Evaluation of Fluoride Adsorptive Removal by Metallic Phosphates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Metallic Phosphates
2.3. Adsorption and Regeneration Experiments
2.4. Analysis and Characterization
3. Results and Discussion
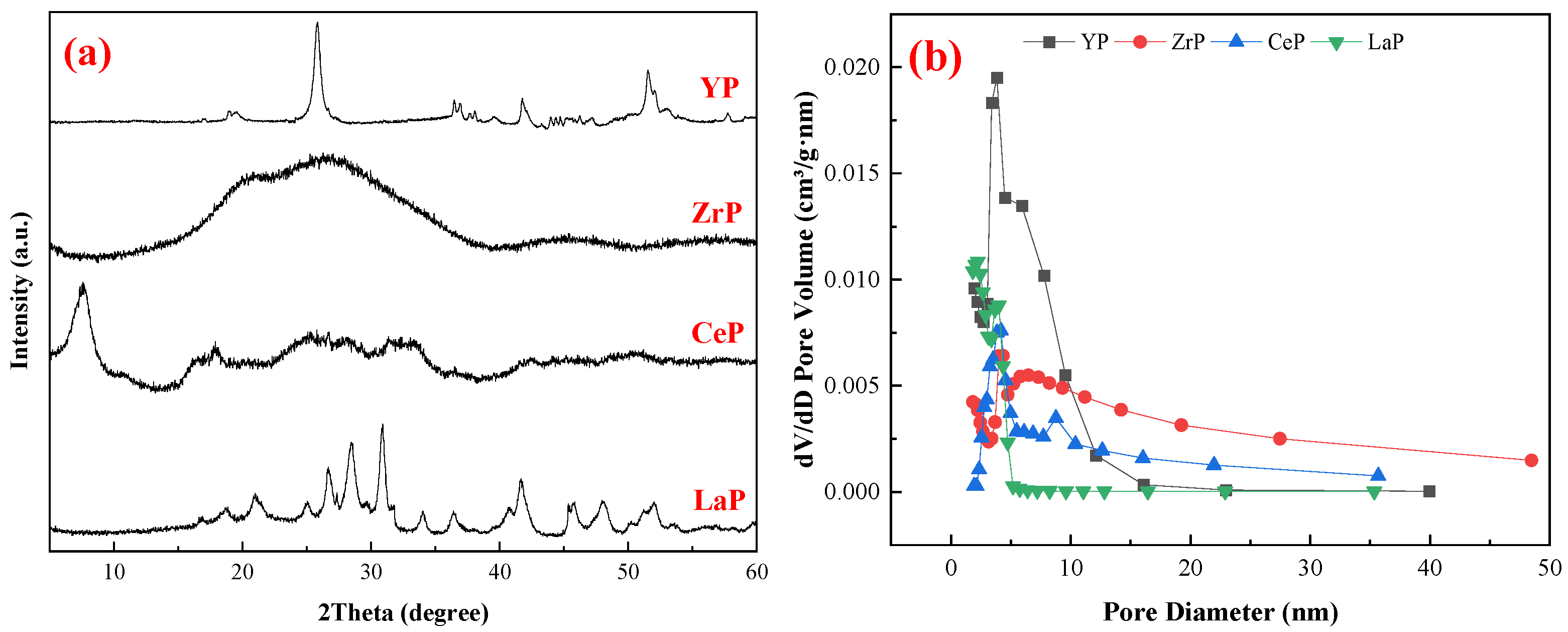
3.1. Characterization of Adsorbents
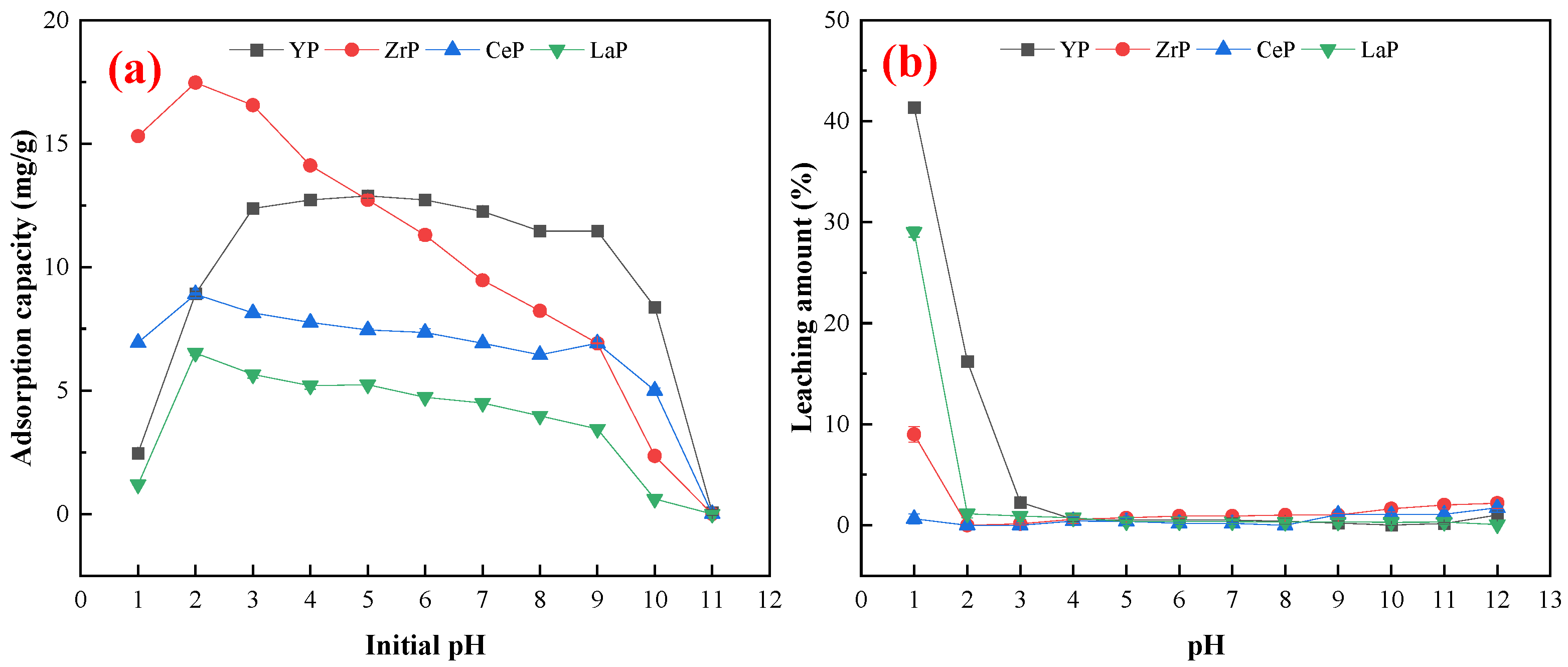
3.2. Effect of pH on Fluoride Adsorption
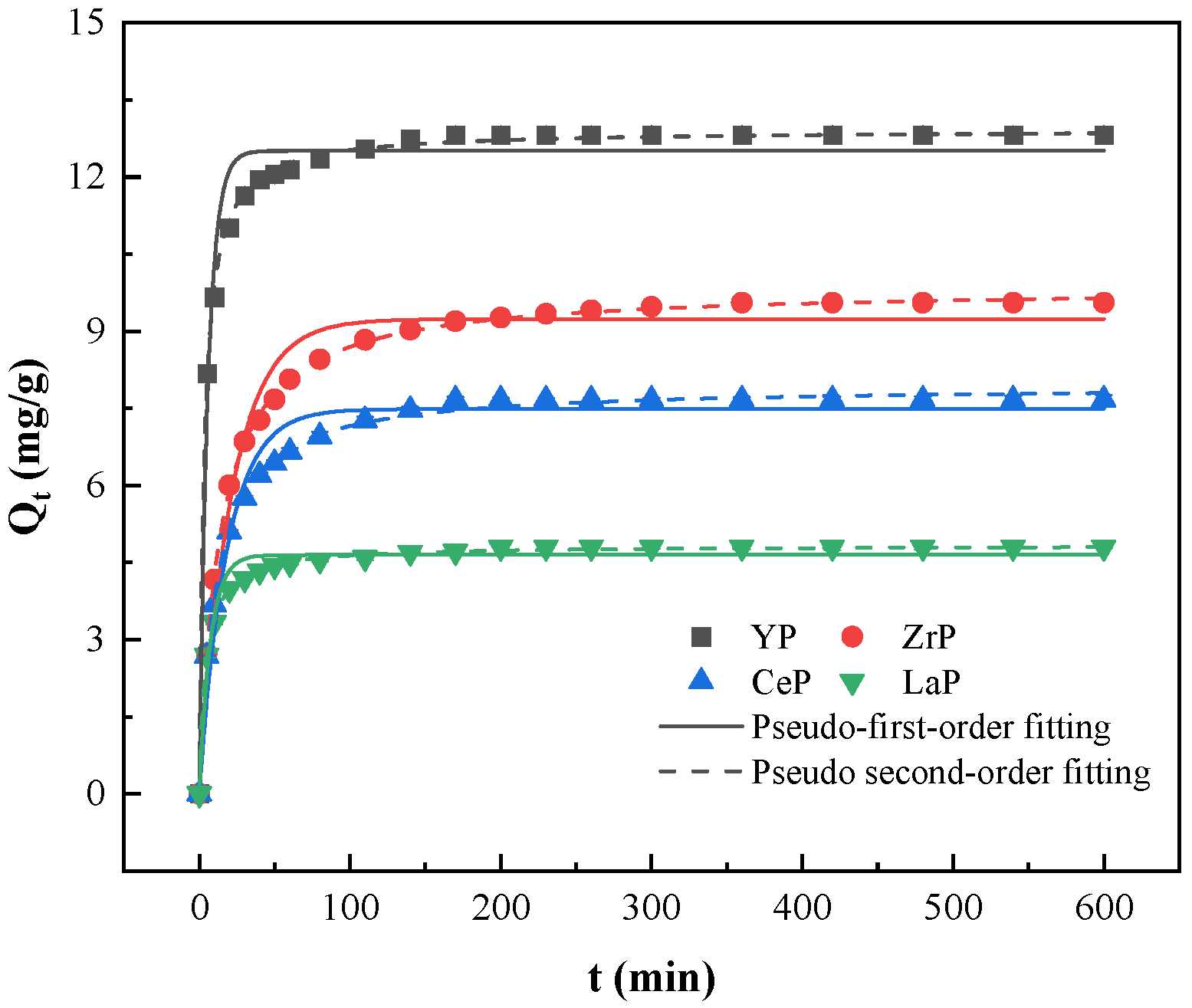
3.3. Adsorption Kinetic Analysis
3.4. Adsorption Isotherm and Thermodynamic Analysis
3.5. Effect of Co-Existing Anions on Fluoride Adsorption
3.6. Effect of Co-Existing Organics on Fluoride Adsorption
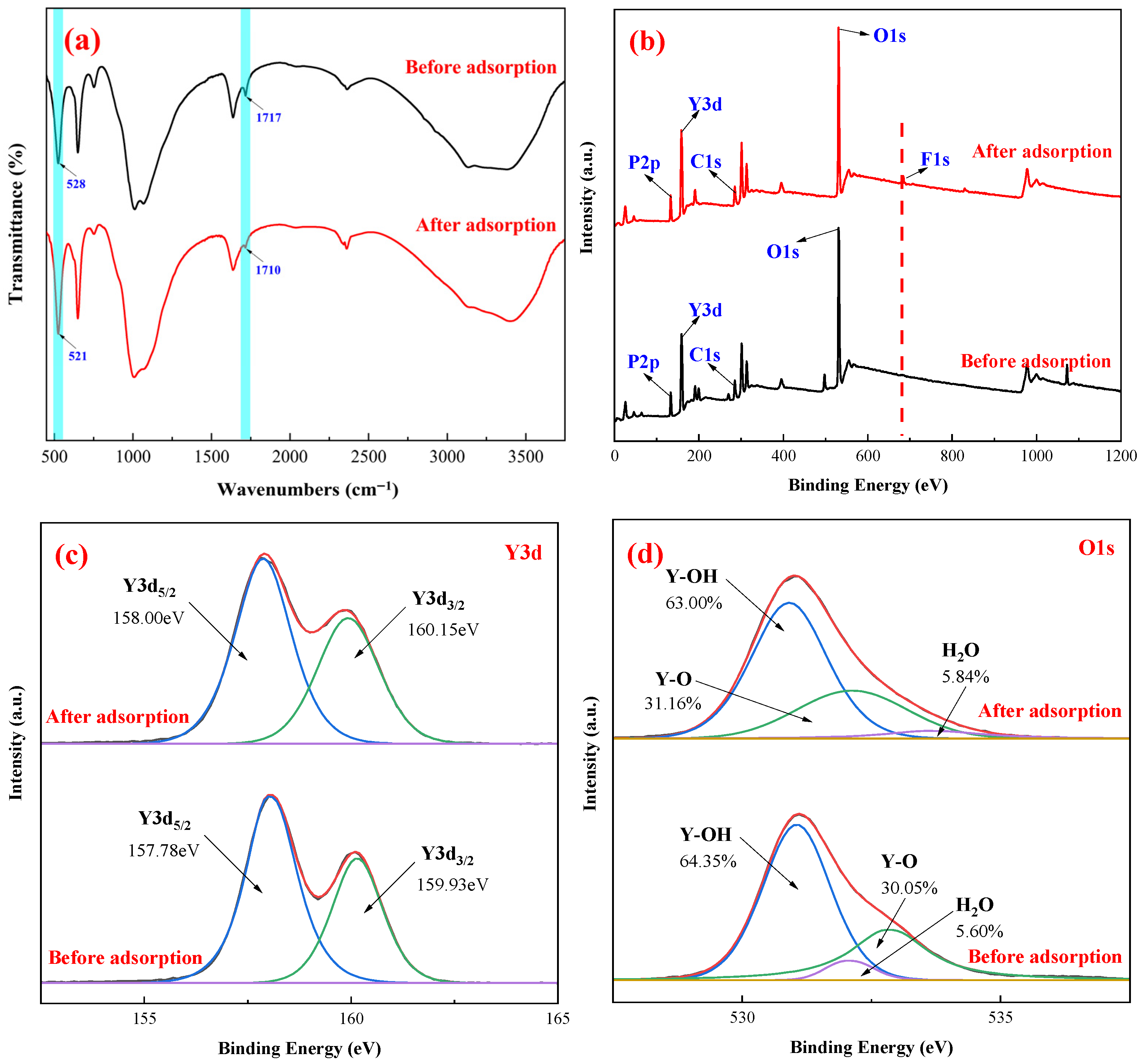
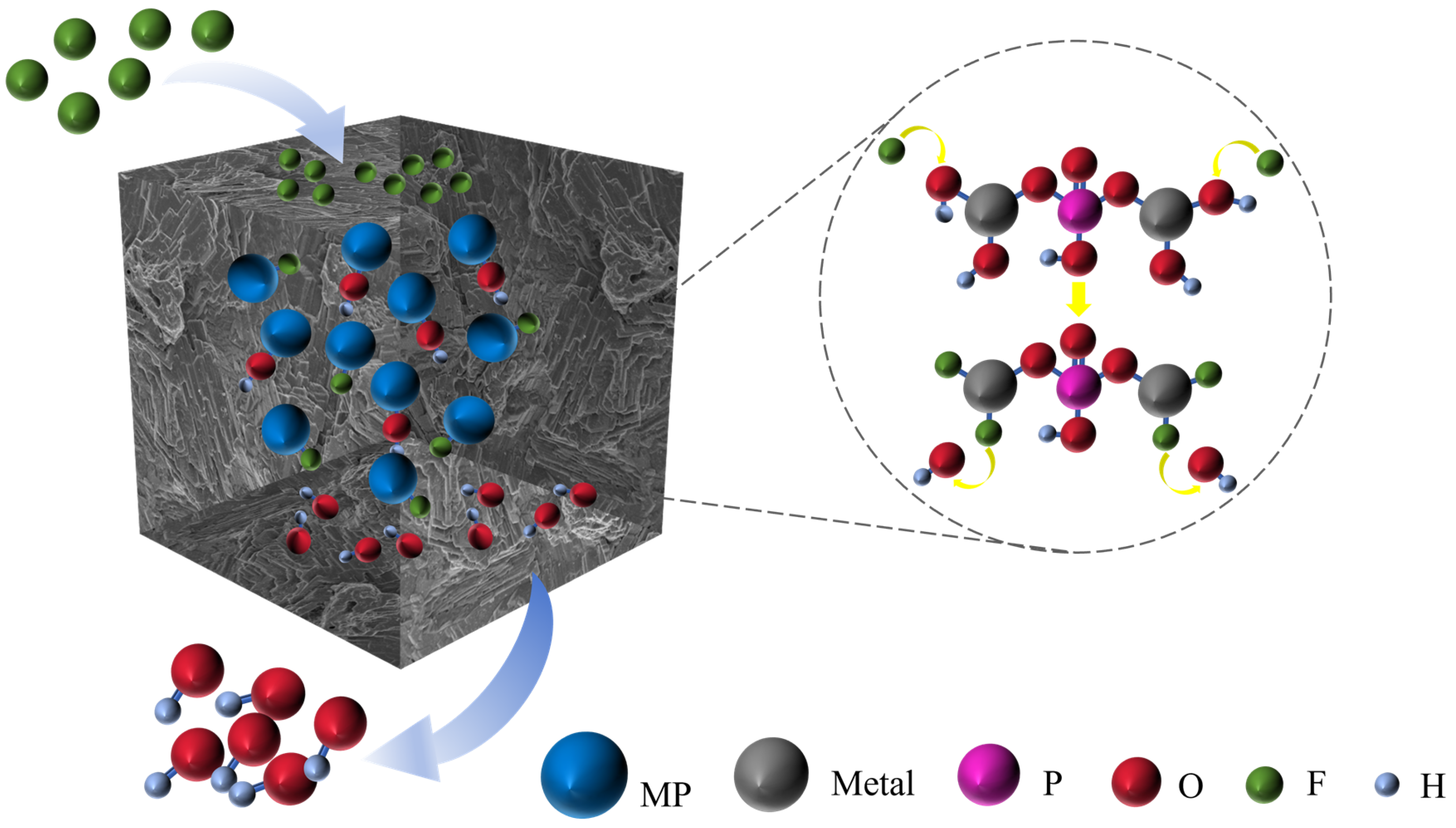
3.7. Fluoride Adsorption Mechanism
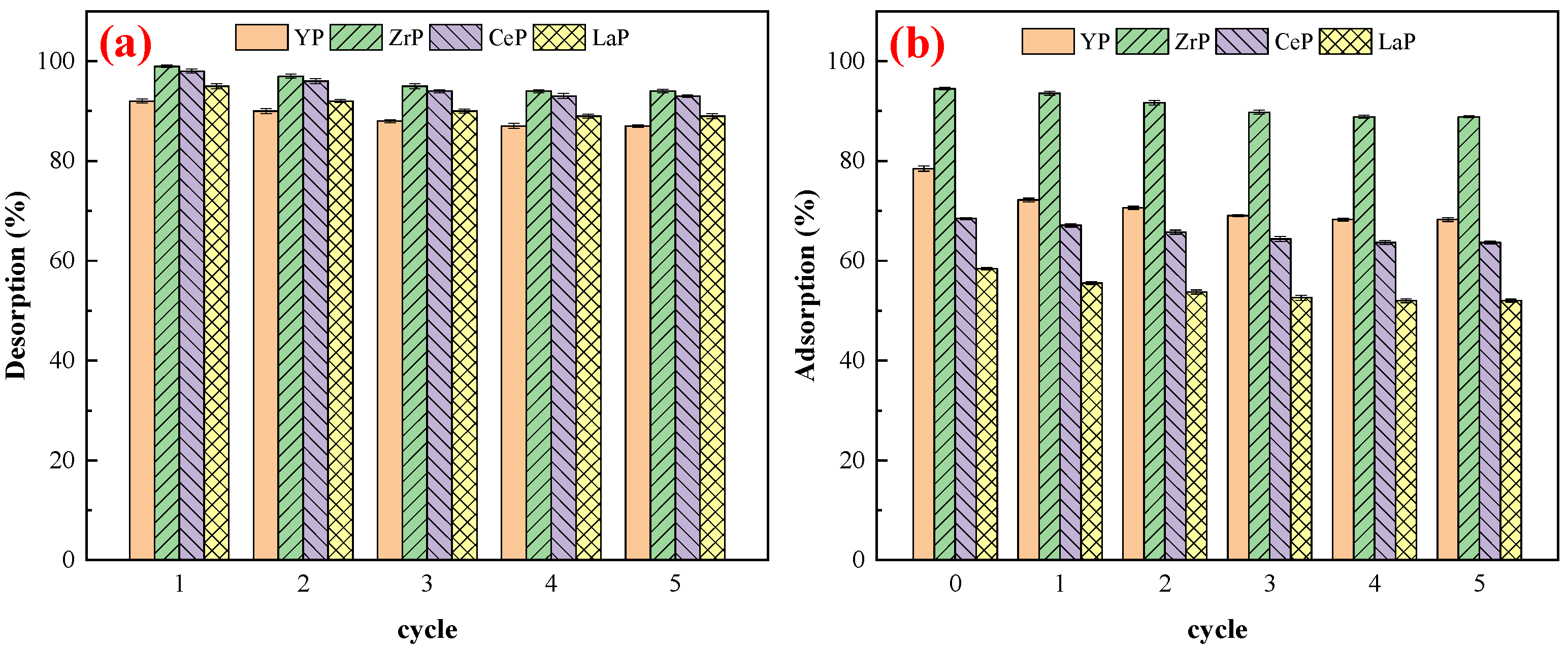
3.8. Adsorption–Desorption Cycles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solangi, I.B.; Memon, S.; Bhanger, M.I. An Excellent FLuoride Sorption Behavior of Modified Amberlite Resin. J. Hazard. Mater. 2010, 176, 186–192. [Google Scholar] [CrossRef]
- Lin, K.-Y.A. Adsorption of Fluoride to UiO-66-NH2 in Water: Stability, Kinetic, Isotherm and Thermodynamic Studies. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef]
- Li, R.; Tian, X.; Ashraf, I.; Chen, B. Fluoride Removal Using a Chelating Resin Containing Phosphonic-Sulfonic Acid Bifunctional Group. J. Chromatogr. A 2020, 1613, 460697. [Google Scholar] [CrossRef]
- Mumtaz, N.; Pandey, G.; Labhasetwar, P.K. Global Fluoride Occurrence, Available Technologies for Fluoride Removal, and Electrolytic Defluoridation: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2357–2389. [Google Scholar] [CrossRef]
- Ahmad, S.; Singh, R.; Arfin, T.; Neeti, K. Fluoride Contamination, Consequences and Removal Techniques in Water: A Review. Environ. Sci. Adv. 2022, 1, 620–661. [Google Scholar] [CrossRef]
- Arya, A.; Iqbal, M.; Yadav, V.; Agarwal, T.; Gawali, R.; Jana, S.K.; Datta, D. Fluoride Ion Removal Using Amine Modified Polymeric Resin: Batch and Column Studies. Mater. Today 2022, 57, 1626–1636. [Google Scholar] [CrossRef]
- Solangi, I.B.; Memon, S.; Bhanger, M.I. Removal of FLuoride from Aqueous Environment by Modified Amberlite Resin. J. Hazard. Mater. 2009, 171, 815–819. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Manousi, N.; Zachariadis, G.A.; Katsoyiannis, I.A.; Deliyanni, E.A. Recently Developed Adsorbing Materials for Fluoride Removal from Water and Fluoride Analytical Determination Techniques: A Review. Sustainability 2021, 13, 7061. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A. Removal of Fluoride and Uranium by Nanofiltration and Reverse Osmosis: A Review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, L.; Li, H.; Yan, W.; Feng, J. Synergistic Fluoride Adsorption by Composite Adsorbents Synthesized from Different Types of Materials—A Review. Front. Chem. 2022, 10, 900660. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, N. Development of Amine Functionalized Co-Polymeric Resins for Selective Fluoride Sorption. J. Fluor. Chem. 2013, 153, 143–150. [Google Scholar] [CrossRef]
- Raychoudhury, T.; Boindala, S.P.; Kalidindi, S. Performance Evaluation of Metal Impregnated Activated Carbon Composite for Removal of Fluoride under Varying Solution Chemistry. Water Supply 2017, 17, 1377–1385. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.; Aman, A.K.; Singh, R.K. Equilibrium Sorption of Fluoride on the Activated Alumina in Aqueous Solution. DWT 2020, 197, 224–236. [Google Scholar] [CrossRef]
- Rathnayake, A.; Hettithanthri, O.; Sandanayake, S.; Mahatantila, K.; Rajapaksha, A.U.; Vithanage, M. Essence of Hydroxyapatite in Defluoridation of Drinking Water: A Review. Environ. Pollut. 2022, 311, 119882. [Google Scholar] [CrossRef] [PubMed]
- Zare, K.; Banihashemi, A.; Javanbakht, V.; Mohammadifard, H. Fluoride Removal from Aqueous Solutions Using Alginate Beads Modified with Functionalized Silica Particles. J. Mol. Struct. 2022, 1252, 132217. [Google Scholar] [CrossRef]
- Karekar, J.M.; Katamble, R.T.; Divekar, S.V. Adsorption Studies of Fluoride on Weak Base Anion Exchangers and Surface Modified Strong Acid Cation Exchangers in Aqueous Medium. DWT 2020, 204, 356–365. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, F.; Yu, T.; Yan, Y.; Zhou, Z.; Liang, Y.; Wang, Y. Ultra-Rapid Fluoride Removal by Magnetic Calcium-Doped Layered Yttrium Hydroxides at Environmental-Relevant Concentrations. Chem. Eng. Sci. 2023, 280, 119035. [Google Scholar] [CrossRef]
- Xiao, D.; Jiang, C. The Unique Fluoride Sorption Behavior onto Nanosized Zirconium Oxides. Appl. Mech. Mater. 2014, 556–562, 209–212. [Google Scholar] [CrossRef]
- Nur, T.; Loganathan, P.; Nguyen, T.C.; Vigneswaran, S.; Singh, G.; Kandasamy, J. Batch and Column Adsorption and Desorption of Fluoride Using Hydrous Ferric Oxide: Solution Chemistry and Modeling. Chem. Eng. J. 2014, 247, 93–102. [Google Scholar] [CrossRef]
- Kang, D.; Yu, X.; Ge, M. Morphology-Dependent Properties and Adsorption Performance of CeO2 for Fluoride Removal. Chem. Eng. J. 2017, 330, 36–43. [Google Scholar] [CrossRef]
- Du, Y.; Qiu, S.; Zhang, X.; Nie, G. Nanoconfined Hydrous Titanium Oxides with Excellent Acid Stability for Selective and Efficient Removal of as(V) from Acidic Wastewater. Chem. Eng. J. 2020, 400, 125907. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Sun, M.; Paul Chen, J. Facile Synthesis of Highly Active Hydrated Yttrium Oxide towards Arsenate Adsorption. J. Colloid Interface Sci. 2016, 474, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, Y.; Li, W.; Gao, X.; Pan, B. Fluoride Uptake by Three Lanthanum Based Nanomaterials: Behavior and Mechanism Dependent upon Lanthanum Species. Sci. Total Environ. 2019, 683, 609–616. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Chen, J. Preparation of Nano-Cerium Phosphate and Its Adsorption Properties for Fluoride in Acidic Wastewater-All Databases. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/CSCD:7601798 (accessed on 12 November 2024).
- Zhang, Q.; Du, Q.; Jiao, T.; Pan, B.; Zhang, Z.; Sun, Q.; Wang, S.; Wang, T.; Gao, F. Selective Removal of Phosphate in Waters Using a Novel of Cation Adsorbent: Zirconium Phosphate (ZrP) Behavior and Mechanism. Chem. Eng. J. 2013, 221, 315–321. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Yang, L.; Luo, J. Synthesis and Characterization of a Reusable Layered Tin Titanium Phosphate for Removing Cu(II). J. Solid State Chem. 2022, 314, 123362. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, T.-J.; Jiang, Y.; Fok, J. Synthesis and Properties of a High-Capacity Iron Oxide Adsorbent for Fluoride Removal from Drinking Water. Appl. Surf. Sci. 2017, 425, 272–281. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Xie, Y.; Xie, W.; He, Z.; Zhong, H. A Critical Review on Adsorption and Recovery of Fluoride from Wastewater by Metal-Based Adsorbents. Environ. Sci. Pollut. Res. 2022, 29, 82740–82761. [Google Scholar] [CrossRef]
- Musa, N.; Allam, B.K.; Singh, N.B.; Banerjee, S. Investigation on Water Defluoridation via Batch and Continuous Mode Using Ce–Al Bimetallic Oxide: Adsorption Dynamics, Electrochemical and LCA Analysis. Environ. Pollut. 2023, 328, 121639. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Nagahashi, E.; Kadowaki, N.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Removal of Fluoride Using Magnesium and Iron Complex Hydroxides. Water Supply 2020, 20, 2815–2825. [Google Scholar] [CrossRef]
- Xie, X.; Gao, L. Effect of Crystal Structure on Adsorption Behaviors of Nanosized TiO2 for Heavy-Metal Cations. Curr. Appl. Phys. 2009, 9, S185–S188. [Google Scholar] [CrossRef]
- Sahu, S.; Mallik, L.; Pahi, S.; Barik, B.; Sahu, U.K.; Sillanpää, M.; Patel, R.K. Facile Synthesis of Poly O-Toluidine Modified Lanthanum Phosphate Nanocomposite as a Superior Adsorbent for Selective Fluoride Removal: A Mechanistic and Kinetic Study. Chemosphere 2020, 252, 126551. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A Zirconium-Based Nanoparticle: Essential Factors for Sustainable Application in Treatment of Fluoride Containing Water. J. Colloid Interface Sci. 2014, 416, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Modaresahmadi, K.; Khodadoust, A.P.; Wescott, J. Adsorption of Fluoride from Water Using Al–Mg–Ca Ternary Metal Oxide-Coated Sand. Water Supply 2023, 23, 4699–4713. [Google Scholar] [CrossRef]
- Turchi, M.; Galmarini, S.; Lunati, I. Amorphous Matters: Heterogeneity and Defects of Nanopore Silica Surfaces Enhance CO2 Adsorption. J. Non-Cryst. Solids 2024, 624, 122709. [Google Scholar] [CrossRef]
- Borgohain, X.; Boruah, A.; Sarma, G.K.; Rashid, M.H. Rapid and Extremely High Adsorption Performance of Porous MgO Nanostructures for Fluoride Removal from Water. J. Mol. Liq. 2020, 305, 112799. [Google Scholar] [CrossRef]
- Patnaik, P.C.; Swain, S.K.; Patel, S.B.; Patnaik, T.; Muller, F.; Delpeux-Ouldriane, S.; Das, S.; Dey, R.K. Kinetics and Thermodynamics of Defluoridation of Drinking Water Using High Performance Hybrid Zirconium (IV)-Hexamethylenediamine: A Comparative Aspect with Ion-Exchanger Amorphous Zirconium (IV) Phosphate. Surf. Interfaces 2018, 13, 22–32. [Google Scholar] [CrossRef]
- Dong, H.; Tang, H.; Shi, X.; Yang, W.; Chen, W.; Li, H.; Zhao, Y.; Zhang, Z.; Hua, M. Enhanced Fluoride Removal from Water by Nanosized Cerium Oxides Impregnated Porous Polystyrene Anion Exchanger. Chemosphere 2022, 287, 131932. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, F.; Xu, C.; Gao, J.; Chen, D.; Li, A. Enhanced Removal of Cu(II) and Ni(II) from Saline Solution by Novel Dual-Primary-Amine Chelating Resin Based on Anion-Synergism. J. Hazard. Mater. 2015, 287, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xie, Y.; Liu, L.; Li, D.; Guan, F. Nature of humic acid and its application progress. Appl. Chem. 2023, 52, 3418–3422, 3427. [Google Scholar]
- Zhou, Z.; Xiaoyan, L.; Jin, Q.; Jing, W.; Xuegang, L. Porous Zirconium Alginate Beads Adsorbent for Fluoride Adsorption from Aqueous Solutions. RSC Adv. 2014, 5, 2100–2112. [Google Scholar] [CrossRef]
- Deng, H.; Yu, X. Adsorption of Fluoride, Arsenate and Phosphate in Aqueous Solution by Cerium Impregnated Fibrous Protein. Chem. Eng. J. 2012, 184, 205–212. [Google Scholar] [CrossRef]
- Sihag, S.; Pal, J. Synthesis and Characterization of Zinc Oxide Nanocomposite for Fluoride Ion Removal from Aqueous Solution. Environ. Monit. Assess. 2023, 195, 1205. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Mohan, D.; Pittman, C.U.; Yang, S. Remediating Fluoride from Water Using Hydrous Zirconium Oxide. Chem. Eng. J. 2012, 198–199, 236–245. [Google Scholar] [CrossRef]
- He, J.; Cui, A.; Ni, F.; Deng, S.; Shen, F.; Yang, G. A Novel 3D Yttrium Based-Graphene Oxide-Sodium Alginate Hydrogel for Remarkable Adsorption of Fluoride from Water. J. Colloid Interface Sci. 2018, 531, 37–46. [Google Scholar] [CrossRef]
- He, C.; Sun, Y.; Gu, Y.; Ji, H. Enhanced Fluoride Removal Using Mg-Zr Binary Metal Oxide Nanoparticles Confined in a Strong-Base Anion Exchanger. Chemosphere 2024, 358, 141980. [Google Scholar] [CrossRef]
- Fabregat, V.; Pagán, J.M. Technical–Economic Feasibility of a New Method of Adsorbent Materials and Advanced Oxidation Techniques to Remove Emerging Pollutants in Treated Wastewater. Water 2024, 16, 814. [Google Scholar] [CrossRef]
- Biswas, K.; Bandhoyapadhyay, D.; Ghosh, U.C. Adsorption Kinetics of Fluoride on Iron(III)-Zirconium(IV) Hybrid Oxide. Adsorption 2007, 13, 83–94. [Google Scholar] [CrossRef]
- Kumar, E.; Bhatnagar, A.; Ji, M.; Jung, W.; Lee, S.-H.; Kim, S.-J.; Lee, G.; Song, H.; Choi, J.-Y.; Yang, J.-S.; et al. Defluoridation from Aqueous Solutions by Granular Ferric Hydroxide (GFH). Water Res. 2009, 43, 490–498. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, Y.; Wang, H.; Wang, T.; Wang, Y. Performance of Granular Zirconium–Iron Oxide in the Removal of Fluoride from Drinking Water. Water Res. 2011, 45, 3571–3578. [Google Scholar] [CrossRef]
- Wang, M.; Yu, X.; Yang, C.; Yang, X.; Lin, M.; Guan, L.; Ge, M. Removal of Fluoride from Aqueous Solution by Mg-Al-Zr Triple-Metal Composite. Chem. Eng. J. 2017, 322, 246–253. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Sharma, A.K.; Philip, L. Manganese-Oxide-Coated Alumina: A Promising Sorbent for Defluoridation of Water. Water Res. 2006, 40, 3497–3506. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, N.; Yang, G.; Tian, L.; Wang, R. Removal of Fluoride Ions from Aqueous Solution by the Calcination Product of Mg–Al–Fe Hydrotalcite-like Compound. Desalination 2011, 268, 20–26. [Google Scholar] [CrossRef]
- Vences-Alvarez, E.; Velazquez-Jimenez, L.H.; Chazaro-Ruiz, L.F.; Diaz-Flores, P.E.; Rangel-Mendez, J.R. Fluoride Removal in Water by a Hybrid Adsorbent Lanthanum–Carbon. J. Colloid Interface Sci. 2015, 455, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Xu, X.; Gao, B.; Yue, Q. Highly Selective and Efficient Removal of Fluoride from Aqueous Solution by ZrLa Dual-Metal Hydroxide Anchored Bio-Sorbents. J. Clean. Prod. 2018, 199, 36–46. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.-S.; He, J.-Y.; Xu, W.-H.; Huang, X.-J.; Liu, J.-H. Enhanced Fluoride Removal from Water by Sulfate-Doped Hydroxyapatite Hierarchical Hollow Microspheres. Chem. Eng. J. 2016, 285, 616–624. [Google Scholar] [CrossRef]
- Minju, N.; Venkat Swaroop, K.; Haribabu, K.; Sivasubramanian, V.; Senthil Kumar, P. Removal of Fluoride from Aqueous Media by Magnesium Oxide-Coated Nanoparticles. Desalin. Water Treat. 2015, 53, 2905–2914. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Gu, Y.; Ma, M.; Sun, Y. Evaluation of Fluoride Adsorptive Removal by Metallic Phosphates. Appl. Sci. 2025, 15, 10454. https://doi.org/10.3390/app151910454
Wang R, Gu Y, Ma M, Sun Y. Evaluation of Fluoride Adsorptive Removal by Metallic Phosphates. Applied Sciences. 2025; 15(19):10454. https://doi.org/10.3390/app151910454
Chicago/Turabian StyleWang, Ruijie, Yingpeng Gu, Mengfei Ma, and Yue Sun. 2025. "Evaluation of Fluoride Adsorptive Removal by Metallic Phosphates" Applied Sciences 15, no. 19: 10454. https://doi.org/10.3390/app151910454
APA StyleWang, R., Gu, Y., Ma, M., & Sun, Y. (2025). Evaluation of Fluoride Adsorptive Removal by Metallic Phosphates. Applied Sciences, 15(19), 10454. https://doi.org/10.3390/app151910454








