Biocompatibility of Biomedical Materials: Reliability of Cell Viability Tests in the Context of Retinal Prostheses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. MTT and LDH Tests
2.3. Fluorescent Staining
2.4. Live and Dead Staining
2.5. Statistical Analysis
3. Results
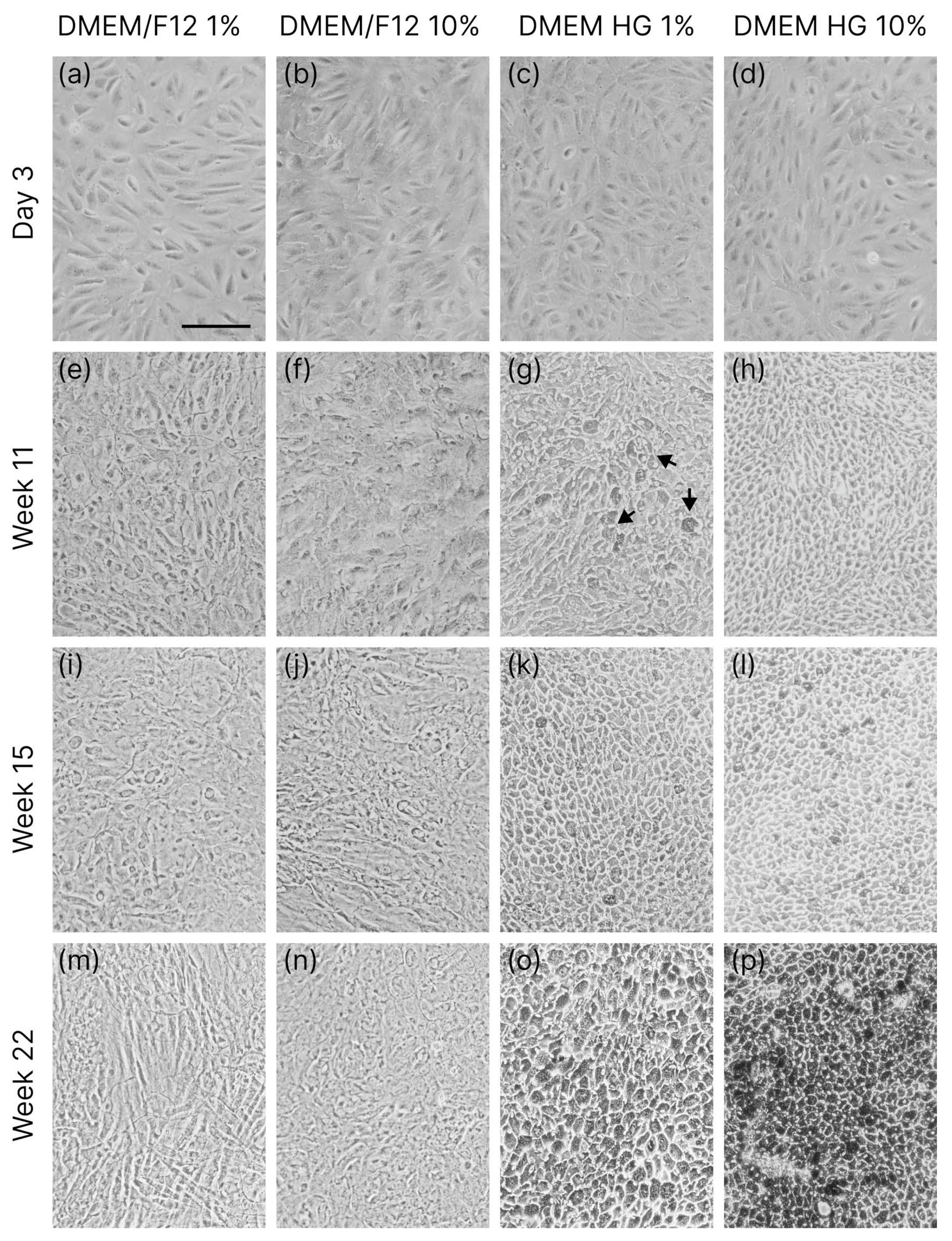
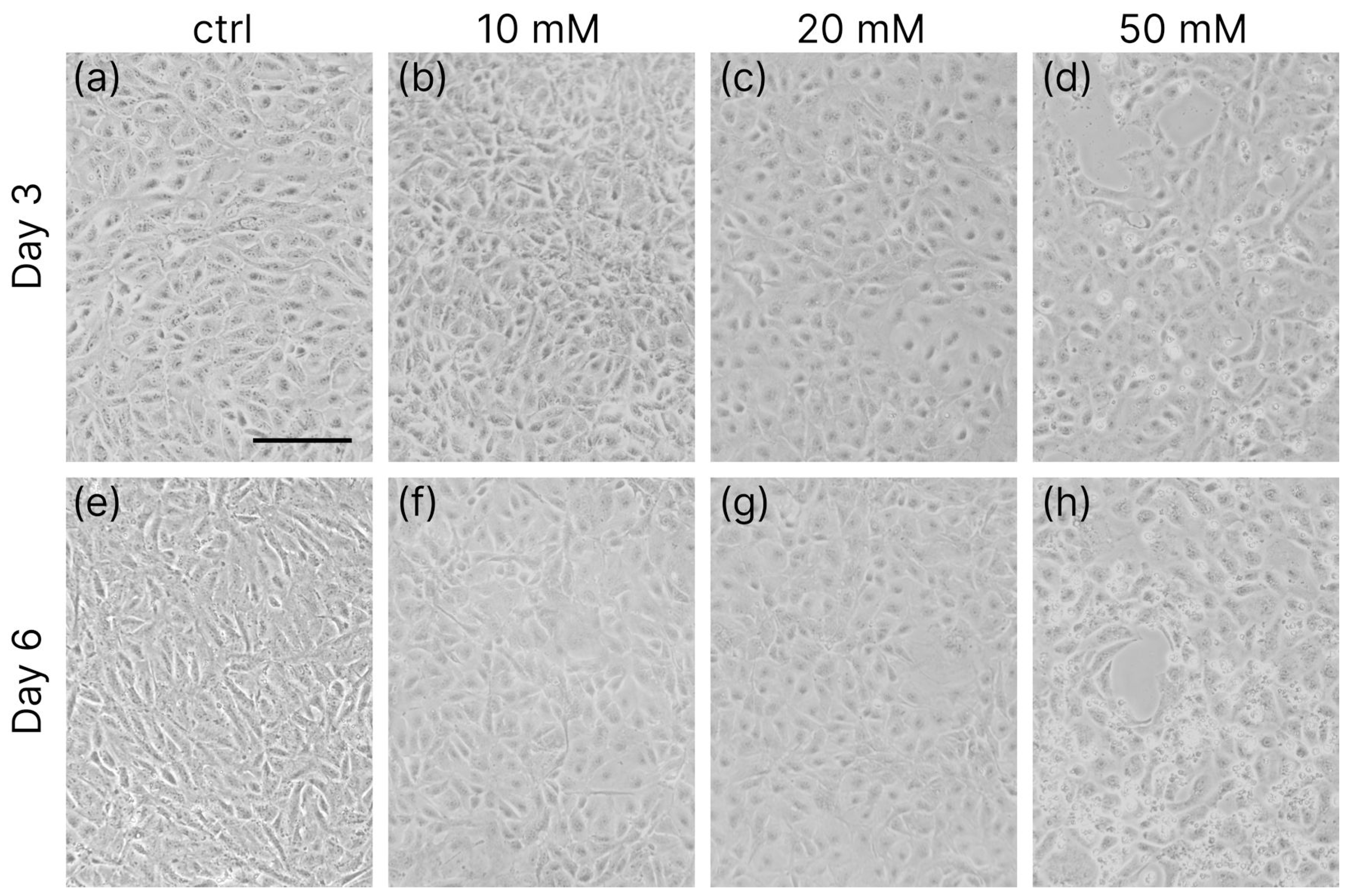
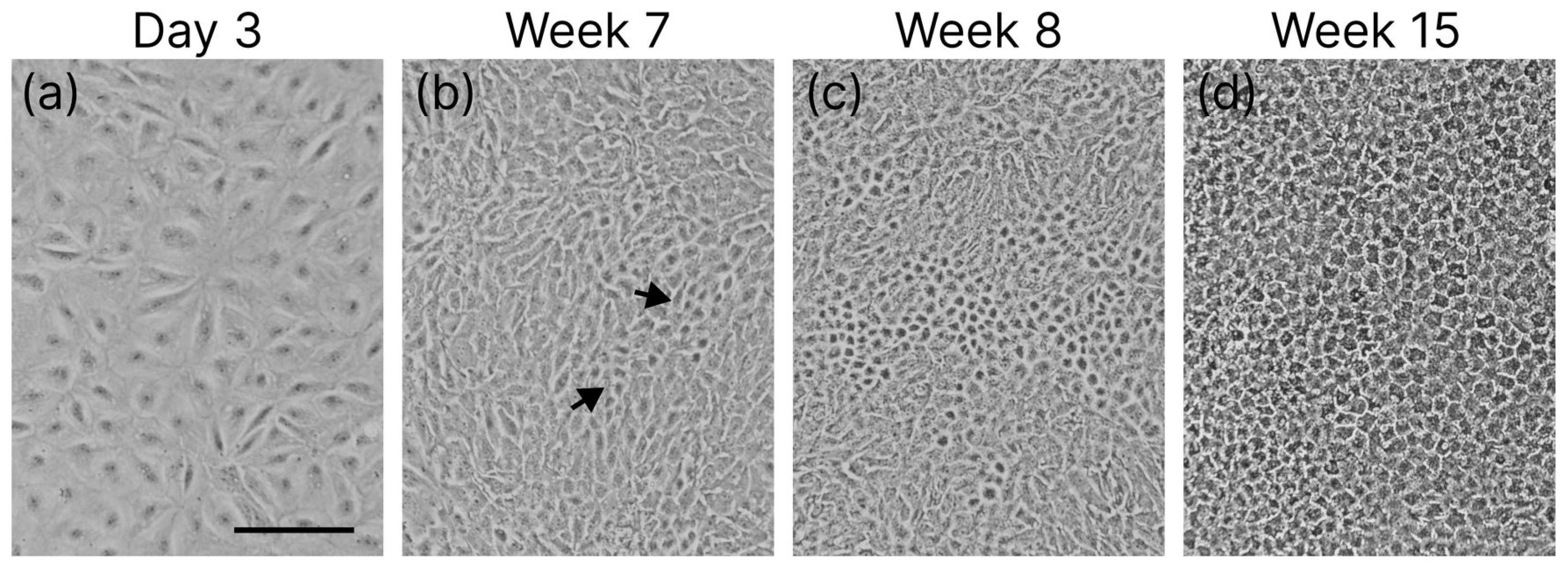
3.1. Cell Differentiation
3.2. Enzymatic Viability Tests
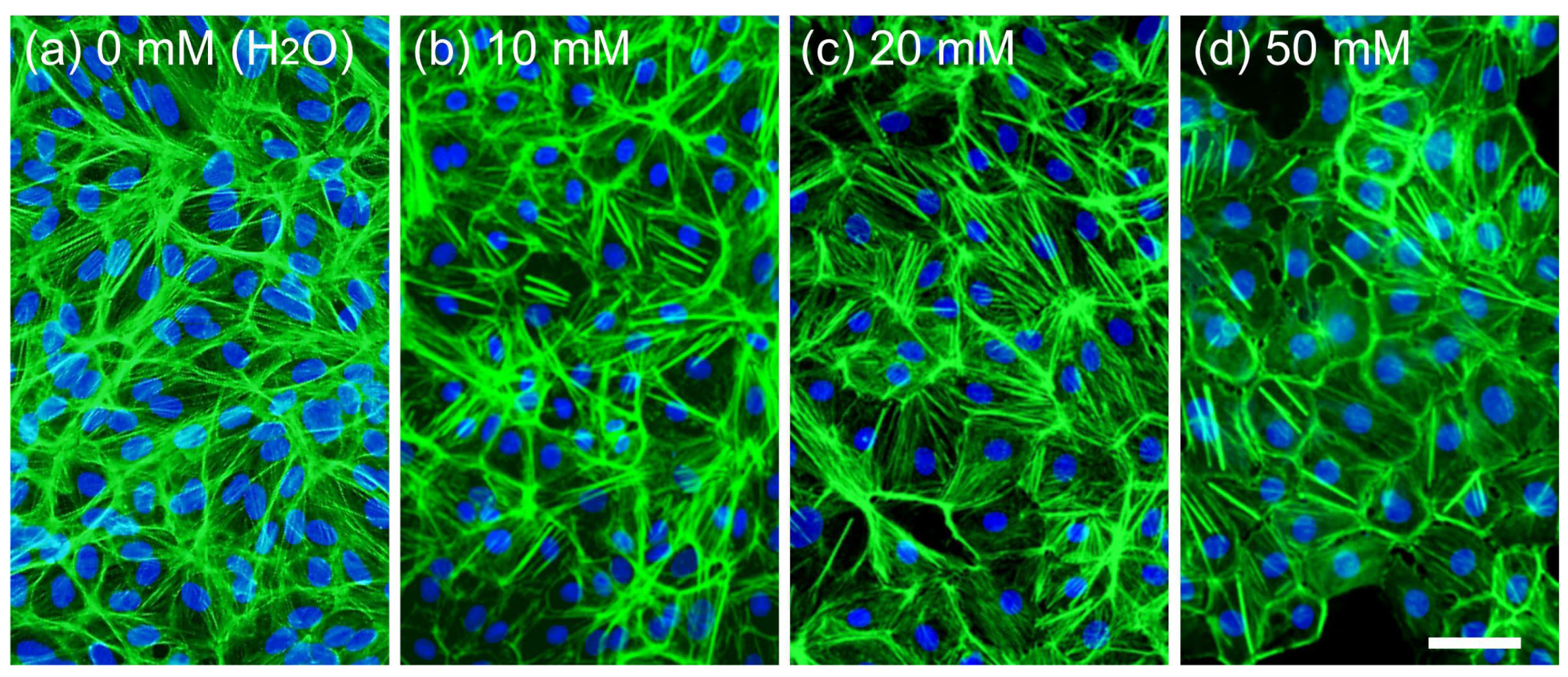
3.3. Live and Dead Cell Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide |
| LDH | Lactate dehydrogenase |
| RPE | Retinal pigment epithelium |
| NAD+/NADH | Nicotinamide adenine dinucleotide |
| DMEM | Dulbecco′s Modified Eagle′s Medium |
| FBS | Fetal Bovine Serum |
| DPBS | Dulbecco’s Phosphate Buffered Saline |
| HG | High glucose |
References
- Sousa, A.; Neves, S.C.; Gonçalves, I.C.; Barrias, C.C. 12—In Vitro Interaction of Polymeric Biomaterials with Cells; Tanzi, M.C., Farè, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 285–315. ISBN 978-0-08-100737-2. [Google Scholar]
- Huzum, B.; Puha, B.; Necoara, R.; Gheorghevici, S.; Puha, G.; Filip, A.; Sirbu, P.; Alexa, O. Biocompatibility assessment of biomaterials used in orthopedic devices: An overview (Review). Exp. Ther. Med. 2021, 22, 1315. [Google Scholar] [CrossRef]
- Barmshuri, M.; Kholdebarin, B.; Sadeghi, S. Applications of comet and MTT assays in studying Dunaliella algae species. Algal Res. 2023, 70, 103018. [Google Scholar] [CrossRef]
- Abel, S.D.A.; Baird, S.K. Honey is cytotoxic towards prostate cancer cells but interacts with the MTT reagent: Considerations for the choice of cell viability assay. Food Chem. 2018, 241, 70–78. [Google Scholar] [CrossRef]
- de la Fuente-Jiménez, J.L.; Rodríguez-Rivas, C.I.; Mitre-Aguilar, I.B.; Torres-Copado, A.; García-López, E.A.; Herrera-Celis, J.; Arvizu-Espinosa, M.G.; Garza-Navarro, M.A.; Arriaga, L.G.; García, J.L.; et al. A Comparative and Critical Analysis for In Vitro Cytotoxic Evaluation of Magneto-Crystalline Zinc Ferrite Nanoparticles Using MTT, Crystal Violet, LDH, and Apoptosis Assay. Int. J. Mol. Sci. 2023, 24, 12860. [Google Scholar] [CrossRef] [PubMed]
- Bopp, S.K.; Lettieri, T. Comparison of four different colorimetric and fluorometric cytotoxicity assays in a zebrafish liver cell line. BMC Pharmacol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Edmondson, J.M.; Armstrong, L.S.; Martinez, A.O. A rapid and simple MTT-based spectrophotometric assay for determining drug sensitivity in monolayer cultures. J. Tissue Cult. Methods 1988, 11, 15–17. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef]
- Smith, S.M.; Wunder, M.B.; Norris, D.A.; Shellman, Y.G. A simple protocol for using a LDH-Based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef]
- Lobner, D. Comparison of the LDH and MTT assays for quantifying cell death: Validity for neuronal apoptosis? J. Neurosci. Methods 2000, 96, 147–152. [Google Scholar] [CrossRef]
- Kendig, D.M.; Tarloff, J.B. Inactivation of lactate dehydrogenase by several chemicals: Implications for in vitro toxicology studies. Toxicol. Vitr. 2007, 21, 125–132. [Google Scholar] [CrossRef]
- Singhal, M.; Shaha, S.; Katsikogianni, M. Comparative Analysis of Cytotoxicity Assays, from Traditional to Modern Approaches. In Cytotoxicity—A Crucial Toxicity Test for In Vitro Experiments; Erkekoğlu, P., Ed.; IntechOpen: London, UK, 2024; ISBN 978-1-83634-031-7. [Google Scholar]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2017; ISBN 978-1-78923-419-0. [Google Scholar]
- Van den Bossche, S.; Vandeplassche, E.; Ostyn, L.; Coenye, T.; Crabbé, A. Bacterial Interference With Lactate Dehydrogenase Assay Leads to an Underestimation of Cytotoxicity. Front. Cell. Infect. Microbiol. 2020, 10, 494. [Google Scholar] [CrossRef] [PubMed]
- Parhamifar, L.; Andersen, H.; Moghimi, S.M. Lactate dehydrogenase assay for assessment of polycation cytotoxicity. Methods Mol. Biol. 2019, 1943, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Khalef, L.; Lydia, R.; Filicia, K.; Moussa, B. Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments. Cell Biochem. Funct. 2024, 42, e4007. [Google Scholar] [CrossRef] [PubMed]
- Madorran, E.; Ambrož, M.; Knez, J.; Sobočan, M. An Overview of the Current State of Cell Viability Assessment Methods Using OECD Classification. Int. J. Mol. Sci. 2025, 26, 220. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, W.; Guo, J.; Zhu, Q.; Chen, H.; Xia, Y.; Zhu, G. Mitochondrion: A sensitive target for Pb exposure. J. Toxicol. Sci. 2021, 46, 345–358. [Google Scholar] [CrossRef]
- Friedlander, M. Fibrosis and diseases of the eye. J. Clin. Investig. 2007, 117, 576–586. [Google Scholar] [CrossRef]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The role of inflammation in age-related macular degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Tenbrock, L.; Wolf, J.; Boneva, S.; Schlecht, A.; Agostini, H.; Wieghofer, P.; Schlunck, G.; Lange, C. Subretinal fibrosis in neovascular age-related macular degeneration: Current concepts, therapeutic avenues, and future perspectives. Cell Tissue Res. 2022, 387, 361–375. [Google Scholar] [CrossRef]
- Higashijima, F.; Hasegawa, M.; Yoshimoto, T.; Kobayashi, Y.; Wakuta, M.; Kimura, K. Molecular mechanisms of TGFβ-mediated EMT of retinal pigment epithelium in subretinal fi brosis of age-related macular degeneration. Front. Ophthalmol. 2023, 2, 1060087. [Google Scholar] [CrossRef]
- Bellapianta, A.; Cetkovic, A.; Bolz, M.; Salti, A. Retinal Organoids and Retinal Prostheses: An Overview. Int. J. Mol. Sci. 2022, 23, 2922. [Google Scholar] [CrossRef]
- Singh, M.S.; Issa, P.C.; Butler, R.; Martin, C.; Lipinski, D.M.; Sekaran, S.; Barnard, A.R.; MacLaren, R.E. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. USA 2013, 110, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Che, J.H.; Seo, J.M.; Jeong, J.; Kim, E.T.; Lee, S.W.; Koo, K.I.; Suaning, G.J.; Lovell, N.H.; Dan Cho, D., II; et al. In vitro biocompatibility of various polymer-based microelectrode arrays for retinal prosthesis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Hazim, R.A.; Paniagua, A.E.; Tang, L.; Yang, K.; Kim, K.K.O.; Stiles, L.; Divakaruni, A.S.; Williams, D.S. Vitamin B3, nicotinamide, enhances mitochondrial metabolism to promote differentiation of the retinal pigment epithelium. J. Biol. Chem. 2022, 298, 102286. [Google Scholar] [CrossRef] [PubMed]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S.; Angeles, L.; Angeles, L. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar]
- Ahmado, A.; Carr, A.J.; Vugler, A.A.; Semo, M.; Gias, C.; Lawrence, J.M.; Chen, L.L.; Chen, F.K.; Turowski, P.; da Cruz, L.; et al. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7148–7159. [Google Scholar] [CrossRef]
- D’Antonio-Chronowska, A.; D’Antonio, M.; Frazer, K.A. In vitro Differentiation of Human iPSC-derived Retinal Pigment Epithelium Cells (iPSC-RPE). Bio Protoc. 2019, 9, e3469. [Google Scholar] [CrossRef]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed Differentiation of Human Embryonic Stem Cells into Functional Retinal Pigment Epithelium Cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef]
- Hazim, R.A. Enhanced mitochondrial metabolism promotes differentiation of the retinal pigment epithelium following treatment with nicotinamide. Investig. Ophthalmol. Vis. Sci. 2023, 64, 953. [Google Scholar]
- Makarov, M.V.; Trammell, S.A.J.; Migaud, M.E. The chemistry of the vitamin B3 metabolome. Biochem. Soc. Trans. 2018, 47, 131–147. [Google Scholar] [CrossRef]
- Yusri, K.; Jose, S.; Vermeulen, K.S.; Tan, T.C.M.; Sorrentino, V. The role of NAD+ metabolism and its modulation of mitochondria in aging and disease. NPJ Metab. Health Dis. 2025, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, H.; Luo, L.; Wu, W. Nicotinamide Adenine Dinucleotide Supplementation to Alleviate Heart Failure: A Mitochondrial Dysfunction Perspective. Nutrients 2025, 17, 1855. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Rizwan, S.; Toothman, B.; Li, B.; Engel, A.J.; Lim, R.R.; Niernberger, S.; Lu, J.; Ratliff, C.; Xiang, Y.; Eminhizer, M.; et al. Metabolic Phenotyping of Healthy and Diseased Human RPE Cells. Investig. Ophthalmol. Vis. Sci. 2024, 65, 5. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic Basis and Clinical Evidence for the Applications of Nicotinamide (Niacinamide) to Control Skin Aging and Pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, E.N. Self-Organization of the Retina during Eye Development, Retinal Regeneration In Vivo, and in Retinal 3D Organoids In Vitro. Biomedicines 2022, 10, 1458. [Google Scholar] [CrossRef]
- Villarroel, M.; García-Ramírez, M.; Corraliza, L.; Hernández, C.; Simó, R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp. Eye Res. 2009, 89, 913–920. [Google Scholar] [CrossRef]
- Luo, Y.; Zhuo, Y.; Fukuhara, M.; Rizzolo, L.J. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3644–3655. [Google Scholar] [CrossRef]
- Oka, M.S.; Landers, R.A.; Bridges, C.D.B. A serum-free defined medium for retinal pigment epithelial cells. Exp. Cell Res. 1984, 154, 537–547. [Google Scholar] [CrossRef]
- Kumar, P.; Satyam, A.; Fan, X.; Collin, E.; Rochev, Y.; Rodriguez, B.J.; Gorelov, A.; Dillon, S.; Joshi, L.; Raghunath, M.; et al. Macromolecularly crowded in vitro microenvironments accelerate the production of extracellular matrix-rich supramolecular assemblies. Sci. Rep. 2015, 5, 8729. [Google Scholar] [CrossRef]
- Torres, Y.; Gluais, M.; Da Silva, N.; Rey, S.; Grémare, A.; Magnan, L.; Kawecki, F.; L’Heureux, N. Cell-assembled extracellular matrix (CAM) sheet production: Translation from using human to large animal cells. J. Tissue Eng. 2021, 12, 2041731420978327. [Google Scholar] [CrossRef]
- Cieślik, A.; Shymborska, Y.; Tymetska, S.; Stetsyshyn, Y.; Bernasik, A.; Brzychczy-Włoch, M.; Drożdż, K.; Szajna, K.; Krok, F.; Budkowski, A.; et al. Cell sheet engineering platforms integrating antibacterial and thermo-responsive functionalities: Cu-nanoparticle-loaded P4VP brushes for retinal cell sheet harvesting. Chem. Eng. J. 2025, 513, 162985. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000; ISBN 0-87893-106-6. [Google Scholar]
- Ho, A.; Dowdy, S.F. Regulation of G1 cell-cycle progression by oncogenes and tumor suppressor genes. Curr. Opin. Genet. Dev. 2002, 12, 47–52. [Google Scholar] [CrossRef]
- Michelet, F.; Balasankar, A.; Teo, N.; Stanton, L.W.; Singhal, S. Rapid generation of purified human RPE from pluripotent stem cells using 2D cultures and lipoprotein uptake-based sorting. Stem Cell Res. Ther. 2020, 11, 47. [Google Scholar] [CrossRef]
- Liu, H.; Huang, S.S.; Lingam, G.; Kai, D.; Su, X.; Liu, Z. Advances in retinal pigment epithelial cell transplantation for retinal degenerative diseases. Stem Cell Res. Ther. 2024, 15, 390. [Google Scholar] [CrossRef]
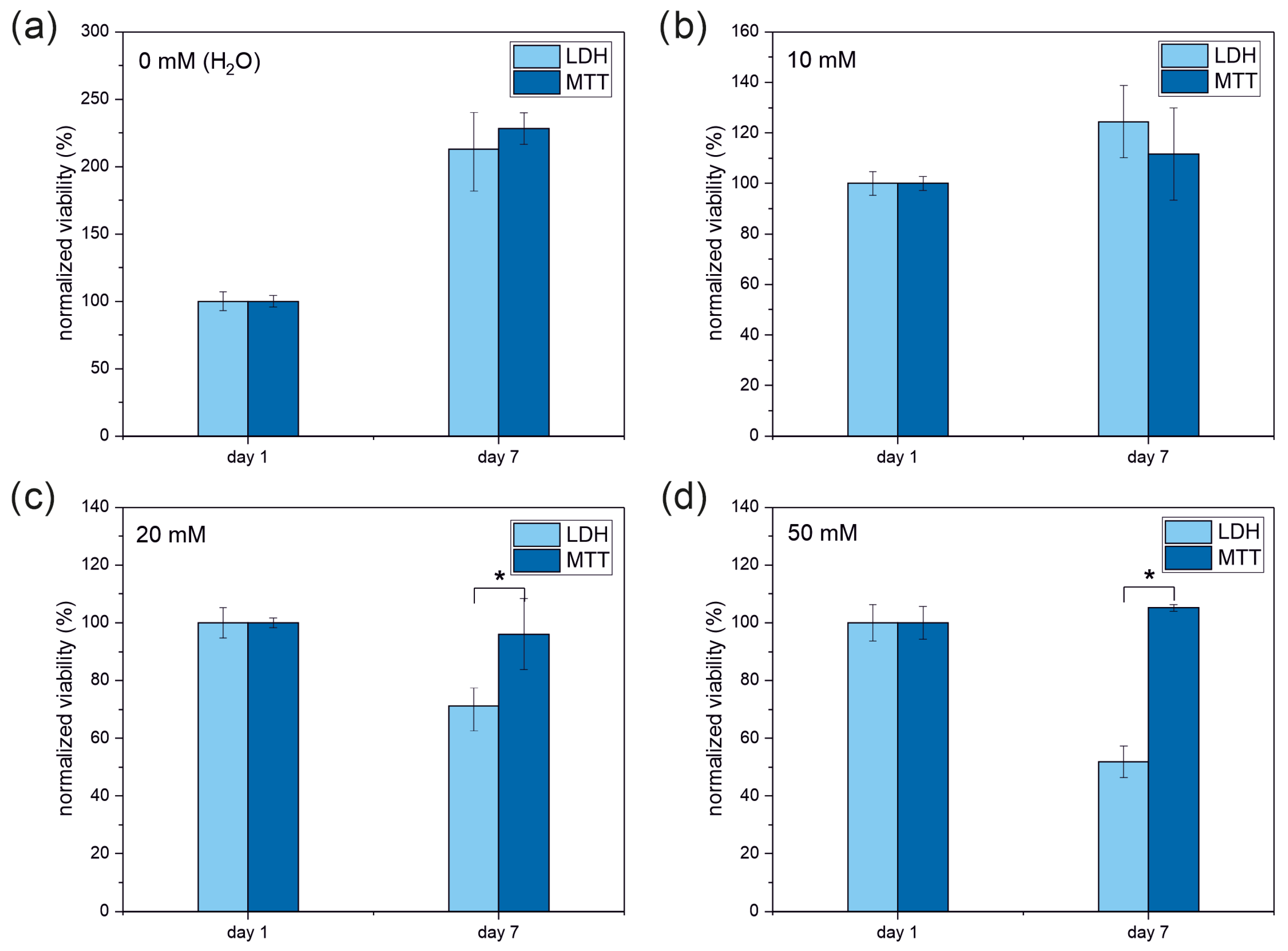
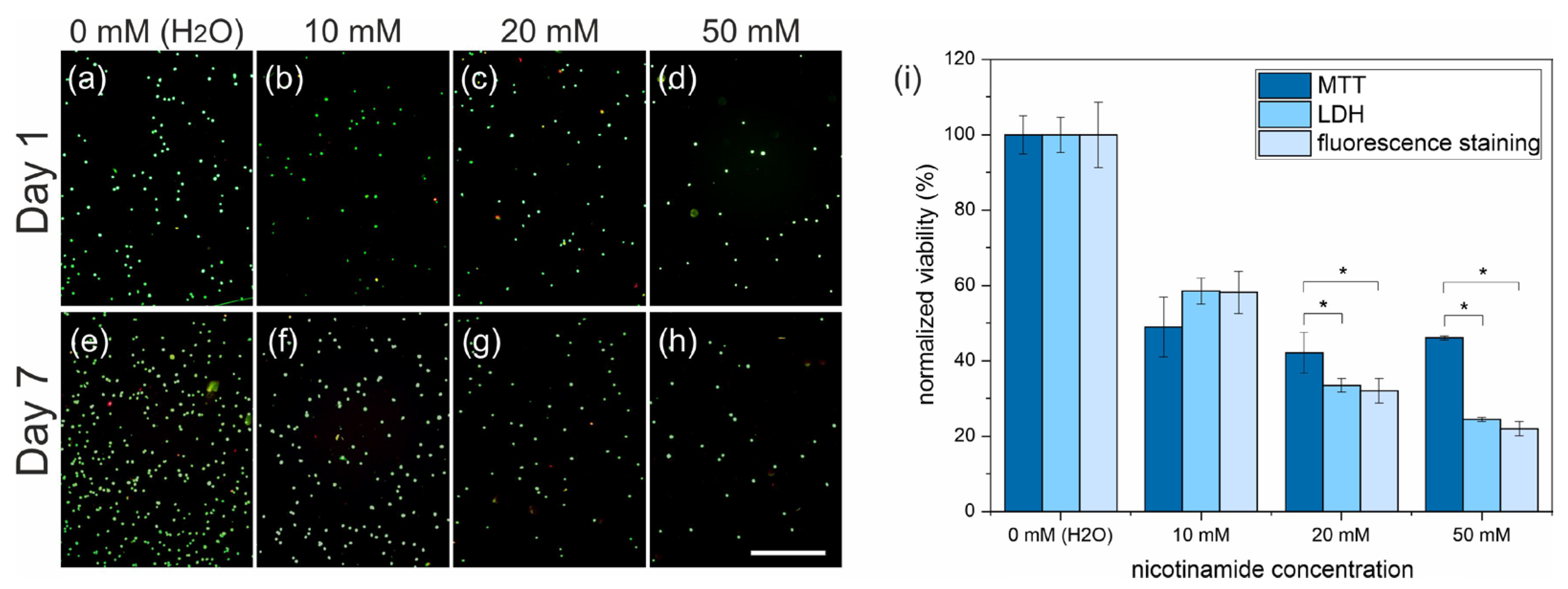
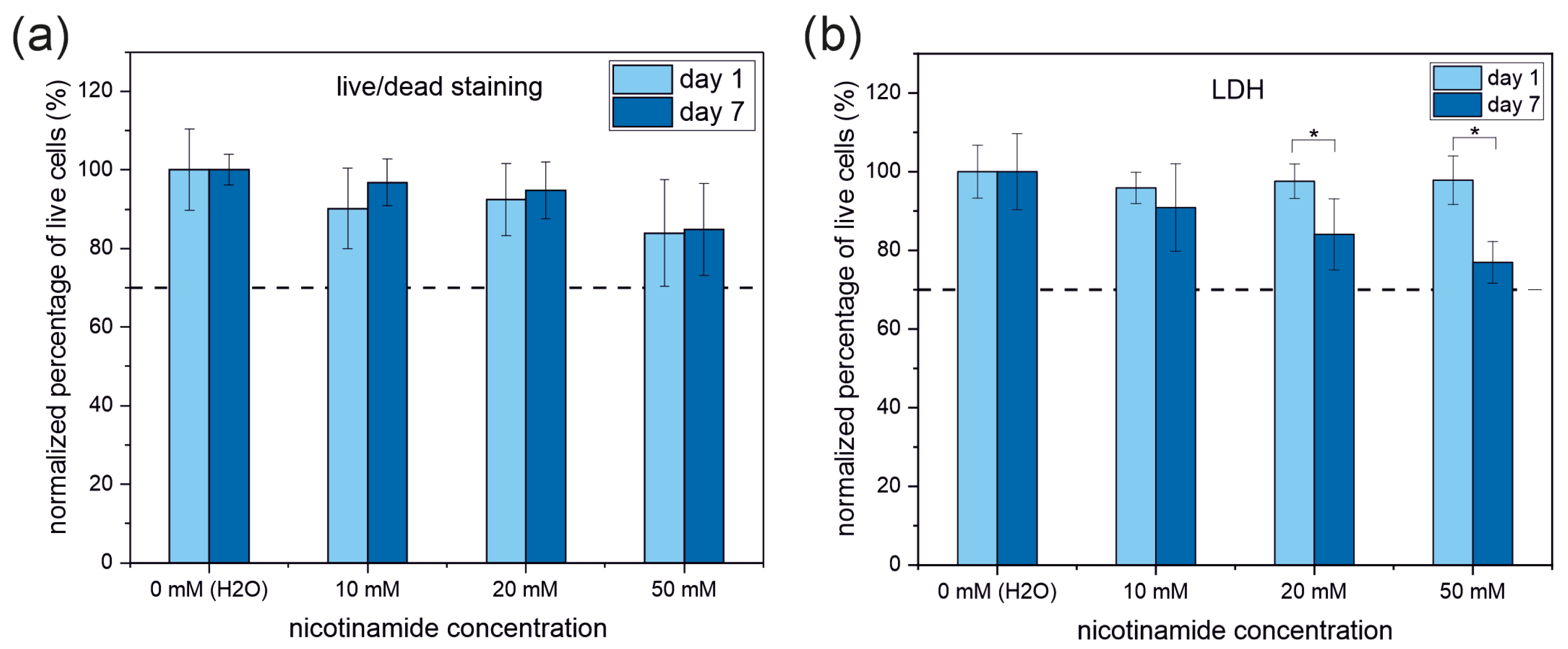
| MTT | LDH | Live/Dead | |
|---|---|---|---|
| 10 mM | 49 ± 7 | 58 ± 3 | 58 ± 5 |
| 20 mM | 42 ± 4 (*) | 33 ± 1 | 31 ± 3 |
| 50 mM | 46 ± 1 (*) | 24 ± 1 | 21 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieślik, A.; Raczkowska, J. Biocompatibility of Biomedical Materials: Reliability of Cell Viability Tests in the Context of Retinal Prostheses. Appl. Sci. 2025, 15, 10684. https://doi.org/10.3390/app151910684
Cieślik A, Raczkowska J. Biocompatibility of Biomedical Materials: Reliability of Cell Viability Tests in the Context of Retinal Prostheses. Applied Sciences. 2025; 15(19):10684. https://doi.org/10.3390/app151910684
Chicago/Turabian StyleCieślik, Anna, and Joanna Raczkowska. 2025. "Biocompatibility of Biomedical Materials: Reliability of Cell Viability Tests in the Context of Retinal Prostheses" Applied Sciences 15, no. 19: 10684. https://doi.org/10.3390/app151910684
APA StyleCieślik, A., & Raczkowska, J. (2025). Biocompatibility of Biomedical Materials: Reliability of Cell Viability Tests in the Context of Retinal Prostheses. Applied Sciences, 15(19), 10684. https://doi.org/10.3390/app151910684






