inoculated with the cocktail of S. enterica strains in a final population of 1.7 log CFU/mL were used as control cases (S). Another control case was prepared by adding 10 mL of orange juice inoculated with 9.0 log CFU/mL of probiotics Lcb. casei Shirota and Lcb. rhamnosus GG with free (FC) and encapsulated (EC) cells. Cases involving both LAB and pathogen inoculation were prepared by adding 10 mL of orange juice, and then inoculation of LAB (9.0 log CFU/mL) as free cells (FCS) or encapsulated cells in WPI:GA (ECS) and inoculation of the cocktail strains of S. enterica (1.7 log CFU/mL) followed. In the final solution containing WPI:GA (ECS) and the juice, the population of non-encapsulated probiotic strains was always checked in MRS Agar plates. All samples were stored at 4 °C and 12 °C in high-precision incubator chambers (MIR153, Sanyo Electric Co., Osaka, Japan) for 5 days.
2.5. In Vitro Digestion Model System
The digestion protocol of the juice followed the INFOGEST harmonized protocol with some modifications [
21]. This protocol replicates the three primary stages of in vivo digestion: oral, gastric, and duodenal stages. In the present study, gastrointestinal digested samples (gastric–duodenal digestion) were used, excluding the oral phase, since according to Minekus et al. (2015), for liquid foods like orange juice the oral phase is not mandatory (food remains only some seconds in this phase) [
22]. As described in the INFOGEST protocol, orange juice was mixed with SGF solution to obtain a final ratio of food to SGF of 1:1 (
v/
v) The recommended time of digestion is 2 h at 37 °C under gentle stirring (130 rpm) (Orbital and Linear Digital shaker, RS Lab/RSLAB-7). The pH of the solution was monitored constantly and if not found at the appropriate value was re-adjusted with 1 M HCl to pH = 3 during digestion. After 2 h, this solution was subsequently diluted in a ratio of 1:1 with simulated intestinal fluid. The recommended time for intestinal digestion was 2 h at 37 °C under gentle stirring (130 rpm). The pH of the solution was monitored constantly and if not found at the appropriate value was re-adjusted with 1 M NaOH to achieve a final pH value of 8 during digestion.
The enzymes used during the gastric and intestinal phases were purchased from Sigma-Aldrich (St. Louis, MO, USA) and were pepsin from porcine gastric mucosa (2500 U/mg protein, EC 3.4.23.1) and pancreatin from porcine and pancreas (8 × USP, EC 232-468-9), respectively. Additionally, porcine bile extract (Porcine Pepsin, EC 3.4.23.1, Sigma-Aldrich, St louis MO, USA) was used as a source of bile salts in the intestinal phase.
Every 24 h, orange juice samples stored at 4 °C and 12 °C were collected to undergo the in vitro static model of the gastrointestinal track (GIT). During the simulation, microbiological analysis was performed every 1 h for a total duration of 4 h. The static in vitro protocol for gastrointestinal digestion was applied to all the samples every 24 h for 6 time points (0, 1, 2, 3, 4, and 5 days) for the samples stored at 4 °C and 12 °C. This protocol included a gastric and an intestinal phase, lasting 2 h, respectively. In the case of the encapsulated probiotic cells, prior to the microbiological analysis, the coacervate was degraded through the addition of a 5 N NaOH solution in a final pH of 7.0 while vigorously agitated using a vortex device (Vortex Shaker 3, IKA, Staufen, Germany). At this pH value, the biopolymers constituting the aggregate structures do not undergo electrostatic interaction due to both carrying negatively charged groups (resulting in repulsive forces) and thus releasing the LAB cells in the solution for enumeration. Throughout the intestinal phase (pH 8.1), the former step of coacervate degradation was not necessary since the capsule at this high pH was already degraded and the LABs were already released into the solution during this stage.
2.6. Microbiological Analyses and pH Measurement
For the microbiological analysis, every 24 h, samples from each temperature (4 °C and 12 °C) were withdrawn and then 1 mL of the juice sample was directly transferred into test tubes containing 9.0 mL of ¼ strength Ringer’s solution (Neogen, NCM0191K). The resulting suspensions were serially diluted in the above diluent and 1 mL or 0.1 mL of the sample was poured or spread on the following Agar media: (i) MRS Agar (Neogen NCM0079) overlaid with the same medium and incubated at 30 °C for 48 to 72 h for the detection of LAB, and (ii) XLD (Xylose Lysine Deoxycholate, Neogen NCM0021A, MO, USA) for the detection of Salmonella spp. incubated at 37 °C for 16–18 h. To reduce the detection limit of Salmonella, 1.0 mL of the juice and 1 mL of the gastric or intestinal digesta was equally spread into three XLD Petri dishes (to achieve detection limits approximately of 1, 2, and 4 CFU/mL, respectively). In all samples containing the pathogen, the enrichment method was applied, according to the ISO 6579-1:2017 method for Salmonella spp. Samples were examined at time 0 (immediately after storage) and during the two stages of the GIT simulation, i.e., after the 1st and 2nd h of exposure in the gastric phase and after the 1st and 2nd h of the gastric phase.
The pH value of the orange juice samples was recorded and monitored at time 0 of each storage day and during the GIT simulation (see
Section 2.5) with a digital pH meter Russel RL150 (Russell Inc., Cork, Ireland) and with a glass electrode (Metrohm AG, Herisau, Switzerland).
2.7. Sensory Evaluation
A sensory evaluation of the juice samples (without the addition of the pathogen) was conducted by a group of ten semi-trained assessors [
23]. The evaluation took place under artificial light in the organoleptic assessment room of the Institute of Technology of Agricultural Products (ITAP, HAO DIMITRA, Lycovrissi, Greece). Each panelist received 40 mL of orange juice served at room temperature in plastic containers, with samples coded using three-digit numbers to ensure blinding. Samples included untreated juice (control) as well as juice enriched with 9.0 log CFU/mL of the selected LAB, either in free or encapsulated form. Panelists assessed the attributes of odor, taste, color, and overall acceptance using a 0–9 hedonic scale (the descriptive terms range from 9 ‘like extremely’ to 0 ‘dislike extremely’) during storage at 4 and 12 °C. Unsalted crackers and water were provided between samples to cleanse the palate. In total, 240 samples were assessed throughout the study.
The study was reviewed and approved by the Bioethics Committee of the Agricultural University of Athens under approval # EIDE Reference Number 75 4 October 2022, and informed consent was obtained from each subject prior to their participation in the study.
2.8. Statistical Analysis
Data were indicated as means: mean ± SD of four replicates × two batches (4 × 2). Microbiological and sensory results were analyzed for statistical significance with significance established at p < 0.05. Post hoc analysis Tukey’s HSD test was performed to determine significant differences among results. Statistical analyses were performed with SPSS 20.0 software (SPSS Inc., Chicago, IL, USA)
4. Discussion
Fruit juices may be considered as good carriers for probiotics [
24]. However, due to their low pH, probiotics may face survival challenges. Microencapsulation can serve as a protective barrier, reducing their interaction with the external harsh environment (e.g., pH) and consequently enhancing probiotic viability. This was shown by Ding and Shah (2008) [
25], who analyzed the viability of free and encapsulated (in calcium alginate beads) probiotics (eight strains) in orange juice stored at 4 °C for up to six weeks [
25]. They observed that encapsulated probiotics maintained high viability of ca. 5 logs during storage; however, free cells declined by 2 logs during the first 14 days. Da Silva et al. (2021) used crosslinked coacervation techniques to produce microcapsules containing
Lactobacillus acidophilus LA-02 to study its viability during cold storage using as a food matrix different fruit juice (orange, apple) [
26]. From the results, it was evident that the encapsulated probiotic exhibited greater viability in contrast to the free cells since it was vital and in high population after 63 days of storage. In our study, free probiotic cells demonstrated higher survival rates in orange juice compared to previous studies (e.g., Ding and Shah, 2008 [
25], Da Silva et al., 2021 [
26]). This may be explained by several factors. First, the juice used in this study was pasteurized orange juice, with mild initial acidity (pH of 4.3), which was less stressful for the added free cells in comparison to lower pH values reported in other juice matrices. Second, pasteurization reduced the enzyme activity and the native microbiota of the juice to very low levels (as was also shown in TVC plate Agar), thereby limiting potential competition and suppression of probiotic growth and making a more favorable environment for survival of the free cells. Furthermore, the storage time in this study was relatively short (<10 days), and this shorter timeframe may have contributed to the observed higher survival rates compared to studies assessing longer storage periods, as these studies have already been discussed.
The high survival rate of encapsulated probiotics in foods and in GIT after consumption is essential for producing functional foods. Probiotics that can resist gastric and intestinal fluids may enhance immune system functionality [
27]. According to the results of this study, complex coacervation (WPI:GA) enhanced probiotics viability during the GIT simulation compared to the free cells (FC). At pH 7, the coacervate degrades, and the encapsulated cells are released into the solution, suggesting that bacterial cells can be released from the coacervate in the large intestine where the pH is approximately neutral [
20]. In FC samples, there was a notable 5 log reduction after 2 h in the gastric phase. In contrast, encapsulated probiotics (EC) experienced a lower reduction of 2 logs (final population of 7.0 log CFU/mL), which falls within the range recommended by [
28] to confer health benefits. Kiran et al. (2023) [
29] used whey or zein protein nano-encapsulation protocol to encapsulate
Lcb. rhamnosus that was consequently added to yogurt. Their study evaluated its survival in the different encapsulation matrixes under GIT simulation, and it was shown that its viability decreased from 9.79 to 8.05 log CFU/mL when it was encapsulated, compared to free cells that were reduced by ca. 7 logs, demonstrating that nano-encapsulation improved probiotic survival in GIT simulation compared to FC [
29]. Jin et al. (2020) [
30] explored the influence of different coating agents such as xanthan, carrageenan, and acacia gum on the survival of microencapsulated
Lacticaseibacillus casei Shirota under conditions simulating gastric and intestinal fluids. The authors observed that the coated beads enhanced probiotic viability (to population levels over 7.0 log CFU/mL) compared to the population of free cells where their survival was reduced to 3.0 log CFU/mL [
30]. Chen et al. (2017) [
31] demonstrated that the survival of microbeads in WPI with Transglutaminase (TGase) was improved in SGF due to the coating with WPI. Different types of acids and pH conditions varying from 2.5 to 8.2 comprise stomach and intestine environments. This is crucial for probiotic survival during gastric digestion and travel into the intestine, where the nutrients are absorbed [
31]. According to Kiran et al. (2023) [
29], the intestine environment causes higher mortality to the free cells, compared to the encapsulated cells [
29]. This is in accordance with the results of the current study, since it was shown that during intestinal simulation, FC showed the least survivability, where their population decreased to 3.9 ± 0.02 log CFU/mL at the end of the intestinal phase. In contrast, in EC only a 2.0 log CFU/mL population reduction was detected, highlighting the beneficial protection of encapsulation. According to Jin et al. (2020) [
30], bile salts and simulated intestinal fluid negatively impacted probiotic’s viability and led to increased mortality of free cells. The bile resistance varies from strain to strain, and the viability of probiotics enhances when they are encapsulated, since the coating acts as a barrier during the diffusion process in the intestinal fluid [
30]. Kiran et al. (2023) [
29] aimed to evaluate the effect of two different encapsulation matrixes (WPI, Xanthan) in protecting probiotic strains incorporated into yogurt during storage and after GIT simulation. It was found that the population of probiotic strains remained at high levels when they were encapsulated in WPI matrixes (8.88 log CFU/mL) and in xanthan (8.51 log CFU/mL), while free cells reduced to 3.37 log CFU/mL [
29]. Silva et al. (2022) [
32] focused on the combined encapsulation, using gelatin and GA as coacervation agents, of probiotics and plant extracts to evaluate probiotic release and survival (with an initial population over 8.5 logs in the encapsulation system) and bioactive compounds during in vitro digestion. It was shown that the free cells population was significantly reduced to 5.0 log CFU/mL when the encapsulated probiotic strains remained in higher levels (7.0 log CFU/mL) at the end of GIT simulation [
32]. All the above studies highlighted the protective effect of microencapsulation during GIT simulation, as was also shown in the current study.
In both cases (free and encapsulated probiotics), the probiotics remained at high population levels, adequate for conferring a health benefit on the host. However, in the case of free LAB probiotics, they altered the sensory attributes of orange juice, increasing the acid taste and making it less acceptable, while the encapsulated probiotics did not affect the sensory characteristics of orange juice, as was shown above. Indeed, the sensory profile of juices fortified with probiotics depends upon the selected microorganism and the juice kind, whether the juice is stored in cold conditions or at room temperature, as well as the inclusion of other additional compounds. The review by Rodríguez et al. (2009) focused on the relationship between LAB and phenolics present in foods, highlighting their possible application or their enzymes in enhancing the sensory and nutritional profile of food products [
33]. Also, the study by Luckow et al. (2006) [
34] investigated the impact of probiotics on the sensory properties of juices and their influence on consumer acceptance. They concluded that adding tropical fruit juices (e.g., pineapple, mango or passion fruit) to probiotic fortified orange juice enhanced its sensory profile by masking the probiotic off-flavors that can be perceived by consumers [
34]. In our study, sensory evaluation was conducted to ensure that the addition of encapsulated probiotics did not negatively affect consumer acceptance of the juice. It was shown that the encapsulated probiotics maintained the overall sensory quality of the juices (in terms of taste, aroma, and appearance), making them comparable to the control juice samples. This outcome is critical, since consumer acceptance is a prerequisite for the successful marketability of functional beverages. Encapsulation not only supported probiotic survival during storage and gastrointestinal transit but also minimized/masked undesirable sensory changes that were evident when juice was produced with free probiotic cells (using the same population levels). In addition, the sensory results were also crucial for designing a subsequent clinical study, aimed at assessing how probiotic juice consumption may influence patient’s clinical profiles across various health indicators [
18].
EFSA surveillance data indicated that
Salmonella Enteritidis remained the most identified pathogen in reported foodborne outbreaks and illnesses across the EU in 2023 [
13]. It was linked to the highest number of outbreaks ranked among the top cases for hospitalizations. Furthermore,
Salmonella was also the most frequent agent involved in multi-country outbreaks that year [
13]. Fresh fruit and vegetables can become contaminated with
Salmonella through exposure to contaminated soil, manure, compost, water or operators [
35,
36]. As regards antimicrobial potential of the selected probiotics against
Salmonella, relevant studies [
36,
37] have shown that the two strains could antagonize or reduce
Salmonella. However, most of the studies available in the literature focus on animal/food models or in vitro studies but not on juices, which was the case of this work. In the current study,
S. enterica inoculated in orange juice samples did not show significant reduction (
p > 0.05), and the population remained close to the initial population level throughout the 5-day storage at 4 °C, posing a threat to food safety and increasing the risk of potential consumer exposure to foodborne pathogens. Álvarez-Ordóñez et al. (2013) [
37] investigated how both acid-adapted and non-adapted
S. typhimurium cells survived in different food matrixes, i.e., yogurt and orange juice, stored at temperatures ranging from 4 °C to 37 °C. They found out that the pathogen’s survival throughout storage was affected by the type of food matrix, the temperature conditions, and whether the cells had undergone acid adaptation. Also, it was found that non-acid-adapted cells survived better in orange juice than acid-adapted cells during storage at 4 and 10 °C [
37]. Sharma et al. (2001) [
3] explored how calcium fortification in orange juice influenced
Salmonella spp. (initial level of inoculation 5.0 log CFU/mL) survival during 32 days of refrigerated storage. Additionally, they assessed whether
Salmonella Muenchen, a serotype that has been previously linked to an orange juice outbreak, exhibited distinct survival behavior in orange juice compared to other serotypes not associated with juice-related sources. Results showed that the population of
Salmonella Muenchen remained at high levels (3.2 log CFU/mL) in comparison to the examined serotypes that declined [
3]. As regards storage temperature, previous studies have not found significant differences in the survival of
S. enterica throughout a range of storage temperatures between 4 °C and 22 °C in orange juices [
35,
36], a result that was also observed in the current study. When
S. enterica was co-cultured with free probiotic strains and stored at 4 °C, its population declined to 0.8 log CFU/mL at the end of storage. Marianelli et al. (2010) [
38] evaluated the antagonistic properties of different probiotic strains (
Lcb. rhamnosus GG,
Limosilactobacillus reuteri,
Alkalihalobacillus clausii) against
S. typhimurium 1344 in BHI broth. They observed that the greatest antimicrobial activity was exhibited by
Lcb. rhamnosus GG. According to their study, the antimicrobial activity of probiotic LAB strains is known to be multifactorial. In the case of
Lcb.
rhamnosus GG, the antimicrobial effect against the pathogen may involve a combination of lactic acid production and non-lactic acid molecules which act synergistically to inhibit the pathogen [
38]. Fazeli et al. (2007) [
39] examined whether different free lactic acid bacteria (
Lcb. casei,
Limosilactobacillus fermentum,
L. acidophilus, and
Lpb. plantarum) present in watermelon juice could eradicate
S. typhimurium. Results showed that
Lcb. casei was the most potent inhibitor of
S. typhimurium. Also, through Fazeli et al.’s (2007) experiment, it was shown that all the LAB present in probiotic watermelon juices could exert their anti-pathogenic properties, and the antagonistic action mechanism of
Lcb. casei seemed to be dependent on the acidic environment due to lactic acid itself or to an active substance at a low pH [
39]. The population of
S. enterica co-cultured with encapsulated LAB strains remained at the initial inoculum level after 5 days of storage at 4 °C. This could be attributed to the protective effect of the complex coacervation to the LAB cells that keeps them isolated from the juice. Specifically, the WPI:GA coacervation matrix encapsulates the probiotic bacteria within a biopolymer structure, preserving their viability during stress conditions such as storage in low pH food and gastrointestinal simulation. However, this encapsulation also limits their direct interaction with
S. enterica, preventing them from exerting their antimicrobial effects. As a result, the pathogen’s population remained stable, unlike in samples containing free probiotic strains where a reduction in
S. enterica was observed.
The protective effect of the food matrix on pathogens during gastric transit is well recognized. This was also confirmed by Akritidou et al. (2022), who studied the survival of two major foodborne pathogens (
Salmonella typhimurium and
Listeria monocytogenes) under varying gastric pH conditions (2.0–3.5) and bile salts concentration (2.5 mM–10.0 mM) using an in vitro digestion model simulating the ingestion of a contaminated food system [
27]. Their findings indicated that
S. typhimurium exhibited greater sensitivity to acid pH than
L. monocytogenes but was more resistant to bile salts. Specifically, at pH 2.0,
S. typhimurium was reduced to undetectable levels, highlighting its vulnerability to strong gastric acidity. Koseki et al. (2010) [
40] developed inactivation kinetics of major bacterial pathogens (
L. monocytogenes,
E. coli O157:H7, and
Salmonella spp.) in a gastric environment using simulated gastric fluids (SGF) adjusted to various pH values. Their results showed that acid resistance varied depending on the type of bacteria and strain and for
S. typhimurium; the pH ranged between 2.0 and 2.8. The population of
S. typhimurium seemed to face a significant reduction (>2 log CFU/mL) in all examined cases (fresh-cut lettuce, minced tuna, scrambled egg) [
40]. The results of the current study showed that the population of
S. enterica showed some resistance and had a reduction equal to 0.7 log CFU/mL at the end of gastric simulation and remained stable until the end of the intestinal phase. Yuk et al. (2006) [
41] evaluated the acid resistance of five
Salmonella serovars (Agona, Gaminara, Michigan, Montevideo, and Poona) after their addition in simulated gastric fluid (SGF) for 100 s. The
Salmonella serovars had been previously acid-adapted in juices (orange, tomato, and apple juice) by storage under refrigerator (7 °C) and under room temperature (20 °C) conditions for 24 h. Their results showed that all acid-adapted
Salmonella serovars displayed enhanced survival times compared to non-adapted controls. The enhanced acid resistance of the examined serovars during SGF raises food safety concerns, as it may allow them to survive stomach acidity and subsequent colonize the intestinal tract, potentially leading to Salmonellosis [
41]. The population of
S. enterica, when was in co-culture with free probiotic strains during GIT simulation, became undetectable, as was described previously. This reduction may be a result of the combination of harsh gastric conditions and the antimicrobial and antagonistic activity of the probiotic strains [
36]. Castillo et al. (2013) [
42] aimed to study the ability of three different LAB strains to activate the intestinal immune response and provide protection against
Salmonella typhimurium infection in a mouse model. Their finding showed that protection against
Salmonella infection was evident only by the continuous administration (previous and post challenge) of the probiotic strain
Lcb. paracasei CRL 431. This protective effect was associated with a reduced inflammatory reaction in the intestine [
42]. Makras et al. (2006) [
43] examined the production dynamics of antimicrobial compounds by
Lactobacillus strains during controlled batch fermentations. Results showed that in some cases, the anti-Salmonella effect was entirely attributed to lactic acid production, whereas in others, it resulted from lactic acid in combination with an unidentified inhibitory compound [
43]. In the presence of encapsulated probiotic cells, the
S. enterica population survived throughout the gastric phase, showing resistance without being affected by the probiotics. During the intestinal phase (pH = 8.1), the capsule decomposed and thus the probiotic cells were released. As a result, the
S. enterica population decreased to levels below the detection limit of the enumeration method within the 1st hour of intestinal simulation and was undetectable after enrichment. This is a crucial phase for the probiotics to exhibit their antimicrobial activity against
Salmonella, which can invade the mucosa of the small and large intestine and produce toxins [
44].

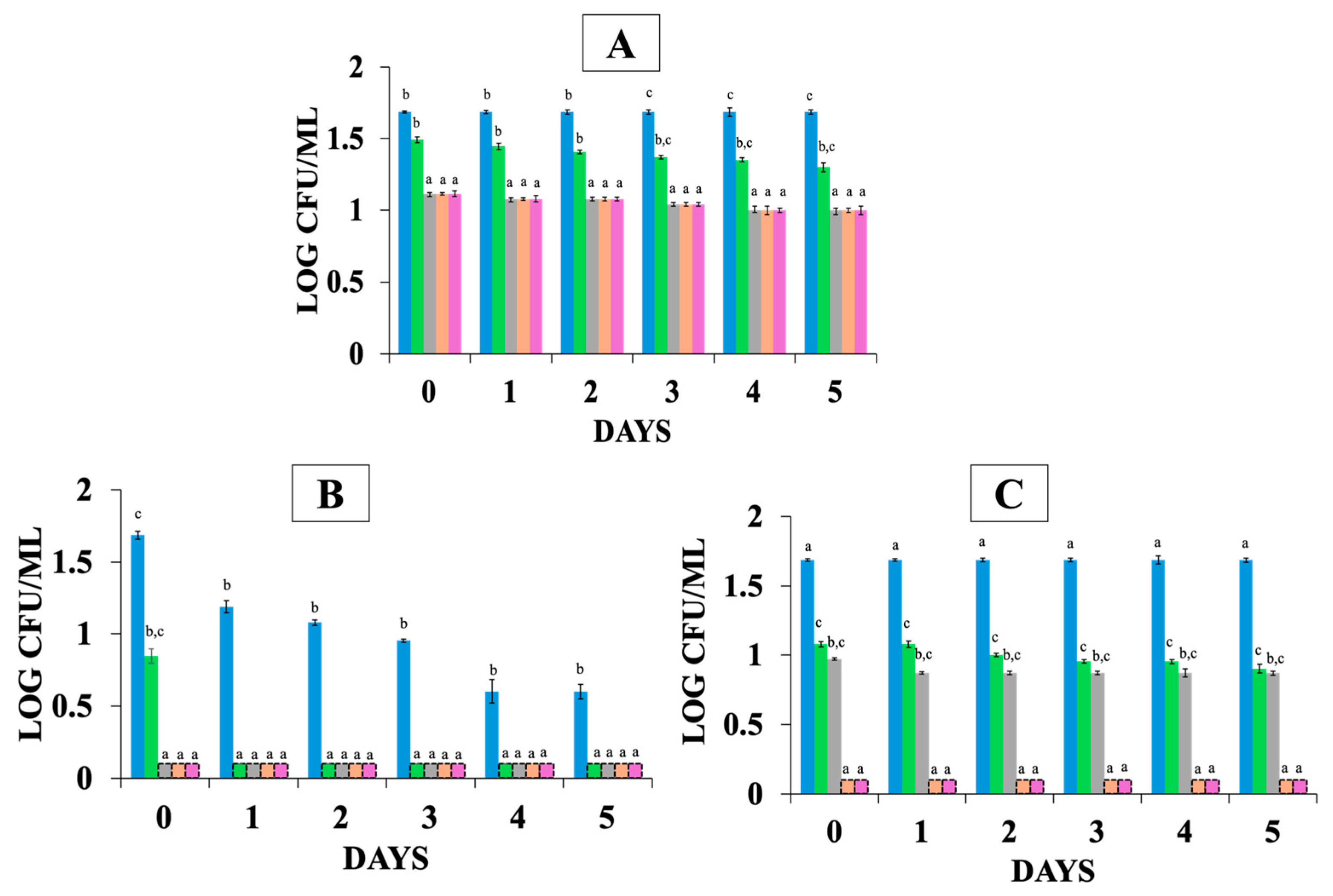
 = t = 0 and after the exposure in gastrointestinal tract (GIT) simulation;
= t = 0 and after the exposure in gastrointestinal tract (GIT) simulation;  = gastric phase after 1 h,
= gastric phase after 1 h,  = gastric phase after 2 h,
= gastric phase after 2 h,  = intestinal phase after 1 h,
= intestinal phase after 1 h,  = intestinal phase 2 h, in orange juice stored at the following: (A) 4 °C with free probiotic strains, (B) 4 °C with encapsulated probiotic strains, (C) 12 °C with free probiotic strains, (D) 12 °C with encapsulated probiotic strains. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
= intestinal phase 2 h, in orange juice stored at the following: (A) 4 °C with free probiotic strains, (B) 4 °C with encapsulated probiotic strains, (C) 12 °C with free probiotic strains, (D) 12 °C with encapsulated probiotic strains. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
 = t = 0 and after the exposure in gastrointestinal tract (GIT) simulation;
= t = 0 and after the exposure in gastrointestinal tract (GIT) simulation;  = gastric phase after 1 h,
= gastric phase after 1 h,  = gastric phase after 2 h,
= gastric phase after 2 h,  = intestinal phase after 1 h,
= intestinal phase after 1 h,  = intestinal phase 2 h, in orange juice stored at the following: (A) 4 °C with free probiotic strains, (B) 4 °C with encapsulated probiotic strains, (C) 12 °C with free probiotic strains, (D) 12 °C with encapsulated probiotic strains. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
= intestinal phase 2 h, in orange juice stored at the following: (A) 4 °C with free probiotic strains, (B) 4 °C with encapsulated probiotic strains, (C) 12 °C with free probiotic strains, (D) 12 °C with encapsulated probiotic strains. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).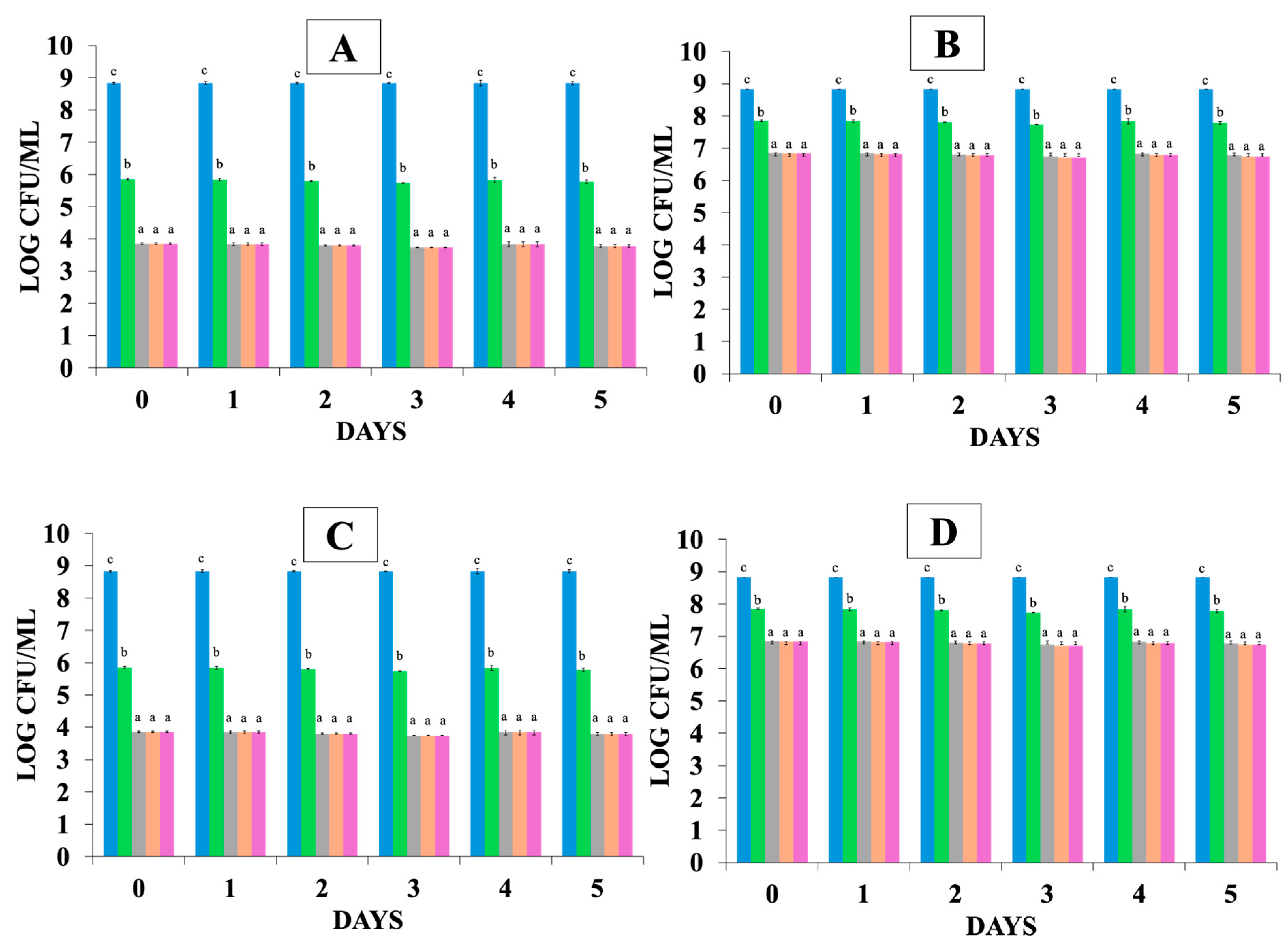
 FC: free probiotic,
FC: free probiotic,  EC: with encapsulated probiotic bacteria,
EC: with encapsulated probiotic bacteria,  C: control, at day 0 and day 5 of storage at 4 °C.
C: control, at day 0 and day 5 of storage at 4 °C.
 FC: free probiotic,
FC: free probiotic,  EC: with encapsulated probiotic bacteria,
EC: with encapsulated probiotic bacteria,  C: control, at day 0 and day 5 of storage at 4 °C.
C: control, at day 0 and day 5 of storage at 4 °C.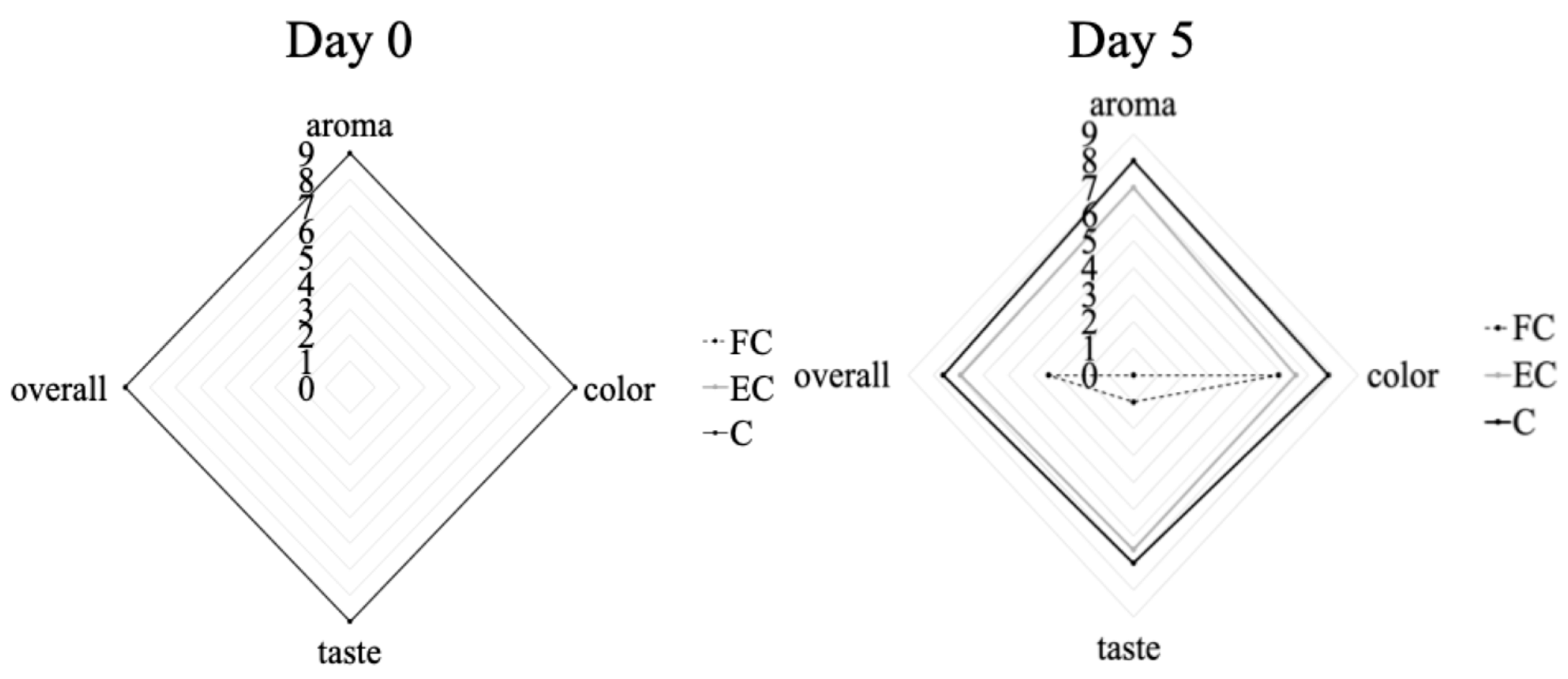
 FC: free probiotic,
FC: free probiotic,  EC: with encapsulated probiotic bacteria,
EC: with encapsulated probiotic bacteria,  C: control, at day 0 and day 5 of storage at 12 °C.
C: control, at day 0 and day 5 of storage at 12 °C.
 FC: free probiotic,
FC: free probiotic,  EC: with encapsulated probiotic bacteria,
EC: with encapsulated probiotic bacteria,  C: control, at day 0 and day 5 of storage at 12 °C.
C: control, at day 0 and day 5 of storage at 12 °C.
 = t = 0 and after the exposure in GIT simulation;
= t = 0 and after the exposure in GIT simulation;  = gastric phase after 1 h,
= gastric phase after 1 h,  = gastric phase after 2 h,
= gastric phase after 2 h,  = intestinal phase after 1 h,
= intestinal phase after 1 h,  = intestinal phase 2 h, where (A) control (S—without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
= intestinal phase 2 h, where (A) control (S—without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
 = t = 0 and after the exposure in GIT simulation;
= t = 0 and after the exposure in GIT simulation;  = gastric phase after 1 h,
= gastric phase after 1 h,  = gastric phase after 2 h,
= gastric phase after 2 h,  = intestinal phase after 1 h,
= intestinal phase after 1 h,  = intestinal phase 2 h, where (A) control (S—without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
= intestinal phase 2 h, where (A) control (S—without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
 t = 0 and after the exposure in GIT simulation;
t = 0 and after the exposure in GIT simulation;  = gastric phase after 1 h,
= gastric phase after 1 h,  = gastric phase after 2 h,
= gastric phase after 2 h,  = intestinal phase after 1 h,
= intestinal phase after 1 h,  = intestinal phase 2 h, where (A) S—control (without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
= intestinal phase 2 h, where (A) S—control (without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
 t = 0 and after the exposure in GIT simulation;
t = 0 and after the exposure in GIT simulation;  = gastric phase after 1 h,
= gastric phase after 1 h,  = gastric phase after 2 h,
= gastric phase after 2 h,  = intestinal phase after 1 h,
= intestinal phase after 1 h,  = intestinal phase 2 h, where (A) S—control (without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).
= intestinal phase 2 h, where (A) S—control (without probiotics), (B) FCS—with free probiotic cells, (C) ECS—with encapsulated probiotic cells. Different letters (a, b, c) indicate statistically significant differences (p < 0.05).