AI-Enabled Secure and Scalable Distributed Web Architecture for Medical Informatics
Abstract
1. Introduction
2. Related Work
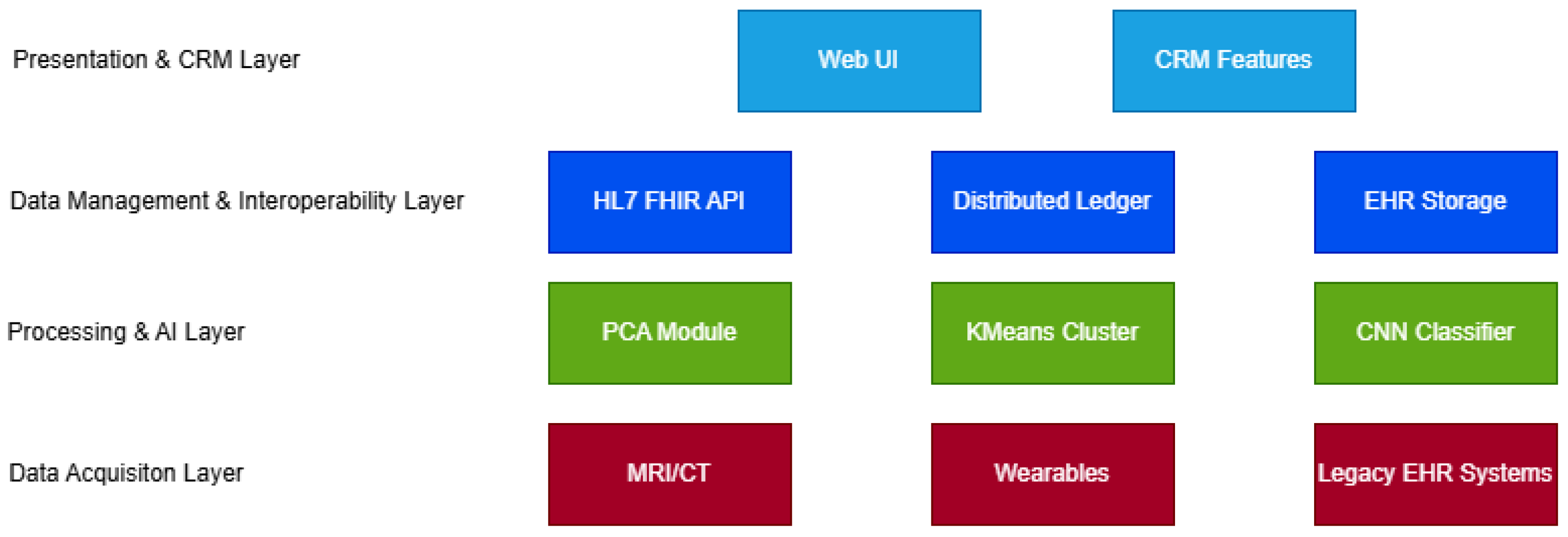
3. Proposed Architecture
3.1. Data Acquisition Layer
3.2. Processing and AI Layer
- Principal component analysis (PCA): Used for dimensionality reduction in DTI (diffusion tensor imaging) data to minimize noise and reduce computational complexity [21].
- K-Means clustering: Applied as a lightweight unsupervised method to segment potential anomalies or lesion clusters for subsequent analysis.
3.3. Data Management and Interoperability Layer
3.4. Presentation and Customer Relationship Management (CRM)-Enhanced Interaction Layer
3.5. Deployment Model
3.6. Technical Implementation Details
| Algorithm 1: Docker Compose configuration for AI inference service |
Data: Containerized AI diagnostic service Result: Deployment of CNN inference module in a distributed environment Service Definition: ai_inference Image: medical/cnn_inference:v2 Deployment Settings: replicas = 3 cpus = 2.0 memory = 4G Port Mapping: 5000:5000 Network: ehr_net |
3.7. AI Diagnostic Pipeline: Pseudocode
| Algorithm 2: AI-Based Diagnostic Pipeline for DTI Data Processing |
Input: DTI_Image Output: Diagnosis_Label Step 1: Preprocessing Normalize(DTI_Image) Resize(DTI_Image, 128 × 128) Step 2: Dimensionality Reduction PCA_Model ← Train_PCA(DTI_Training_Set) Reduced_Features ← PCA_Model.Transform(DTI_Image) Step 3: Unsupervised Clustering KMeans_Model ← Fit_KMeans(Reduced_Features, k = 4) Clustered_Image ← KMeans_Model.Labels Step 4: Lesion Classification using CNN CNN_Model ← Load_Trained_Model(‘cnn_dti_v2.h5’) Diagnosis_Label ← CNN_Model.Predict(Clustered_Image) return Diagnosis_Label |
3.8. Performance Evaluation Metrics
- —True Positives.
- —False Positives.
- —True Negatives.
- —False Negatives.
3.9. System Robustness Evaluation
- Fault tolerance: Measured system availability during individual node failures (achieved achieved 99.2% uptime).
- Network latency impact: Evaluated performance degradation under varying network conditions (10 ms to 500 ms latency between nodes).
- Load distribution: Assessed Docker Swarm’s load balancing efficiency under concurrent requests (up to 100 simultaneous DTI processing requests).
- Recovery time: Measured mean time to recovery (MTTR) after simulated failures (average 3.4 s).
4. Case Study and Evaluation
- Latency and throughput under concurrent inference loads.
- F1 score and precision of CNN classifications.
- User satisfaction from simulated clinical sessions.
4.1. Practical Integration with Romanian eHealth Infrastructure
4.2. Comparative Analysis with State-of-the-Art Approaches
5. Conclusions and Future Work
5.1. Research Summary
5.2. Research Value and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ileana, M.; Oproiu, M.I.; Marian, C.V. Using Docker Swarm to Improve Performance in Distributed Web Systems. In Proceedings of the 2024 International Conference on Development and Application Systems (DAS), Suceava, Romania, 22–24 May 2024; IEEE: New York, NY, USA; pp. 1–6. [Google Scholar]
- Ileana, M.; Petrov, P.; Milev, V. Optimizing Customer Experience by Exploiting Real-Time Data Generated by IoT and Leveraging Distributed Web Systems in CRM Systems. IoT 2025, 6, 24. [Google Scholar] [CrossRef]
- Ileana, M.; Petrov, P.; Milev, V. Optimizing CRM Platforms with Distributed Cloud Architectures for Scalable Performance and Security. In Proceedings of the 8th International Symposium on Innovative Approaches in Smart Technologies (ISAS), İstanbul, Turkiye, 6–7 December 2024; pp. 10–15. [Google Scholar]
- Ileana, M.; Petrov, P.; Milev, V. Improving CRM Functionality in Medical Systems through Blockchain and Distributed Web Systems. In Proceedings of the ISAS Symposium 2025, Ho Chi Minh, Vietnam, 17–19 October 2025. [Google Scholar]
- Sfat, R.; Marin, I.; Goga, N.; Popa, R.; Darla, I.C.; Marian, C.V. Conceptualization of an Intelligent HL7 Application Based on Questionnaire Generation and Editing. In Proceedings of the 2021 IEEE International Black Sea Conference on Communications and Networking (BlackSeaCom), Bucharest, Romania, 24–28 May 2021; IEEE: New York, NY, USA, 2021; pp. 1–4. [Google Scholar]
- Sfat, R.; Marian, C.V. Medical Systems Open Data Exchange Interconnection and Web Questionnaires Based on the HL7 FHIR Standards. In Proceedings of the 2022 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 17–18 November 2022; IEEE: New York, NY, USA, 2022; pp. 1–4. [Google Scholar]
- Cacovean, D.; Ileana, M. Advanced Neurological Imaging Analysis Using Diffusion Tensor Techniques and Distributed Web Systems. Rev. Roum. Sci. Techn.-Sér. Électrotech. Énerg. 2025, 70, 269–274. [Google Scholar] [CrossRef]
- Ileana, M. Elevating Medical Efficiency and Personalized Care Through the Integration of Artificial Intelligence and Distributed Web Systems. In Proceedings of the International Conference on Intelligent Tutoring Systems, Thessaloniki, Greece, 10–13 June 2024; Springer Nature: Cham, Switzerland, 2024; pp. 3–11. [Google Scholar]
- Stanciu, S.G.; Boriga, R.; Dascalescu, A.C.; Hristu, R.; Stanciu, G.A. Bag-of-Features Approaches for Combined Classification of Laser Scanning Microscopy and Spectroscopy Data Sets. In Proceedings of the 2016 International Conference on Laser Optics (LO), St. Petersburg, Russia, 27 June–1 July 2016; IEEE: New York, NY, USA, 2016; p. S2-13. [Google Scholar]
- Baashar, Y.; Alhussian, H.; Patel, A.; Alkawsi, G.; Alzahrani, A.I.; Alfarraj, O.; Hayder, G. Customer Relationship Management Systems (CRMS) in the Healthcare Environment: A Systematic Literature Review. Comput. Stand. Interfaces 2020, 71, 103442. [Google Scholar] [CrossRef]
- Gruendner, J.; Schwachhofer, T.; Sippl, P.; Wolf, N.; Erpenbeck, M.; Gulden, C.; Toddenroth, D. KETOS: Clinical Decision Support and Machine Learning as a Service-A Training and Deployment Platform Based on Docker, OMOP-CDM, and FHIR Web Services. PLoS ONE 2019, 14, e0223010. [Google Scholar]
- Gulden, C.; Macho, P.; Reinecke, I.; Strantz, C.; Prokosch, H.U.; Blasini, R. RecruIT: A Cloud-Native Clinical Trial Recruitment Support System Based on Health Level 7 Fast Healthcare Interoperability Resources (HL7 FHIR) and the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM). Comput. Biol. Med. 2024, 174, 108411. [Google Scholar] [CrossRef]
- Cezar, D.; Gheorghe, G.; Gabriel, P.; Mariuca-Roxana, G. Device for Securing IoT in the Wireless Environment. In Proceedings of the 2024 16th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Iasi, Romania, 27–28 June 2024; IEEE: New York, NY, USA, 2024; pp. 1–6. [Google Scholar]
- Kin, D.T.; Taskin, C.; Yazar, S.; Colak, A. Real-Time Low Energy Indoor Positioning System to Efficient Use of Operating Theaters with Medical Asset and Staff Tracking. Sci. Technol. 2024, 27, 151–165. [Google Scholar]
- Ashari, R.; Suakanto, S.; Hamami, F.; Raffei, A.F.M.; Nuryatno, E. Development of an Application for Visualizing Medical Asset Distribution to Enhance Hospital Asset Management Efficiency. In Proceedings of the 2024 7th International Conference of Computer and Informatics Engineering (IC2IE), Bali, Indonesia, 12–13 September 2024; IEEE: New York, NY, USA, 2024; pp. 1–6. [Google Scholar]
- Sadeghi, M.; Mahmoudi, A. Synergy between Blockchain Technology and Internet of Medical Things in Healthcare: A Way to Sustainable Society. Inf. Sci. 2024, 660, 120049. [Google Scholar] [CrossRef]
- Dinu, A.; Gheorghe, S.; Danciu, G.M.; Ogrutan, P.L. Debugging FPGA Projects Using Artificial Intelligence. Sci. Technol. 2021, 24, 299–320. [Google Scholar]
- Qiu, C.; Gong, S.; Gao, W. Three Artificial Intelligence-Based Solutions Predicting Concrete Slump. UPB. Sci. Bull. Series C 2019, 81, 3–14. [Google Scholar]
- Cristescu, M.C. Machine Learning Techniques for Improving the Performance Metrics of Functional Verification. Sci. Technol. 2021, 24, 99–116. [Google Scholar]
- Marin, I.; Pavaloiu, B.I.; Marian, C.V.; Racovita, V.; Goga, N. Early Detection of Preeclampsia Based on a Machine Learning Approach. In Proceedings of the 2019 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2019; IEEE: New York, NY, USA, 2019; pp. 1–4. [Google Scholar]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; Sánchez, C.I. A Survey on Deep Learning in Medical Image Analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mora-Cantallops, M.; García-Barriocanal, E.; Sicilia, M.Á. Trustworthy AI Guidelines in Biomedical Decision-Making Applications: A Scoping Review. Big Data Cogn. Comput. 2024, 8, 73. [Google Scholar] [CrossRef]
- Shakor, M.Y.; Khaleel, M.I. Recent Advances in Big Medical Image Data Analysis Through Deep Learning and Cloud Computing. Electronics 2024, 13, 4860. [Google Scholar] [CrossRef]
- Guedes, J.; Duarte, J.; Guimarães, T.; Santos, M.F. Revisioning Healthcare Interoperability System for ABI Architectures: Introspection and Improvements. Information 2024, 15, 569. [Google Scholar] [CrossRef]
- Miotto, R.; Wang, F.; Wang, S.; Jiang, X.; Dudley, J.T. Deep Learning for Healthcare: Review, Opportunities and Challenges. Brief. Bioinform. 2018, 19, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Unanah, O.V.; Mbanugo, O.J. Integration of AI into CRM for Effective US Healthcare and Pharmaceutical Marketing. World J. Adv. Res. Rev. 2025, 25, 609–630. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sharma, V.; Kumar, D. Customer Relationship Management in Healthcare: Strategies for Adoption in a Public Health System. J. Mark. Theory Pract. 2024, 1–26. [Google Scholar] [CrossRef]
- Veeramachaneni, V. Edge Computing: Architecture, Applications, and Future Challenges in a Decentralized Era. Recent Trends Comput. Graph. Multimed. Technol. 2025, 7, 8–23. [Google Scholar]
- Khemachi, A.; Mendas, F.Z. Parallel and Distributed System for Medical Data Processing. Doctoral Dissertation, Ibn Khaldoun University, Tiaret, Algeria, 2024. [Google Scholar]
- Lina, Y.; Wenlong, S. Efficient Processing of Large-Scale Medical Data in IoT: A Hybrid Hadoop–Spark Approach for Health Status Prediction. Int. J. Adv. Comput. Sci. Appl. 2024, 15, 74. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Zhang, K.; Fung, K.M.; Thai, T.C.; Moore, K.; Qiu, Y. Recent Advances and Clinical Applications of Deep Learning in Medical Image Analysis. Med. Image Anal. 2022, 79, 102444. [Google Scholar] [CrossRef]
- Dhar, T.; Dey, N.; Borra, S.; Sherratt, R.S. Challenges of Deep Learning in Medical Image Analysis-Improving Explainability and Trust. IEEE Trans. Technol. Soc. 2023, 4, 68–75. [Google Scholar] [CrossRef]
- Liu, X.; Gao, K.; Liu, B.; Pan, C.; Liang, K.; Yan, L.; Yu, Y. Advances in Deep Learning-Based Medical Image Analysis. Health Data Sci. 2021, 2021, 8786793. [Google Scholar] [CrossRef] [PubMed]
- Zarour, M.; Ansari, M.T.J.; Alenezi, M.; Sarkar, A.K.; Faizan, M.; Agrawal, A.; Khan, R.A. Evaluating the Impact of Blockchain Models for Secure and Trustworthy Electronic Healthcare Records. IEEE Access 2020, 8, 157959–157973. [Google Scholar] [CrossRef]
- Haddad, A.; Habaebi, M.H.; Islam, M.R.; Hasbullah, N.F.; Zabidi, S.A. Systematic Review on AI-Blockchain Based E-Healthcare Records Management Systems. IEEE Access 2022, 10, 94583–94615. [Google Scholar] [CrossRef]
- Rathore, N.; Kumari, A.; Patel, M.; Chudasama, A.; Bhalani, D.; Tanwar, S.; Alabdulatif, A. Synergy of AI and Blockchain to Secure Electronic Healthcare Records. Secur. Priv. 2025, 8, e463. [Google Scholar] [CrossRef]
- Erickson, S.M.; Rockwern, B.; Koltov, M.; McLean, R.M. Putting Patients First by Reducing Administrative Tasks in Health Care: A Position Paper of the American College of Physicians. Ann. Intern. Med. 2017, 166, 659–661. [Google Scholar] [CrossRef]
- Karoui, H.; Rwandarwacu, V.P.; Niyonzima, J.; Makuza, A.; Nkuranga, J.B.; D’Acremont, V.; Kulinkina, A.V. Identifying Clinical Skill Gaps of Healthcare Workers Using a Digital Clinical Decision Support Algorithm During Outpatient Pediatric Consultations in Primary Health Centers in Rwanda. PLoS ONE 2025, 20, e0318284. [Google Scholar] [CrossRef]
- Chowhan, S.S.; Bagrecha, M.S.; Sharma, S.K.; Issac, A.S.; Bennadi, D.; Satodiya, V.; Tiwari, R. Bridging the Healthcare Skill Gap: A Higher Education Perspective-A Data Base Research. J. Pharm. Bioallied Sci. 2024, 16 (Suppl. 3), S2356–S2359. [Google Scholar] [CrossRef]
- Rancea, A.; Anghel, I.; Cioara, T. Edge Computing in Healthcare: Innovations, Opportunities, and Challenges. Future Internet 2024, 16, 329. [Google Scholar] [CrossRef]
- Awasthi, R.; Ramachandran, S.P.; Mishra, S.; Mahapatra, D.; Arshad, H.; Atreja, A.; Mathur, P. Artificial Intelligence in Healthcare: 2024 Year in Review. medRxiv 2025, 2025-02. [Google Scholar] [CrossRef]
- Stoumpos, A.I.; Kitsios, F.; Talias, M.A. Digital Transformation in Healthcare: Technology Acceptance and Its Applications. Int. J. Environ. Res. Public Health 2023, 20, 3407. [Google Scholar] [CrossRef] [PubMed]
- Dal Mas, F.; Massaro, M.; Rippa, P.; Secundo, G. The Challenges of Digital Transformation in Healthcare: An Interdisciplinary Literature Review, Framework, and Future Research Agenda. Technovation 2023, 123, 102716. [Google Scholar] [CrossRef]
- Obaidat, M.A.; Rawashdeh, M.; Alja’afreh, M.; Abouali, M.; Thakur, K.; Karime, A. Exploring IoT and Blockchain: A Comprehensive Survey on Security, Integration Strategies, Applications and Future Research Directions. Big Data Cogn. Comput. 2024, 8, 174. [Google Scholar] [CrossRef]
- Popescu, D.A. An Enhanced Genetic Algorithm for Optimized Educational Assessment Test Generation Through Population Variation. Big Data Cogn. Comput. 2025, 9, 98. [Google Scholar] [CrossRef]
- Pan, S.J.; Yang, Q. A Survey on Transfer Learning. IEEE Trans. Knowl. Data Eng. 2010, 22, 1345–1359. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ileana, M.; Petrov, P.; Milev, V. AI-Enabled Secure and Scalable Distributed Web Architecture for Medical Informatics. Appl. Sci. 2025, 15, 10710. https://doi.org/10.3390/app151910710
Ileana M, Petrov P, Milev V. AI-Enabled Secure and Scalable Distributed Web Architecture for Medical Informatics. Applied Sciences. 2025; 15(19):10710. https://doi.org/10.3390/app151910710
Chicago/Turabian StyleIleana, Marian, Pavel Petrov, and Vassil Milev. 2025. "AI-Enabled Secure and Scalable Distributed Web Architecture for Medical Informatics" Applied Sciences 15, no. 19: 10710. https://doi.org/10.3390/app151910710
APA StyleIleana, M., Petrov, P., & Milev, V. (2025). AI-Enabled Secure and Scalable Distributed Web Architecture for Medical Informatics. Applied Sciences, 15(19), 10710. https://doi.org/10.3390/app151910710








