Phytochemical Profile and Biological Activities of Biscutella laevigata: A Comparative Study of Leaves, Seeds, and Microshoot Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Parent Plant Material
2.2. Initiation of In Vitro Cultures
2.3. Experimental In Vitro Cultures
2.4. Extraction
2.5. Analysis of GSL
2.6. Total Phenolic Assay
2.7. Polyphenol Profiling by HPLC-DAD
2.8. Assessment of Biological Activity
2.8.1. Total Antioxidant Potential Assay by ABTS
2.8.2. Total Antioxidant Potential by DPPH
2.8.3. The Chelating Capacity of Iron Ions Fe2+
2.8.4. Elastase Inhibition Ability Test
2.8.5. Anti-Microbial Assay
2.9. Study on Cosmetic Formulation
2.9.1. Preparation of Emulsion
2.9.2. Physiochemical Tests
Stability Tests and pH Measurement
Rheological Properties Testing
Evaluation of Antioxidant Properties Using the DPPH Method
2.10. Statistical Analysis
3. Results
3.1. Microshoot Appearance
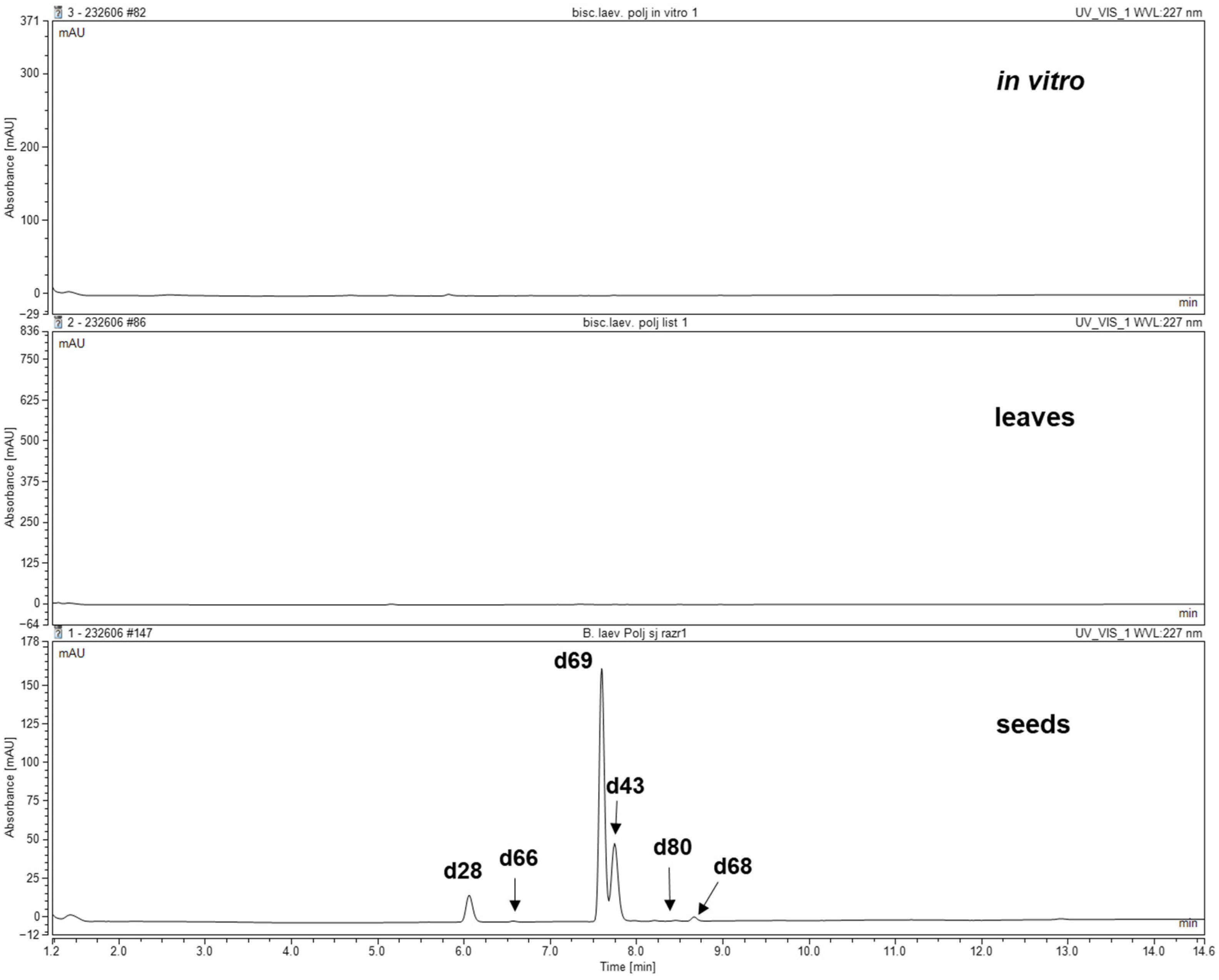
3.2. GSLs Profiling and Individual Contents
3.3. Total and Individual Polyphenol Profiling
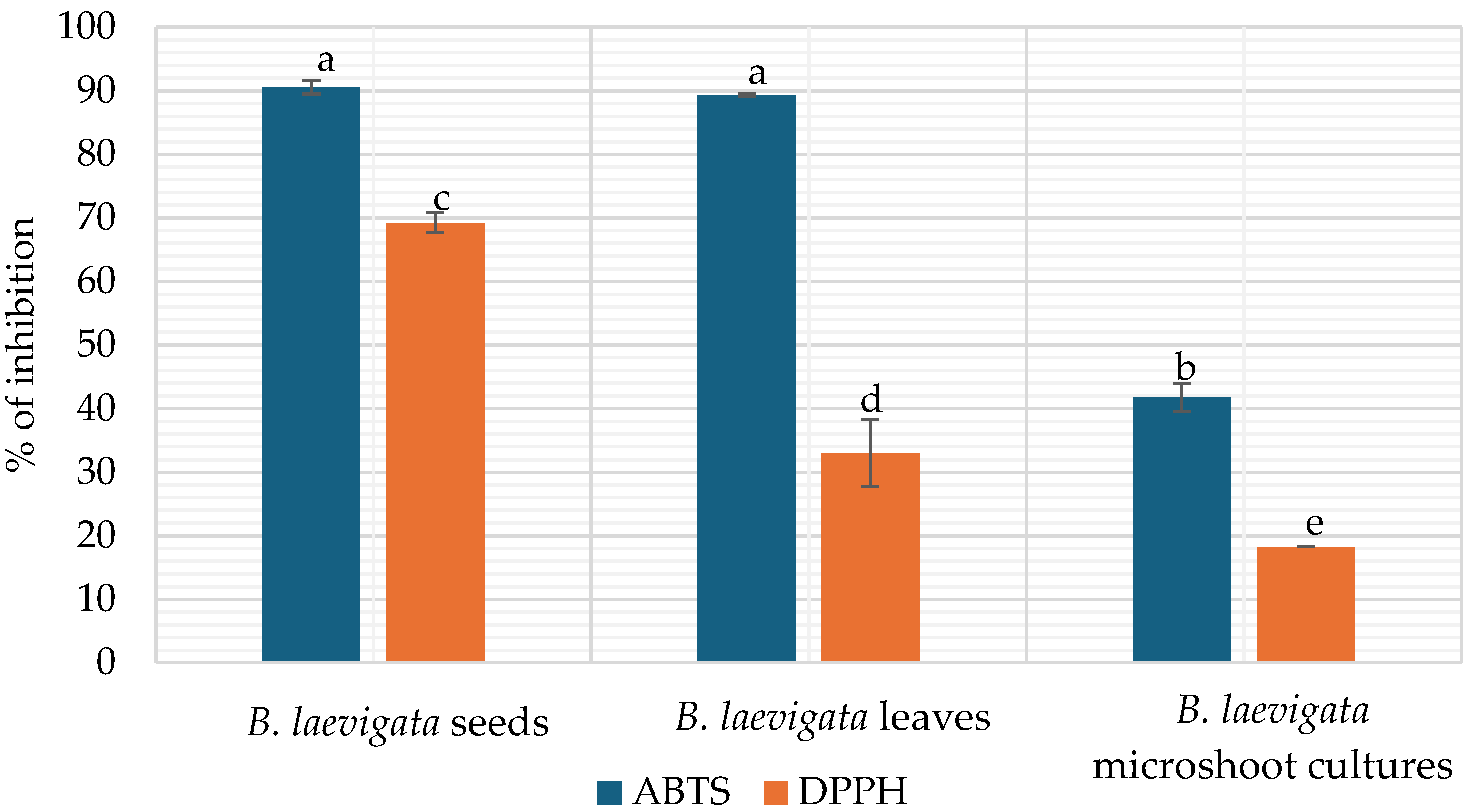
3.4. Assessment of Biological Activity
3.4.1. The Antioxidant Potential Assessed by ABTS and DPPH
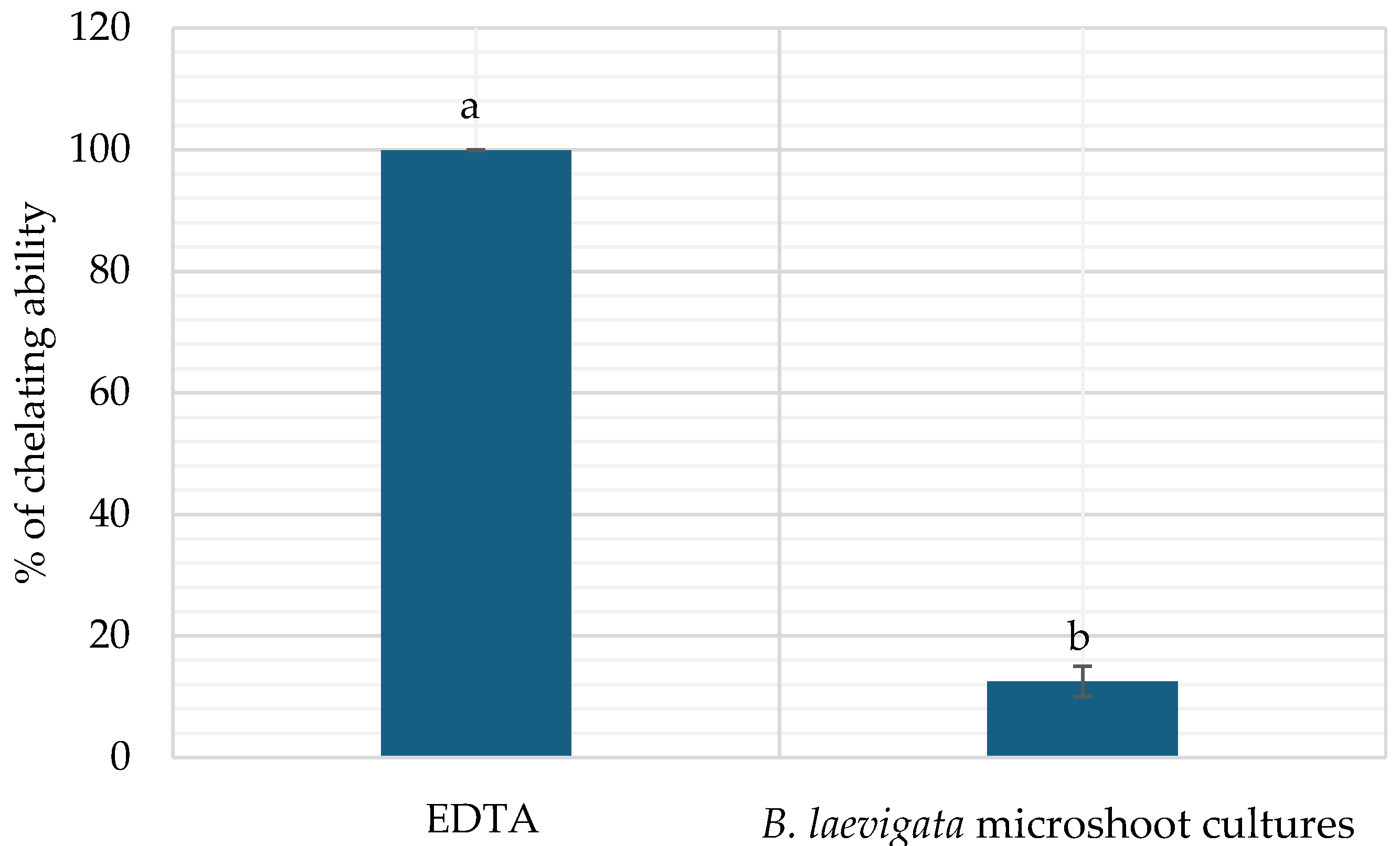
3.4.2. The Chelating Capacity of Iron Ions Fe2+
3.4.3. Elastase Inhibition Ability Assay
3.4.4. Anti-Microbial Assay
3.5. Study on Cosmetic Formulation
3.5.1. Physicochemical Tests
Stability Tests and pH Measurement
Rheological Study of Emulsions
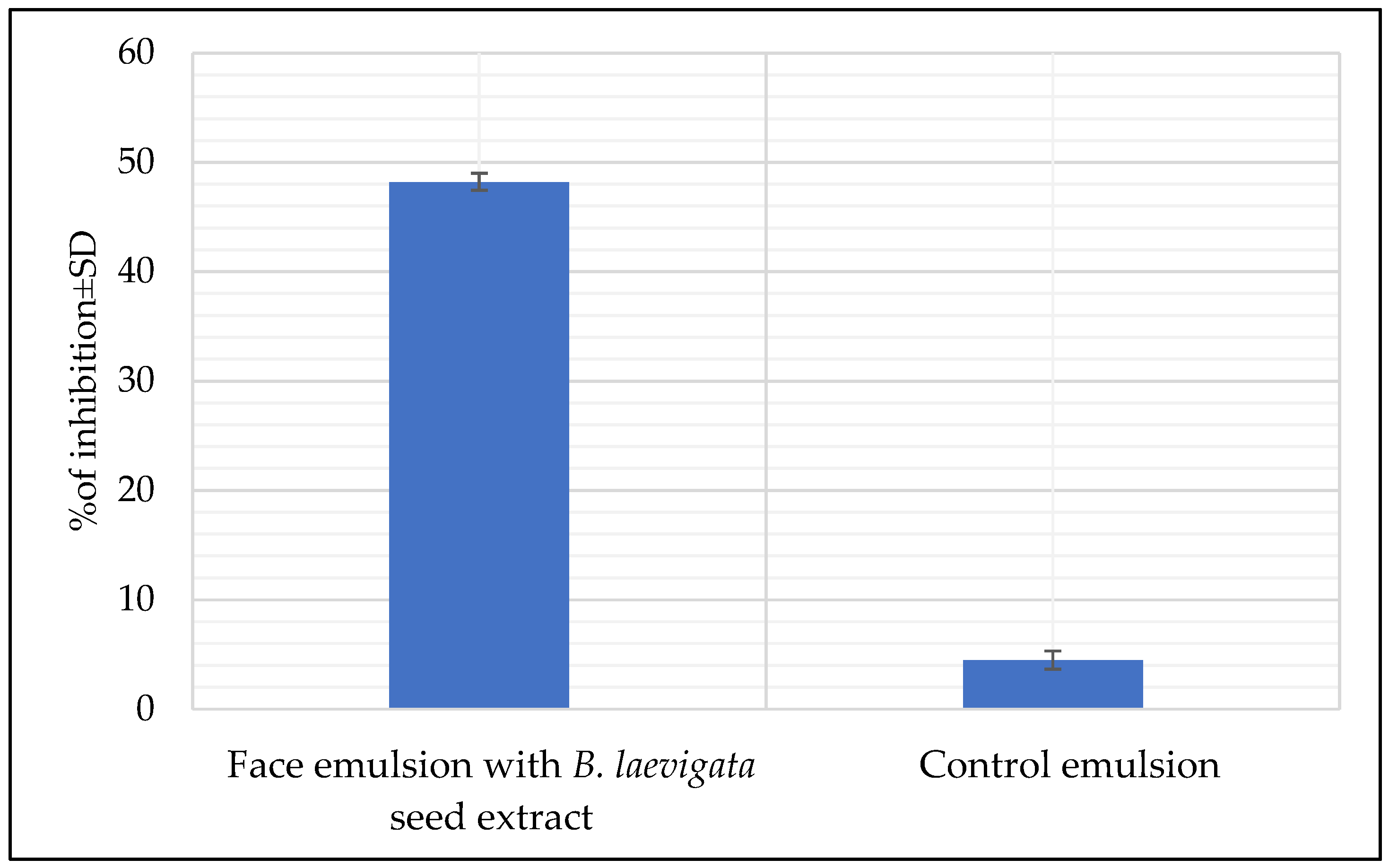
Evaluation of Antioxidant Properties of Emulsions Using the DPPH Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae Microgreens: A Novel and Promissory Source of Sustainable Bioactive Compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef]
- Rostanski, A.; Szarek-Łukaszewska, G.; Jędrzejczyk-Korycińska, M. The Buckler Mustard (Biscutella laevigata L.)—Species Description. In Buckler Mustard (Biscutella laevigata L.) an Extraordinary Plant on Ordinary Mine Heaps Near Olkusz; Szarek-Łukaszewska, G., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Krakow, Poland, 2020; pp. 79–93. [Google Scholar]
- Bróż, E.; Przemyski, A. The Red List of Vascular Plants in the Wyżyna Małopolska Upland (S Poland). In Rare, Relict and Endangered Plants and Fungi in Poland; Mirek, Z., Nikel, A., Eds.; W. Szafer Institute of Botany, Polish Academy of Sciences: Krakow, Poland, 2009; pp. 123–136. [Google Scholar]
- Zarzycki, K.; Szeląg, Z. Red List of the Vascular Plants in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szeląg, Z., Eds.; W. Szafer Institute of Botany, Polish Academy of Sciences: Krakow, Poland, 2006; pp. 9–20. [Google Scholar]
- Kaźmierczakowa, R.; Bloch-Orłowska, J.; Celka, Z.; Cwener, A.; Dajdok, Z.; Michalska-Hejduk, D.; Pawlikowski, P.; Szczęśniak, E.; Ziarnek, K. Polish Red List of Pteridophytes and Flowering Plants; Instytut Ochrony Przyrody PAN: Krakow, Poland, 2016. [Google Scholar]
- Ciarkowska, K.; Hanus-Fajerska, E.; Gambuś, F.; Muszyńska, E.; Czech, T. Phytostabilization of Zn-Pb Ore Flotation Tailings with Dianthus Carthusianorum and Biscutella laevigata after Amending with Mineral Fertilizers or Sewage Sludge. J. Environ. Manag. 2017, 189, 75–83. [Google Scholar] [CrossRef] [PubMed]
- LaCoste, C.; Robinson, B.; Brooks, R.; Anderson, C.; Chiarucci, A.; Leblanc, M. The Phytoremediation Potential of Thallium-Contaminated Soils Using Iberis and Biscutella Species. Int. J. Phytoremediation 1999, 1, 327–338. [Google Scholar] [CrossRef]
- Remigio, A.; Poscic, F.; Nkrumah, P.; Edraki, M.; Spiers, K.; Brueckner, D.; van der Ent, A. Comprehensive Insights in Thallium Ecophysiology in the Hyperaccumulator Biscutella laevigata. Sci. Total Environ. 2022, 838, 155899. [Google Scholar] [CrossRef] [PubMed]
- Boudouda, H.B.; Zeghib, A.; Karioti, A.; Bilia, A.R.; Öztürk, M.; Aouni, M.; Kabouche, A.; Kabouche, Z. Antibacterial, Antioxidant, Anti-Cholinesterase Potential and Flavonol Glycosides of Biscutella raphanifolia (Brassicaceae). Pak. J. Pharm. Sci. 2015, 28, 153–158. [Google Scholar]
- Karuppusamy, S. A Review on Trends in Production of Secondary Metabolites from Higher Plants by in Vitro Tissue, Organ and Cell Cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Muszyńska, E.; Hanus-Fajerska, E.; Piwowarczyk, B.; Augustynowicz, J.; Ciarkowska, K.; Czech, T. From Laboratory to Field Studies—The Assessment of Biscutella laevigata Suitability to Biological Reclamation of Areas Contaminated with Lead and Cadmium. Ecotoxicol. Environ. Saf. 2017, 142, 266–273. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wiszniewska, A.; Muszynska, E. In Vitro Multiplication and Acclimatization of Biscutella laevigata (Brassicaceae) to Cultivation in Greenhouse Conditions. Biotechnologia 2012, 93, 97–101. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Granica, S.; Korona-Glowniak, I.; Ekiert, H.; Szopa, A. Phytochemical and Biological Activity Studies on Nasturtium officinale (Watercress) Microshoot Cultures Grown in RITA® Temporary Immersion Systems. Molecules 2020, 25, 5257. [Google Scholar] [CrossRef]
- Grosser, K.; van Dam, N.M. A Straightforward Method for Glucosinolate Extraction and Analysis with High-Pressure Liquid Chromatography (HPLC). J. Vis. Exp. 2017, 2017, 55425. [Google Scholar] [CrossRef]
- Wathelet, J.P.; Iori, R.; Leoni, O.; Quinsac, O.; Palmieri, S. Guidelines for Glucosinolate Analysis in Green Tissues Used for Biofumigation. Agroindustria 2004, 3, 257–266. [Google Scholar]
- Brown, P.D.; Tokuhisa, J.G.; Reichelt, M.; Gershenzon, J. Variation of Glucosinolate Accumulation among Different Organs and Developmental Stages of Arabidopsis thaliana. Phytochemistry 2003, 62, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Maślanka, A.; Szewczyk, A.; Muszyńska, B. Physiologically Active Compounds in Four Species of Genus Phellinus. Nat. Prod. Commun. 2017, 12, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-Performance Liquid Chromatography and Thin-Layer Chromatography of Phenolic Acids from Gingko biloba L. Leaves Collected within Vegetative Period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kubica, P.; Kokotkiewicz, A.; Malinowska, M.A.; Synowiec, A.; Gniewosz, M.; Hussain, S.; Yaqoob, M.; Bonn, G.K.; Jakschitz, T.; Mahmoud, E.A.; et al. Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena Officinalis Microshoot Cultures and Soil-Grown Plant. Antioxidants 2022, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Sonja, M.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory Effects of Polyphenols from Grape Pomace Extract on Collagenase and Elastase Activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Available online: https://www.eucast.org (accessed on 22 May 2025).
- Agerbirk, N.; Hansen, C.C.; Olsen, C.E.; Kiefer, C.; Hauser, T.P.; Christensen, S.; Jensen, K.R.; Ørgaard, M.; Pattison, D.I.; Lange, C.B.A.; et al. Glucosinolate Profiles and Phylogeny in Barbarea Compared to Other Tribe Cardamineae (Brassicaceae) and Reseda (Resedaceae), Based on a Library of Ion Trap HPLC-MS/MS Data of Reference Desulfoglucosinolates. Phytochemistry 2021, 185, 112658. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate Structural Diversity, Identification, Chemical Synthesis and Metabolism in Plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Pantelić, I.; Savić, S.D. Towards Optimal pH of the Skin and Topical Formulations: From the Current State of the Art to Tailored Products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Dziurka, M.; Komsta, Ł.; Tomczyk, M.; Ekiert, H. The Influence of Nasturtium officinale R. Br. Agar and Agitated Microshoot Culture Media on Glucosinolate and Phenolic Acid Production, and Antioxidant Activity. Biomolecules 2020, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. Cretan Dittany (Origanum dictamnus L.), a Valuable Local Endemic Plant: In Vitro Regeneration Potential of Different Type of Explants for Conservation and Sustainable Exploitation. Plants 2023, 12, 182. [Google Scholar] [CrossRef]
- Mendler-Drienyovszki, N.; Magyar-Tábori, K. Response of Rowan Berry (Sorbus redliana) Shoot Culture to Slow Growth Storage Conditions. Plants 2023, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An Efficient Micropropagation Protocol for an Endangered Ornamental Tree Species (Magnolia sirindhorniae Noot. & Chalermglin) and Assessment of Genetic Uniformity through DNA Markers. Sci. Rep. 2019, 9, 9634. [Google Scholar] [CrossRef]
- Park, C.H.; Sathasivam, R.; Nguyen, B.V.; Baek, S.A.; Yeo, H.J.; Park, Y.E.; Kim, H.H.; Kim, J.K.; Park, S.U. Metabolic Profiling of Primary Metabolites and Galantamine Biosynthesis in Wounded Lycoris radiata Callus. Plants 2020, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Sapre, S.; Ahuja, A.; Tiwari, S. Cell Suspension Culture and in Vitro Screening for Drought Tolerance in Soybean Using Poly-Ethylene Glycol. Plants 2021, 10, 517. [Google Scholar] [CrossRef]
- Dogan, M. Effect of Salt Stress on in Vitro Organogenesis from Nodal Explant of Limnophila aromatica (Lamk.) Merr. and Bacopa monnieri (L.) Wettst. and Their Physio-Morphological and Biochemical Responses. Physiol. Mol. Biol. Plants 2020, 26, 803–816. [Google Scholar] [CrossRef]
- Marzilli, M.; Di Santo, P.; Palumbo, G.; Maiuro, L.; Paura, B.; Tognetti, R.; Cocozza, C. Cd and Cu Accumulation, Translocation and Tolerance in Populus alba Clone (Villafranca) in Autotrophic in Vitro Screening. Environ. Sci. Pollut. Res. 2018, 25, 10058–10068. [Google Scholar] [CrossRef]
- Cole, R. Isothiocyanates, Nitriles and Thiocyanates as Products of Autolysis of Glucosinolates in Cruciferae. Phytochemistry 1976, 15, 759–762. [Google Scholar] [CrossRef]
- Daxenbichler, M.E.; Spencer, G.F.; Carlson, D.G.; Rose, G.B.; Brinker, A.M.; Powell, R.G. Glucosinolate Composition of Seeds from 297 Species of Wild Plants. Phytochemistry 1991, 30, 2623–2638. [Google Scholar] [CrossRef]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling Glucosinolates and Phenolics in Vegetative and Reproductive Tissues of the Multi-Purpose Trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef]
- Lockwood, G.B.; Belkhiri, A. Glucosinolate Spectrum of Some Algerian Cruciferae. Plant Syst. Evol. 1991, 176, 11–20. [Google Scholar] [CrossRef]
- Metz, J.; Ribbers, K.; Tielborger, K.; Muller, C. Long- and Medium-Term Effects of Aridity on the Chemical Defence of a Widespread Brassicaceae in the Mediterranean. Environ. Exp. Bot. 2014, 105, 39–45. [Google Scholar] [CrossRef]
- Czerniawski, P.; Piasecka, A.; Bednarek, P. Evolutionary Changes in the Glucosinolate Biosynthetic Capacity in Species Representing Capsella, Camelina and Neslia genera. Phytochemistry 2021, 181, 112571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, R.; Zheng, J.; Wang, Z.; Gao, T.; Qin, M.; Hu, X.; Wang, Y.; Yang, S.; Li, T. Insights into Glucosinolate Accumulation and Metabolic Pathways in Isatis indigotica Fort. BMC Plant Biol. 2022, 22, 78. [Google Scholar] [CrossRef]
- Arena, D.; Ben Ammar, H.; Major, N.; Kovačević, T.K.; Goreta Ban, S.; Treccarichi, S.; Lo Scalzo, R.; Branca, F. Light Use Efficiency of Broccoli (Brassica oleracea var. italica Plenck) and Rocket (Eruca sativa L.) during the Initial Plant Growth Stages. Sci. Hortic. 2024, 336, 113408. [Google Scholar] [CrossRef]
- Al-Ogaili, N. The Evaluation of Total Flavonoids, Total Phenolic Content and Biological Activity of Iraqi Lipedium sativum L. Crude Extract Obtained by Optimized Ultrasound Assisted Extraction Conditions. Plant Sci. Today 2024, 11, 214–220. [Google Scholar] [CrossRef]
- Arena, P.; Miceli, N.; Marino, A.; Davì, F.; Cavò, E.; Spadaro, V.; Raimondo, F.M.; Cacciola, F.; Laganà Vinci, R.; Mondello, L.; et al. Comparative Study on Phenolic Profile and Biological Activities of the Aerial Parts of Sinapis pubescens L. Subsp. Pubescens (Brassicaceae) Wild from Sicily (Italy). Chem. Biodivers. 2023, 20, e202300309. [Google Scholar] [CrossRef]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on Secondary Metabolite Profiling, Anti-Inflammatory Potential, in Vitro Photoprotective and Skin-Aging Related Enzyme Inhibitory Activities of Malaxis acuminata, a Threatened Orchid of Nutraceutical Importance. J. Photochem. Photobiol. B Biol. 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Miazga-Karska, M.; Klimek, K.; Ekiert, H.; Szopa, A. Precursor-Boosted Production of Metabolites in Nasturtium officinale Microshoots Grown in Plantform Bioreactors, and Antioxidant and Antimicrobial Activities of Biomass Extracts. Molecules 2021, 26, 4660. [Google Scholar] [CrossRef] [PubMed]
- Klimek-Szczykutowicz, M.; Dziurka, M.; Blažević, I.; Đulović, A.; Apola, A.; Ekiert, H.; Szopa, A. Impacts of Elicitors on Metabolite Production and on Antioxidant Potential and Tyrosinase Inhibition in Watercress Microshoot Cultures. Appl. Microbiol. Biotechnol. 2022, 106, 619–633. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Siddiquah, A.; Tungmunnithum, D.; Bose, S.; Younas, M.; Garros, L.; Drouet, S.; Giglioli-Guivarc’h, N.; Hano, C. Isodon rugosus (Wall. Ex Benth.) Codd in Vitro Cultures: Establishment, Phytochemical Characterization and In Vitro Antioxidant and Anti-Aging Activities. Int. J. Mol. Sci. 2019, 20, 452. [Google Scholar] [CrossRef]
- Szewczyk, K.; Pietrzak, W.; Klimek, K.; Miazga-Karska, M.; Firlej, A.; Flisiński, M.; Grzywa-Celińska, A. Flavonoid and Phenolic Acids Content and in Vitro Study of the Potential Anti-Aging Properties of Eutrema japonicum (Miq.) Koidz Cultivated in Wasabi Farm Poland. Int. J. Mol. Sci. 2021, 22, 6219. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Malinowska, M.A.; Gałka, A.; Blažević, I.; Ðulović, A.; Paprocka, P.; Wrzosek, M.; Szopa, A. Nasturtium officinale Microshoot Culture Multiplied in PlantForm Bioreactor—Phytochemical Profiling and Biological Activity. Molecules 2025, 30, 936. [Google Scholar] [CrossRef]
- Milentyeva, I.S.; Le, V.M.; Kozlova, O.V.; Velichkovich, N.S.; Fedorova, A.M.; Loseva, A.I.; Yustratov, V.P. Secondary Metabolites in in Vitro Cultures of Siberian Medicinal Plants: Content, Antioxidant Properties, and Antimicrobial Characteristics. Foods Raw Mater. 2021, 9, 153–163. [Google Scholar] [CrossRef]
- Rajaram, K.; Sriramji, P.; Sureshkumar, P. Micropropagation, Antioxidant and Antimicrobial Effect of Plectranthus bourneae Gamble: An Endangered Medicinal Plant. J. Chem. Pharm. Sci. 2015, 8, 142–147. [Google Scholar]
- Shahrour, W.G.; Shatnawi, M.A.; Al-Alawi, M.; Shibli, R.A.; Alqudah, T.S.; Majdalawi, M.M.; Al-Tawaha, A.R.; Aljammal, A. In Vitro Multiplication, Antimicrobial, and Insecticidal Activity of Capparis spinosa L. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13609. [Google Scholar] [CrossRef]
- Jo, D.M.; Tabassum, N.; Oh, D.K.; Ko, S.C.; Kim, K.W.; Yang, D.; Kim, J.Y.; Oh, G.W.; Choi, G.; Lee, D.S.; et al. Green Medicine: Advancing Antimicrobial Solutions with Diverse Terrestrial and Marine Plant-Derived Compounds. Processes 2024, 12, 2316. [Google Scholar] [CrossRef]
- Akaza, N.; Takasaki, K.; Matsudaira, T.; Usui, A.; Iijima, A.; Miura, S.; Yashiro, Y. Relationship between Skin Fungal and Bacterial Microbiomes and Skin PH. Int. J. Cosmet. Sci. 2023, 45, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Toro, A.; Montero-Vilchez, T.; Muñoz-Baeza, J.; Sanabria-De-la-Torre, R.; Buendia-Eisman, A.; Arias-Santiago, S. The Assessment of Skin Homeostasis Changes after Using Different Types of Excipients in Healthy Individuals. Int. J. Environ. Res. Public Health 2022, 19, 16678. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Lameirão, F.; Delerue-Matos, C.; Rodrigues, F.; Costa, P. Characterization and Stability of a Formulation Containing Antioxidants-Enriched Castanea sativa Shells Extract. Cosmetics 2021, 8, 49. [Google Scholar] [CrossRef]



| Phase | Control Emulsion | Face Emulsion with B. laevigata Seed Extract | ||
|---|---|---|---|---|
| Ingredient | Concentration % | Ingredient | Concentration % | |
| Oil phase | Persea gratissima (Avocado) Oil | 7.5 | Persea gratissima (Avocado) Oil | 7.5 |
| Cocos nucifera Oil | 7.5 | Cocos nucifera Oil | 7.5 | |
| Cetearyl Olivate, Sorbitan Olivate | 5.0 | Cetearyl Olivate, Sorbitan Olivate | 5.0 | |
| Butyrospermum parkii Butter | 3.0 | Butyrospermum parkii Butter | 3.0 | |
| Cera Flava | 2.0 | Cera Flava | 2.0 | |
| Cetyl Alcohol | 1.0 | Cetyl Alcohol | 1.0 | |
| Retinyl Palmitate | 0.5 | Retinyl Palmitate | 0.5 | |
| Tocopherol Acetate | 0.5 | Tocopherol Acetate | 0.5 | |
| Water phase | Aqua | 68.0 | Aqua | 66.0 |
| Biscutella laevigata Seed Extract | - | Biscutella laevigata Seed Extract | 2.0 | |
| Sodium Hyaluronate | 1.0 | Sodium Hyaluronate | 1.0 | |
| D-panthenol | 1.0 | Panthenol | 1.0 | |
| Niacinamide | 0.5 | Niacinamide | 0.5 | |
| Sodium Benzoate | 0.5 | Sodium Benzoate | 0.5 | |
| No. | Glucosinolate (GSL) (Trivial Name) | tR (min) | [M + Na]+ | Glucosinolate Content (mg/100 g DW) |
|---|---|---|---|---|
| Methionine-derived | ||||
| 66 | 7-(Methylsulfinyl)heptyl GSL a | 6.58 | 422 | tr |
| 69 | 8-(Methylsulfinyl)octyl GSL (Glucohirsutin) a,b | 7.59 | 436 | 15.06 ± 1.99 |
| 80 | 8-(Methylsulfonyl)octyl GSL a | 7.99 | 452 | tr |
| 68 | 9-(Methylsulfinyl)octyl GSL (Glucoarabin) a,b | 8.45 | 450 | 0.21 ± 0.01 |
| Tryptophan-derived | ||||
| 28 | 4-Hydroxyindol-3-ylmethyl GSL (4-Hydroxyglucobrassicin) a,b | 6.09 | 407 | 0.48 ± 0.09 |
| 43 | Indol-3-ylmethyl GSL (Glucobrassicin) a,b | 7.75 | 391 | 1.08 ± 0.09 |
| Polyphenols | B. laevigata | |||
|---|---|---|---|---|
| Seeds | Leaves | Microshoot Cultures | ||
| TPC | 25,701.00 ± 41.23 a | 16,244.00 ± 72.09 b | 16,552.00 ± 110.36 b | |
| Phenolic acid | Caffeic acid | 5.60 ± 0.37 c | 5.35 ± 0.46 c | 6.67 ± 0.35 d |
| Ferulic acid | 20.85 ± 0.55 e | 14.23 ± 0.25 f | 5.78 ± 0.25 g | |
| Protocatechuic acid | 6.68 ± 0.18 h | 7.18 ± 0.16 i | 5.28 ± 0.12 j | |
| Vanillic acid | 7.79 ± 0.23 k | 0.88 ± 0.01 l | 2.22 ± 0.20 m | |
| Flavonoid | Astragalin (kaempferol-3-glucoside) | 26.96 ± 2.30 n | 106.29 ± 3.70 o | 0.78 ± 0.02 p |
| Kaempferol | 45.31 ± 2.60 r | 38.18 ± 3.89 r | nd * | |
| Rutin (quercetin 3-rutinoside) | 943.92 ± 21.97 s | 1609.21 ± 15.44 t | 11.66 ± 0.24 u | |
| Microorganism | Extract | Antibiotic/Antifungal Drug | MIC (µg/mL) | MBC or MFC (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. laevigata Seeds | B. laevigata Leaves | B. laevigata Microshoot Cultures | ||||||||
| MIC (µg/mL) | MBC or MFC (µg/mL) | MIC (µg/mL) | MBC or MFC (µg/mL) | MIC (µg/mL) | MBC or MFC (µg/mL) | |||||
| Bacteria | Staphylococcus epidermidis | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | Tetracycline | 128 | 256 |
| ATCC 12228 | ||||||||||
| Staphylococcus aureus | 2500 | 5000 | 2500 | 5000 | 1250 | 5000 | Tetracycline | 1 | 0.5 | |
| ATCC 29213 | ||||||||||
| Escherichia coli | 1250 | 2500 | 625 | 1250 | 625 | 2500 | Tetracycline | 0.25 | 0.25 | |
| ATCC 25922 | ||||||||||
| Cutibacterium acnes | 1250 | 2500 | 2500 | 5000 | 1250 | 2500 | Tetracycline | 0.5 | 16 | |
| ATCC 6919 | ||||||||||
| Cutibacterium acnes | 1250 | 2500 | 1250 | 1250 | 1250 | 2500 | Tetracycline | 0.5 | 16 | |
| ATCC 11827 | ||||||||||
| Fungi | Candida albicans | 1250 | 1250 | 1250 | 1250 | 1250 | 1250 | Fluconazole | 1 | 0.5 |
| ATCC 14053 | ||||||||||
 |  | |||||||||
| Emulsion | Tangential Stress (Pa) | Viscosity (Pa·s) |
|---|---|---|
| Face emulsion with B. laevigata seed extract | 100.71 ± 0.35 | 1.42 ± 0.01 |
| Control emulsion | 109.57 ± 0.97 | 1.55 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimek-Szczykutowicz, M.; Malinowska, M.A.; Śliwa, A.; Blažević, I.; Ðulović, A.; Wiśniewska, K.; Piwowarczyk, R.; Paprocka, P.; Wrzosek, M.; Szopa, A. Phytochemical Profile and Biological Activities of Biscutella laevigata: A Comparative Study of Leaves, Seeds, and Microshoot Cultures. Appl. Sci. 2025, 15, 10462. https://doi.org/10.3390/app151910462
Klimek-Szczykutowicz M, Malinowska MA, Śliwa A, Blažević I, Ðulović A, Wiśniewska K, Piwowarczyk R, Paprocka P, Wrzosek M, Szopa A. Phytochemical Profile and Biological Activities of Biscutella laevigata: A Comparative Study of Leaves, Seeds, and Microshoot Cultures. Applied Sciences. 2025; 15(19):10462. https://doi.org/10.3390/app151910462
Chicago/Turabian StyleKlimek-Szczykutowicz, Marta, Magdalena Anna Malinowska, Anna Śliwa, Ivica Blažević, Azra Ðulović, Karolina Wiśniewska, Renata Piwowarczyk, Paulina Paprocka, Małgorzata Wrzosek, and Agnieszka Szopa. 2025. "Phytochemical Profile and Biological Activities of Biscutella laevigata: A Comparative Study of Leaves, Seeds, and Microshoot Cultures" Applied Sciences 15, no. 19: 10462. https://doi.org/10.3390/app151910462
APA StyleKlimek-Szczykutowicz, M., Malinowska, M. A., Śliwa, A., Blažević, I., Ðulović, A., Wiśniewska, K., Piwowarczyk, R., Paprocka, P., Wrzosek, M., & Szopa, A. (2025). Phytochemical Profile and Biological Activities of Biscutella laevigata: A Comparative Study of Leaves, Seeds, and Microshoot Cultures. Applied Sciences, 15(19), 10462. https://doi.org/10.3390/app151910462










