Abstract
Colovesical fistulas (CVFs) are abnormal connections between the colon and bladder, most commonly associated with complicated diverticular disease. The standard treatment involves bowel resection to remove the fistulous tract and reduce the risk of recurrence. This study aimed to evaluate the feasibility and safety of laparoscopic and robotic conservative surgery for CVFs, avoiding bowel resection. Between 2012 and 2019, 12 consecutive patients underwent conservative treatment: 5 with a laparoscopic approach and 7 with a robotic approach. Perioperative outcomes showed shorter operative time and lower blood loss in the robotic group (101 min vs. 144 min, p = 0.02; 47 mL vs. 176 mL, p = 0.02). No surgical conversions were required, and the complication rate was low. One recurrence occurred in the laparoscopic group due to extensive diverticular disease. Short- and long-term outcomes demonstrated favorable functional results, with a reduced risk of complications compared to traditional bowel resection. The robotic technique provided advantages in operative time and blood loss. A conservative approach is an option for selected patients, particularly those with non-extensive diverticular disease.
1. Introduction
A colovesical fistula (CVF) is an abnormal connection between the bowel and the bladder, most commonly occurring between the sigmoid colon and the bladder dome [1]. Enterovesical fistulas include colovesical, rectovesical, ileovesical, and appendicovesical fistulas. Among these, colovesical fistulas (CVFs) are the most common, accounting for approximately 95% of cases. Its incidence is about 0.5/10,000 cases per year. CVFs are more common in males than females, with a male-to-female ratio 3:1 [2,3]. This difference is attributed to the uterus in females, a barrier between the sigmoid colon and the bladder, reducing the likelihood of fistula formation. However, CVF can also occur in females without previous hysterectomy when the sigmoid colon comes into contact with the bladder dome above a prolapsed uterus. In 65–79% of cases, the primary cause of CVF is a complication of diverticular disease, typically resulting from inflammation following recurrent episodes of diverticulitis or the formation of a diverticular abscess under the bladder. However, only 1–4% of patients with diverticular disease develop a CVF [4,5,6,7]. Other causes include pelvic cancers or a history of colorectal or bladder cancer (10–15%), complications of surgery such as prostatectomy, rectal resection, endoscopic procedures, augmentation cystoplasty, laparoscopic inguinal hernia repair, chemotherapy or radiotherapy complications, and pelvic or abdominal penetrating trauma [8]. Although enteral conditions generally cause CVF, many patients present with urological symptoms [1]. These symptoms often include recurrent urinary tract infections or asymptomatic bacteriuria, which can persist for months. Gouverneur’s syndrome is the hallmark clinical presentation of any enterovesical fistula, characterized by suprapubic pain, urinary frequency, dysuria, and tenesmus. While symptoms may arise from both the gastrointestinal and urinary tracts, they predominantly originate from the latter [9]. The diagnostic process for CVFs starts with the onset of typical symptoms. Pneumaturia is the most common, occurring in 50–70% of cases, followed by fecaluria in up to 51%, recurrent urinary tract infections (45%), dysuria, frequency, urgency, suprapubic pain, hematuria (22%), and orchitis (10%) [6,10,11]. Computed Tomography (CT) is the gold-standard imaging exam for detecting CVFs, as the American College of Radiology recommends. CT scans typically show bladder wall thickening near a loop of thickened intestine, the presence of colonic diverticula, the air within the bladder, and oral contrast material inside the bladder on scans without intravenous contrast [12,13,14,15,16]. Magnetic resonance imaging (MRI) is another highly accurate diagnostic tool, offering specificity and sensitivity rates that may reach 100%.
Although barium enemas may assist in differentiating diverticular disease from colonic cancer, they are not definitive, and further imaging and endoscopic evaluation are required for an accurate diagnosis [17,18,19,20]. Cystoscopy and colonoscopy are valuable for defining the nature and extent of the fistula [4]. Ultrasound imaging may identify the “beak sign,” where abdominal compression reveals an echogenic tract connecting the peristaltic bowel lumen to the urinary bladder [21,22]. Finally, the poppy seed test achieves a 100% diagnostic success rate for CVFs, though it does not provide information about the fistula’s exact location.
CVFs can be managed using both surgical and non-surgical approaches. Medical management includes fasting, parenteral nutrition, antibiotics, steroids, immunomodulatory drugs, and vesical catheter drainage. This approach is suitable for patients who are not candidates for major surgery due to poor overall health, inability to tolerate general anesthesia, or the presence of metastatic cancer [23,24]. The standard surgical treatment includes resection of the affected bowel segment, followed by either immediate or staged anastomosis and repair of the bladder wall defect. This procedure is typically performed using an open surgical approach [25]. In the last decade, minimally invasive techniques have gained traction in treating diverticular fistulas due to their association with reduced morbidity and improved postoperative outcomes. However, mortality and morbidity rates remain significant, influenced by factors such as colonic resection, adhesions, paracolic abscesses, shortening of the colon and mesocolon, colonic wall thickening related to diverticulitis, and complications such as anastomotic leakage. These complications can lead to severe outcomes, including peritonitis, septic shock, and the need for re-intervention. While colorectal cancer often requires complex oncologic resection, diverticulitis-related CVFs may present greater technical difficulties due to chronic inflammation, severe fibrosis, adhesions, and altered tissue planes. These factors can significantly increase operative complexity despite the benign nature of the disease [26].
Previously, we described our laparoscopic conservative technique involving simple fistulectomy avoiding colon resection [3]. We reproduced the same conservative technique through a robotic approach, utilizing the advantages of robotic surgery to overcome the limitations of laparoscopy. This study aimed to evaluate the feasibility and safety of minimally invasive conservative surgery for CVF through a retrospective analysis of perioperative and long-term outcomes from a series of consecutive laparoscopic and robotic conservative treatments.
2. Materials and Methods
Between May 2012 and June 2016, we performed laparoscopic conservative treatments for CVF in consecutive, unselected patients. From July 2016 to June 2019, we continued conservative treatment using the Si or Xi da Vinci (Intuitive Surgical Inc., Sunnyvale, CA, USA) robotic platforms in a successive, unselected patient cohort. Data were prospectively collected, including demographic information, intraoperative parameters, perioperative outcomes, and long-term outcomes. The internal institutional review board approved the study, and written informed consent was obtained from all patients before surgery and data collection.
All patients underwent abdominal and pelvic CT scans with intravenous contrast, colonoscopy, and cystoscopy. A colonoscopy was performed to rule out a gastrointestinal neoplastic cause or active colitis, while a cystoscopy was conducted to exclude bladder cancer. Patients with malignancies were excluded from the study. None of the patients had undergone prior abdominal or pelvic surgery. Among the female patients, all had intact uteri and no history of hysterectomy. All surgical procedures were performed by a single urologist highly skilled in both laparoscopic and robotic techniques, without consultation from other specialists. The surgeon initially treated CVFs laparoscopically before transitioning to a robotic approach after completing an extensive learning curve that included hundreds of laparoscopic and robotic procedures across various pathologies. A cystogram was performed on the seventh postoperative day to assess the integrity of the repair before catheter removal. If leakage was detected, a repeat cystogram was conducted after five days and subsequently as needed.
Demographic variables included age, gender, BMI, and the American Society of Anesthesiologists (ASA) score. Intraoperative and perioperative outcomes assessed included operative time, estimated blood loss, need for conversion, time to bowel movement, postoperative pain, 30-day overall complication rate, hospital stay, and 30-day recurrence rate. Postoperative pain was evaluated using the Visual Analog Scale (VAS), and perioperative complications were classified according to the Clavien-Dindo system, further stratified into minor (Grade I–II) and major (Grade III–V) complications. Long-term outcomes were assessed based on CVF recurrence and other postoperative complications.
Statistical analysis was conducted using SPSS (IBM Corp., Released 2017, Version 25.0, Armonk, NY, USA). Parametric variables are presented as mean ± standard deviation, while categorical non-parametric variables are reported as absolute and relative frequencies (n, %). Non-parametric numerical variables are expressed as median (range). Comparisons between two independent samples were performed using the Mann–Whitney U test and pairwise comparisons for statistically significant results. Significance was set at p < 0.05.
Surgical Technique
In the laparoscopic approach, a 15° Trendelenburg position was applied and a 12 mm trocar for the camera was placed at the umbilicus according to Hasson technique, two 10 mm trocars were placed at the level of the right and left iliac fossa and an assistant 5 mm port was placed along the left mid-clavicular line. For the robotic approach, the patient was positioned in a 25° Trendelenburg position to facilitate access to the abdominal cavity. A da Vinci Xi or Si robotic platform trocar was inserted using the Hasson technique at the midline, 2 cm above the navel. The abdominal cavity was then insufflated with CO2 to a pressure of 12 mmHg, and a 0-degree camera was used. Under direct visualization, four additional trocars were placed at intervals of 7–8 cm across the abdomen: two laterally to the optical trocar and two positioned 2 cm above and medial to the anterior superior iliac spine. In all laparoscopic and robotic cases, the fistulous loop was meticulously isolated (Figure 1), sutured and resected by laparoscopic mechanical stapler (Figure 2) as close as possible to the colonic wall, to preserve bladder tissue and ensure complete excision of the tract. Dissection was performed circumferentially until the tract was clearly visible before stapling. We divided the bladder wall from the enteric tract. We sutured and sinked the wall by interrupted stitches and performed a curettage of the fistula site. The bladder wall was closed with a single layer running suture using Vicryl 2-0. When necrotic and inflammatory tissue surrounding the fistula was extended to bladder wall, the excision of a bladder cuff en bloc and a two layers closure of the defect was performed. Following that, to evaluate the suture’s strength, the bladder was filled by 200 mL of methylene. To minimize the risk of fistula recurrence, an omental flap was consistently interposed between the bladder and the colon (Figure 3).
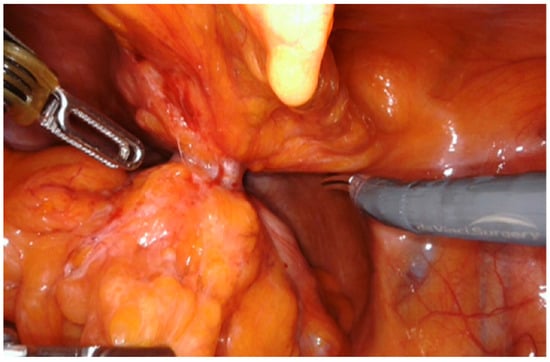
Figure 1.
Isolation of the fistulous tract.
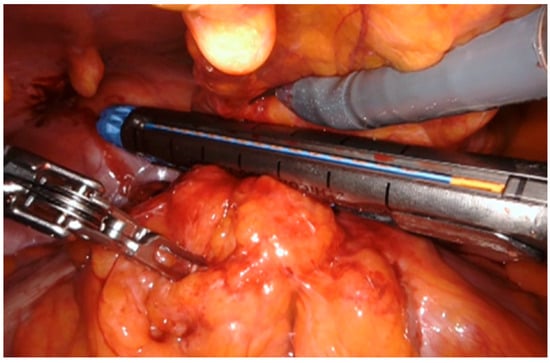
Figure 2.
Suture of the fistulous tract by mechanical stapler.
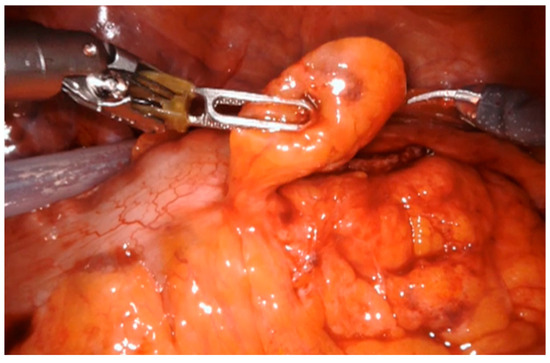
Figure 3.
Placement of fat tissue flap.
3. Results
A total of 12 cases were included in the study. The median age was 51 years (range 34–69) in the laparoscopic group, consisting of three males and two females, and 55 years (range 42–83) in the robotic group, which included five males and two females. The median BMI was 25 (range 22–28) in the laparoscopic group and 26 (range 23–29) in the robotic group. Demographic and perioperative data are summarized in Table 1.

Table 1.
Results.
In the laparoscopic group, 1 (20%) case of CVF occurred following the endoscopic removal of a 2 cm adenomatous polyp within a sigmoid diverticulum. In contrast, the remaining 4 (80%) cases resulted from complicated diverticulitis. In the robotic group, CVF was attributed to non-extensive sigmoid diverticular disease in 6 (85.7%) patients, whereas 1 (14.3%) case was associated with extensive sigmoid diverticular disease. In all cases, the fistulous tract was successfully isolated and resected without requiring bowel resection. A temporary loop ileostomy was performed on one patient due to extensive diverticular disease.
Perioperative outcomes were comparable between the two minimally invasive techniques. No conversions to open surgery were necessary, and no intraoperative complications occurred. Blood transfusions were not required, and no urinary leakage was observed in any patient. The urinary catheter was removed after an average of 8.5 ± 1.4 days in the laparoscopic group and 8.9 ± 1.8 days in the robotic group (p = 0.67) following a confirmatory cystogram. The laparoscopic group had a longer operative time (144 ± 29.4 min vs. 101 ± 21.5 min, p = 0.02) and higher estimated blood loss (176.7 ± 83.4 mL vs. 47 ± 23.8 mL, p = 0.02) compared to the robotic group. No cases of surgical site infection or intra-abdominal abscesses were recorded postoperatively. In the robotic cohort, 2 (28.6%) patients experienced Clavien-Dindo grade II complications: one case of postoperative urinary tract infection treated with antibiotics and one case of transient ileus managed with fasting and nasogastric decompression for three days. In the laparoscopic group, 1 (20%) patient experienced a grade IIIb fistula recurrence. This patient, who had extensive diverticular disease, developed a new CVF two weeks after surgery, presenting with urinary infection, fever, and fecaluria. A CT scan revealed a large abscess involving the bladder and sigmoid colon, necessitating sigmoid resection with Hartmann’s procedure and temporary colostomy via an open surgical approach. No other complications were reported during the follow-up. The median follow-up period was 96 months (range 81–112) for the laparoscopic group and 64 months (range 41–86) for the robotic group.
4. Discussion
This study highlighted the favorable perioperative and long-term outcomes of both laparoscopic and robotic conservative surgeries for CVFs. Both approaches have demonstrated safety and feasibility, with no conversions and a low rate of complications. CVFs typically arise in the context of bowel diseases such as diverticulitis, colorectal carcinoma, and Crohn’s disease [27]. Complicated diverticular disease of the sigmoid colon is widely recognized as the leading cause of CVFs, accounting for approximately 50% to 80% of cases [11,28,29,30,31]. CVFs secondary to colon cancer are also significant, representing 12% to 27% of all cases [4,5,24], highlighting the importance of an accurate preoperative diagnostic workup. Surgical treatment remains the standard of care for CVFs, traditionally involving resection of the fistulous tract and sigmoid colon with contextual or subsequent anastomosis. While the open surgical approach was once preferred, laparoscopic techniques have gained traction, initially for uncomplicated diverticular disease and progressively for more complicated cases, becoming the standard of care [32,33]. Several studies have demonstrated the safety and tolerability of laparoscopic management of CVFs [28,34,35,36,37]. However, interpreting these results can be challenging due to the inclusion of uncomplicated and complicated diverticular diseases and a lack of distinction between CVFs and other types of intestinal fistulas. Compared to open surgery, laparoscopy offers benefits such as reduced postoperative pain, faster recovery of bowel function, and lower complication rates, leading to shorter hospital stays. Nevertheless, high conversion rates to open surgery, ranging from 5% to 65%, and prolonged operative times have been reported [33]. For instance, a study by Hassan et al. reported a 26% conversion rate and a 25% overall complication rate within 30 days post-surgery [38]. In a recent case series focusing on laparoscopic surgery for diverticular fistulas, Martinolich et al. reported a 46.9% conversion rate for CVFs, higher than for other types of colonic fistulas. Common reasons for conversion included bleeding, failure to progress, severe fibrosis, and difficulty with fistula division [39]. Salgado-Nesme et al. evaluated perioperative outcomes in 12 patients with CVFs treated laparoscopically compared to 12 patients treated with open surgery. The laparoscopic group showed reductions in intraoperative bleeding (268 ± 222 mL vs. 327 ± 169 mL), surgical time (212 min vs. 243 min), and length of hospital stay (9 ± 4 days vs. 15 ± 11 days), although these differences were not statistically significant [40]. In our study, we reported perioperative outcomes of five laparoscopic and seven robotic cases using a conservative technique consisting of simple fistulectomy without bowel resection. At a median follow-up of 96 months, our laparoscopic series results were comparable to other laparoscopic series involving colon resection. Specifically, our mean operative time, estimated blood loss, and hospital stay were 144 ± 29.4 min, 176.7 ± 83.4 mL, and 7.6 ± 1.3 days, respectively. A recent systematic review reported operative times ranging from 150 to 321 min, estimated blood loss between 50 and 300 mL, and hospital stays between 4 and 21 days [41]. Moreover, in our series, there was no conversion to open surgery. We observed one fistula recurrence (grade IIIb complication) in the laparoscopic group involving a patient with extensive diverticular disease. In this case, a sigmoid resection with a temporary colostomy was performed. Avoiding bowel resection in the presence of extensive diverticular disease may increase the risk of recurrence. Therefore, we recommend considering bowel resection in such cases. Literature indicates a fistula recurrence rate of about 0.8% and an overall re-intervention rate for postoperative complications of about 2% within 30 days of surgery [33]. In the presence of not extensive diverticular disease, the possible consequences related to bowel resection could be worse and more frequent than the complications related to not resecting the affected intestinal tract, especially for frail patients with poor performance status or high-risk ASA scores. The application of robotic technology in the surgical management of diverticulitis is safe and feasible, even in small retrospective case series [42,43,44,45,46]. In a recent study, Grass et al. evaluated surgical outcomes in 150 patients who underwent robotic left-sided colectomy for both uncomplicated and complicated diverticular disease. The study highlighted the feasibility and safety of the robotic approach for complicated diverticular disease, reporting a mean operative time of 288 min, a conversion rate of 10.3%, a postoperative complication rate of 28.2%, and a median hospital stay of 3 days [47]. The robotic technique maintains the advantages of laparoscopy and offers additional benefits such as magnification of the operative field through three-dimensional, high-definition vision, more precise movements with EndoWrist® instruments (Intuitive Surgical Inc., Sunnyvale, CA, USA) providing seven degrees of motion, primary surgeon camera control, and elimination of tremor. These features allow for the replication of traditional surgical steps with the benefits of a minimally invasive technique, overcoming the limitations of laparoscopy [46,48]. Due to these advantages, the robotic technique provides better visualization of dissection planes in stiff and severely inflamed tissues and difficult anatomic locations, as seen in CVFs. This leads to decreased bleeding, conversion rates, and perioperative complications. Maciel et al., in a retrospective study including 75 patients, compared laparoscopic and robotic colectomy in CVF. The robotic surgery group reported a lower estimated blood loss (101.25 mL vs. 187.65 mL) and conversion rate (0% vs. 14.55%), despite a higher operative time (207.67 min vs. 126.67 min). In contrast, no significant differences in complication rate occurred between the two groups (20% vs. 29%) [49].
In our case series, the robotic conservative technique demonstrated a trend toward superiority compared to both robotic and laparoscopic surgeries involving bowel resection. Specifically, we observed a significantly reduced intraoperative blood loss and a shorter mean operative time. No conversions to laparoscopic or open surgery were required, and no fistula recurrences were recorded. Only two complications occurred, both classified as Grade II according to the Clavien-Dindo classification: postoperative urinary tract infection and transient ileus, both affecting the same patient. These were successfully managed with antibiotic therapy, fasting, and a nasogastric tube for three days. A temporary ileostomy was performed in one patient with extensive diverticular disease, as we consider diversion a valuable safety measure in selected cases, particularly in the presence of fistulas involving poor-quality tissue. Postoperative complications following surgical treatment of CVFs represent a significant challenge, with anastomotic and bladder leaks being among the most concerning adverse events. Anastomotic leakage occurs in approximately 4% of cases, while bladder leakage is reported in 1.8%, both of which can lead to severe morbidity and often necessitate further surgical intervention [50]. A conservative approach, tailored to the patient’s and the disease’s characteristics, may improve surgical outcomes. Avoiding bowel resection facilitates faster recovery, reduces fasting duration, shortens hospital stay, and minimizes the risk of complications. This study represents the first report on long-term surgical outcomes of laparoscopic and robotic approaches for CVF treatment without colectomy. A key strength of our study is the homogeneous patient population, consisting exclusively of cases of diverticular disease complicated by fistula. Our findings highlight the safety and potential benefits of a minimally invasive, conservative approach that avoids colonic resection in CVF management. Overall morbidity and hospital length of stay were lower than surgeries involving colectomy. Notably, our conservative technique appears to be associated with a reduced risk of both short- and long-term complications. This may be attributed to two main factors: first, the extensive expertise of surgeons in both laparoscopic and robotic techniques, and second, the preservation of the sigmoid colon, which may reduce surgical morbidity. Our analysis suggests that laparoscopic and robotic approaches yield comparable short- and long-term surgical outcomes. However, the robotic technique demonstrated advantages in terms of shorter operative time and reduced intraoperative blood loss compared to laparoscopy. If these benefits are confirmed in larger studies, they could potentially offset the higher costs associated with robotic surgery. This study has several limitations. First, the small sample size in both groups could lead to type II errors, limiting our findings’ statistical significance and generalizability. Second, the study’s retrospective nature further constrains the data’s robustness. Future prospective studies with larger cohorts are warranted to validate these preliminary findings.
5. Conclusions
Laparoscopic and robotic conservative surgical approaches for the management of CVF have proven to be both feasible and safe. In patients without extensive diverticular disease, a non-resection strategy may be a reasonable option, particularly for those with elevated risk factors for postoperative complications, such as frail patients. Our preliminary findings indicate low bleeding, complications, and conversion rates within our limited patient cohort. Notably, the robotic approach demonstrated slightly superior outcomes regarding operative time and intraoperative blood loss. However, large-scale prospective studies, multicentric studies with future randomized controlled trials are essential to assess further this minimally invasive conservative strategy’s safety and efficacy, compare the outcomes of laparoscopic and robotic techniques, and define clear patient selection criteria for optimal surgical management.
Author Contributions
Conceptualization, A.P. and E.M.; methodology, A.V.; formal analysis, R.L.M.; investigation, M.M.; writing—original draft preparation, A.P.; writing—review and editing, A.V.; visualization, R.L.M.; supervision, G.C.; project administration, G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study was registered under the protocol “CVF” received a favorable opinion from the Regional Ethics Committee of Umbria (CET Umbria), communicated with protocol No. CE-2738/25 on dated 18 September 2025, R.CET: 4988/25.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data reported in this article are all available at location cited in the reference section.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Golabek, T.; Szymanska, A.; Szopinski, T.; Bukowczan, J.; Furmanek, M.; Powroznik, J.; Chlosta, P. Enterovesical Fistulae: Aetiology, Imaging, and Management. Gastroenterol. Res. Pract. 2013, 2013, 617967. [Google Scholar] [CrossRef]
- Larsen, A.; Johansen, T.E.B.; Solheim, B.M.; Urnes, T. Diagnosis and Treatment of Enterovesical Fistula. Eur. Urol. 1996, 29, 318–321. [Google Scholar] [CrossRef]
- Giovanni, C.; Emanuele, C.; Roberto, C.; Alberto, P.; Emanuele, L.; Alessia, C.; Francesco, B.; Ettore, M. Laparoscopic conservative surgery of colovesical fistula: Is it the right way? Videosurg. Other Miniinvasive Tech. 2013, 2, 162–165. [Google Scholar] [CrossRef]
- Daniels, I.R.; Bekdash, B.; Scott, H.J.; Marks, C.G.; Donaldson, D.R. Diagnostic lessons learnt from a series of enterovesical fistulae. Color. Dis. 2002, 4, 459–462. [Google Scholar] [CrossRef]
- Pollard, S.G.; Macfarlane, R.; Greatorex, R.; Everett, W.G.; Hartfall, W.G. Colovesical fistula. Ann. R. Coll. Surg. Engl. 1987, 69, 163–165. [Google Scholar] [PubMed]
- Najjar, S.F.; Jamal, M.K.; Savas, J.F.; Miller, T.A. The spectrum of colovesical fistula and diagnostic paradigm. Am. J. Surg. 2004, 188, 617–621. [Google Scholar] [CrossRef]
- Bahadursingh, A.M.; Virgo, K.S.; Kaminski, D.L.; Longo, W.E. Spectrum of disease and outcome of complicated diverticular disease. Am. J. Surg. 2003, 186, 696–701. [Google Scholar] [CrossRef]
- Yang, C.-H.O.; Liu, K.-H.; Chen, T.-C.; Chang, P.-L.; Yeh, T.-S. Enterovesical fistula caused by a bladder squamous cell carcinoma. World J. Gastroenterol. 2009, 15, 4215–4217. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Vaughan, A.; Fagan, M.; Schumacher, D.P.; Wekullo, V.; Gehrke, B. Colovesical fistula in men with chronic urinary tract infection: A diagnostic challenge. Clevel. Clin. J. Med. 2023, 90, 165–171. [Google Scholar] [CrossRef]
- McBeath, R.B.; Schiff, M.; Allen, V.; Bottaccini, M.; Miller, J.I.; Ehreth, J.T. A 12-year experience withenterovesical fistulas. Urology 1994, 44, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Melchior, S.; Cudovic, D.; Jones, J.; Thomas, C.; Gillitzer, R.; Thüroff, J. Diagnosis and Surgical Management of Colovesical Fistulas Due to Sigmoid Diverticulitis. J. Urol. 2009, 182, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Shinojima, T.; Nakajima, F.; Koizumi, J. Efficacy of 3-D computed tomographic reconstruction in evaluating anatomical relationships of colovesical fistula. Int. J. Urol. 2002, 9, 230–232. [Google Scholar] [CrossRef]
- Goldman, S.; Fishman, E.; Gatewood, O.; Jones, B.; Siegelman, S. CT in the diagnosis of enterovesical fistulae. Am. J. Roentgenol. 1985, 144, 1229–1233. [Google Scholar] [CrossRef]
- Jarrett, T.W.; Vaughan, E.D. Accuracy of Computerized Tomography in the Diagnosis of Colovesical Fistula Secondary to Diverticular Disease. J. Urol. 1995, 153, 44–46. [Google Scholar] [CrossRef]
- Labs, J.D.; Sarr, M.G.; Fishman, E.K.; Siegelman, S.S.; Cameron, J.L. Complications of acute diverticulitis of the colon: Improved early diagnosis with computerized tomography. Am. J. Surg. 1988, 155, 331–335. [Google Scholar] [CrossRef]
- Keady, C.; Hechtl, D.; Joyce, M. When the bowel meets the bladder: Optimal management of colorectal pathology with urological involvement. World J. Gastrointest. Surg. 2020, 12, 208–225. [Google Scholar] [CrossRef]
- Ravichandran, S.; Ahmed, H.; Matanhelia, S.; Dobson, M. Is There a Role for Magnetic Resonance Imaging in Diagnosing Colovesical Fistulas? Urology 2008, 72, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Semelka, R.C.; Hricak, H.; Kim, B.; Forstner, R.; Bis, K.G.; Ascher, S.M.; Reinhold, C. Pelvic fistulas: Appearances on MR images. Abdom. Imaging 1997, 22, 91–95. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Booth, T.C.; Swallow, D.; Shahabuddin, K.; Thomas, M.; Hanbury, D.; Chang, S.; King, C. Imaging features of colovesical fistulae on MRI. Br. J. Radiol. 2012, 85, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.N.; Knox, R.; Barnard, R.J.; Schofield, P.F. Management of colovesical fistula. Br. J. Surg. 1987, 74, 362–363. [Google Scholar] [CrossRef]
- Sutijono, D. Point-of-Care Sonographic Diagnosis of an Enterovesical Fistula. J. Ultrasound Med. 2013, 32, 883–885. [Google Scholar] [CrossRef]
- Chen, S.; Chou, Y.; Tiu, C.; Chang, T. Sonographic features of colovesical fistula. J. Clin. Ultrasound 1990, 18, 589–591. [Google Scholar] [CrossRef]
- Amin, M.; Nallinger, R.; Polk, H.C. Conservative treatment of selected patients with colovesical fistula due to diverticulitis. Surg. Gynecol. Obstet. 1984, 159, 442–444. [Google Scholar] [PubMed]
- Solkar, M.H.; Forshaw, M.J.; Sankararajah, D.; Stewart, M.; Parker, M.C. Colovesical fistula—Is a surgical approach always justified? Color. Dis. 2005, 7, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Cochetti, G.; Del Zingaro, M.; Boni, A.; Cocca, D.; Panciarola, M.; Tiezzi, A.; Gaudio, G.; Balzarini, F.; Ursi, P.; Mearini, E. Colovesical fistula: Review on conservative management, surgical techniques and minimally invasive approaches. Il G. Chir.-J. Ital. Surg. Assoc. 2018, 39, 195–207. [Google Scholar]
- Van Arendonk, K.J.; Tymitz, K.M.; Gearhart, S.L.; Stem, M.; Lidor, A.O. Outcomes and Costs of Elective Surgery for Diverticular Disease. JAMA Surg. 2013, 148, 316–321. [Google Scholar] [CrossRef]
- Driver, C.P.; Anderson, D.N.; Findlay, K.; Keenan, R.A.; Davidson, A.I. Vesico-colic fistulae in the Grampian region: Presentation, assessment, management and outcome. J. R. Coll. Surg. Edinb. 1997, 42, 182–185. [Google Scholar]
- Marney, L.A.; Ho, Y.-H. Laparoscopic Management of Diverticular Colovesical Fistula: Experience in 15 Cases and Review of the Literature. Int. Surg. 2013, 98, 101–109. [Google Scholar] [CrossRef]
- Pironi, D.; Candioli, S.; Manigrasso, A.; La Torre, V.; Palazzini, G.; Romani, A.M.; Tarroni, D.; Filippini, A. Complicated diverticular disease. Three cases of colovesical fistulas and review of literature. Il G. Chir.-J. Ital. Surg. Assoc. 2006, 27, 15–20. [Google Scholar]
- Young-Fadok, T.M.; Roberts, P.L.; Spencer, M.P.; Wolff, B.G. Colonic Diverticular Disease. Curr. Probl. Surg. 2000, 37, 459–514. [Google Scholar] [CrossRef]
- Desiderio, J.; Trastulli, S.; Listorti, C.; Milani, D.; Cerroni, M.; Cochetti, G.; Cirocchi, R.; Boselli, C.; Parisi, A.; Mearini, E.; et al. Surgical approach of complicated diverticulitis with colovesical fistula: Technical note in a particular condition. Open Med. 2012, 7, 578–583. [Google Scholar] [CrossRef]
- Abraha, I.; Binda, G.A.; Montedori, A.; Arezzo, A.; Cirocchi, R. Laparoscopic versus open resection for sigmoid diverticulitis. Cochrane Database Syst. Rev. 2017, 2017, CD009277. [Google Scholar] [CrossRef]
- Cirocchi, R.; Arezzo, A.; Renzi, C.; Cochetti, G.; D’ANdrea, V.; Fingerhut, A.; Mearini, E.; Binda, G.A. Is laparoscopic surgery the best treatment in fistulas complicating diverticular disease of the sigmoid colon? A systematic review. Int. J. Surg. 2015, 24, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.R.; Detroz, B.; Detry, O.; Degauque, C.; Honoré, P.; Meurisse, M. Laparoscopic Sigmoidectomy for Fistulized Diverticulitis. Dis. Colon Rectum 2005, 48, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.Q.; Divino, C.M.; Vine, A.; Reiner, M.; Katz, L.B.; Salky, B. Laparoscopic surgery for diverticular disease complicated by fistulae. JSLS 2006, 10, 166–168. [Google Scholar]
- Engledow, A.H.; Pakzad, F.; Ward, N.J.; Arulampalam, T.; Motson, R.W. Laparoscopic resection of diverticular fistulae: A 10-year experience. Color. Dis. 2007, 9, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, R.M.; Plaisier, P.W.; van der Hoeven, J.A.B.; Hesp, W.L.E.M. Outcome of Surgery for Colovesical and Colovaginal Fistulas of Diverticular Origin in 40 Patients. J. Gastrointest. Surg. 2012, 16, 1559–1565. [Google Scholar] [CrossRef]
- Hassan, I.; Cima, R.R.; Larson, D.W.; Dozois, E.J.; Byrne, M.M.O.; Larson, D.R.; Pemberton, J.H. The Impact of Uncomplicated and Complicated Diverticulitis on Laparoscopic Surgery Conversion Rates and Patient Outcomes. Surg. Endosc. 2007, 21, 1690–1694. [Google Scholar] [CrossRef]
- Martinolich, J.; Croasdale, D.R.; Bhakta, A.S.; Ata, A.; Chismark, A.D.; Valerian, B.T.; Canete, J.J.; Lee, E.C. Laparoscopic Surgery for Diverticular Fistulas: Outcomes of 111 Consecutive Cases at a Single Institution. J. Gastrointest. Surg. 2019, 23, 1015–1021. [Google Scholar] [CrossRef]
- Salgado-Nesme, N.; Vergara-Fernández, O.; Espino-Urbina, L.A.; Luna-Torres, H.A.; Navarro-Navarro, A. Advantages of Minimally Invasive Surgery for the Treatment of Colovesical Fistula. Rev. Investig. Clin. 2016, 68, 229–304. [Google Scholar] [CrossRef]
- Cirocchi, R.; Cochetti, G.; Randolph, J.; Listorti, C.; Castellani, E.; Renzi, C.; Mearini, E.; Fingerhut, A. Laparoscopic treatment of colovesical fistulas due to complicated colonic diverticular disease: A systematic review. Tech. Coloproctol. 2014, 18, 873–885. [Google Scholar] [CrossRef]
- Weber, P.A.; Merola, S.; Wasielewski, A.; Ballantyne, G.H. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis. Colon Rectum 2002, 45, 1689–1694; discussion 1695–1696. [Google Scholar] [CrossRef]
- Delaney, C.P.; Lynch, C.A.; Senagore, A.J.; Fazio, V.W. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis. Colon Rectum 2003, 46, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Braumann, C.; Jacobi, C.A.; Menenakos, C.; Borchert, U.; Rueckert, J.C.; Mueller, J.M. Computer-Assisted Laparoscopic Colon Resection with the Da Vinci® System: Our First Experiences. Dis. Colon Rectum 2005, 48, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.L.; Woodland, J.H.; Vegunta, R.K.; Crawford, D.L. Robotic versus laparoscopic colectomy. Surg. Endosc. 2007, 21, 1701–1708. [Google Scholar] [CrossRef]
- Ragupathi, M.; Ramos-Valadez, D.I.; Patel, C.B.; Haas, E.M. Robotic-assisted laparoscopic surgery for recurrent diverticulitis: Experience in consecutive cases and a review of the literature. Surg. Endosc. 2011, 25, 199–206. [Google Scholar] [CrossRef]
- Grass, F.; Crippa, J.; Mathis, K.L.; Kelley, S.R.; Larson, D.W. Feasibility and safety of robotic resection of complicated diverticular disease. Surg. Endosc. 2019, 33, 4171–4176. [Google Scholar] [CrossRef]
- Mearini, E.; Cirocchi, R.; Cochetti, G. Robot-Assisted Surgery in Urology: The Show Must Go On. Appl. Sci. 2019, 9, 844. [Google Scholar] [CrossRef]
- Maciel, V.; Lujan, H.J.; Plasencia, G.; Zeichen, M.; Mata, W.; Jorge, I.; Lee, D.; Viamonte, M.; Hartmann, R.F. Diverticular Disease Complicated with Colovesical Fistula: Laparoscopic Versus Robotic Management. Int. Surg. 2014, 99, 203–210. [Google Scholar] [CrossRef]
- Zizzo, M.; Tumiati, D.; Bassi, M.C.; Zanelli, M.; Sanguedolce, F.; Porpiglia, F.; Fiori, C.; Campobasso, D.; Ruiz, C.C.; Bergamaschi, F.A.; et al. Management of colovesical fistula: A systematic review. Minerva Urol. Nephrol. 2022, 74, 400–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).