Determinants of Quality of Life in Older Adults: The Role of Sarcopenia, Physical Fitness, and Lifestyle Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic Characteristics
2.2.2. Quality of Life (SarQoL)
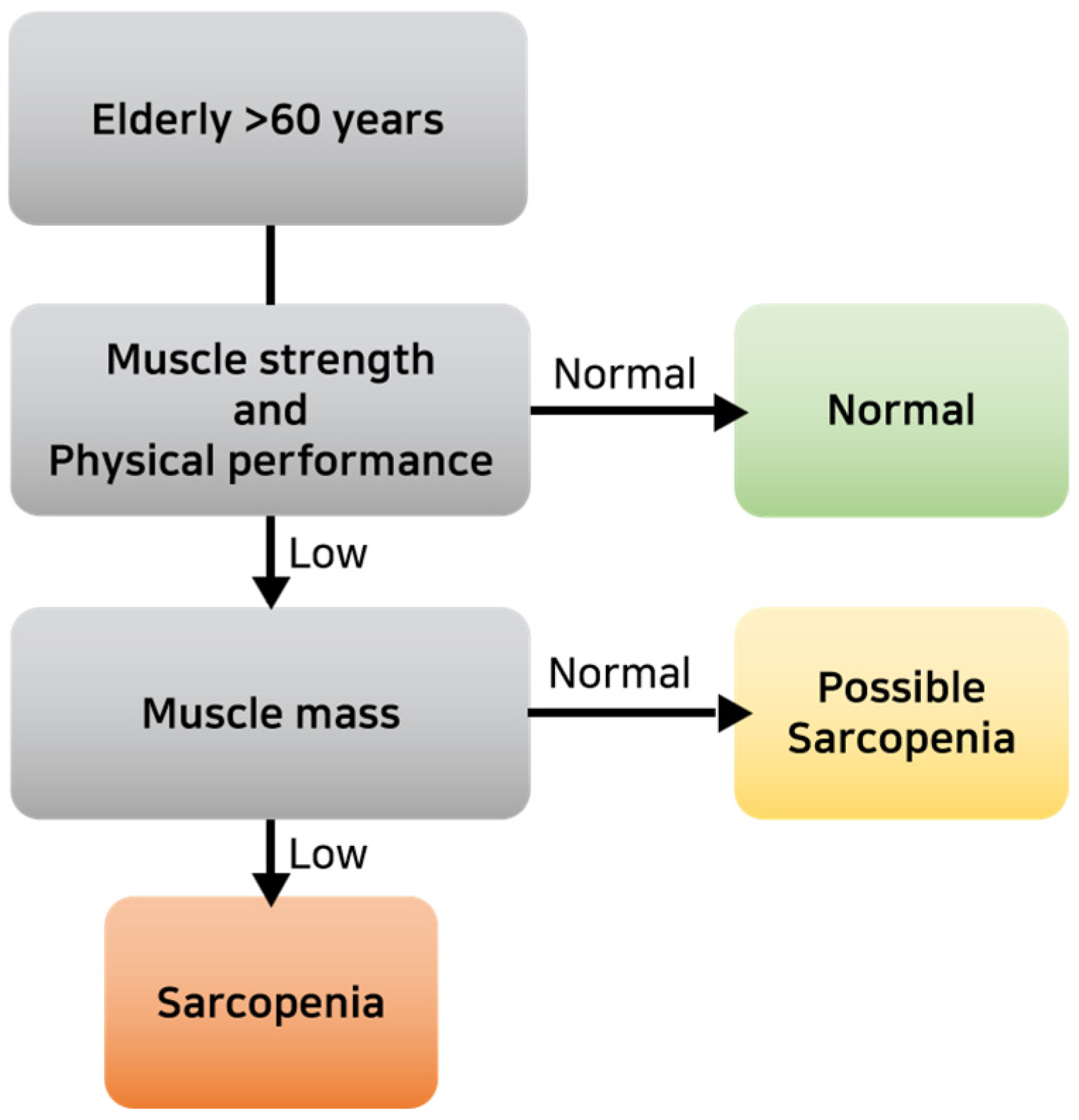
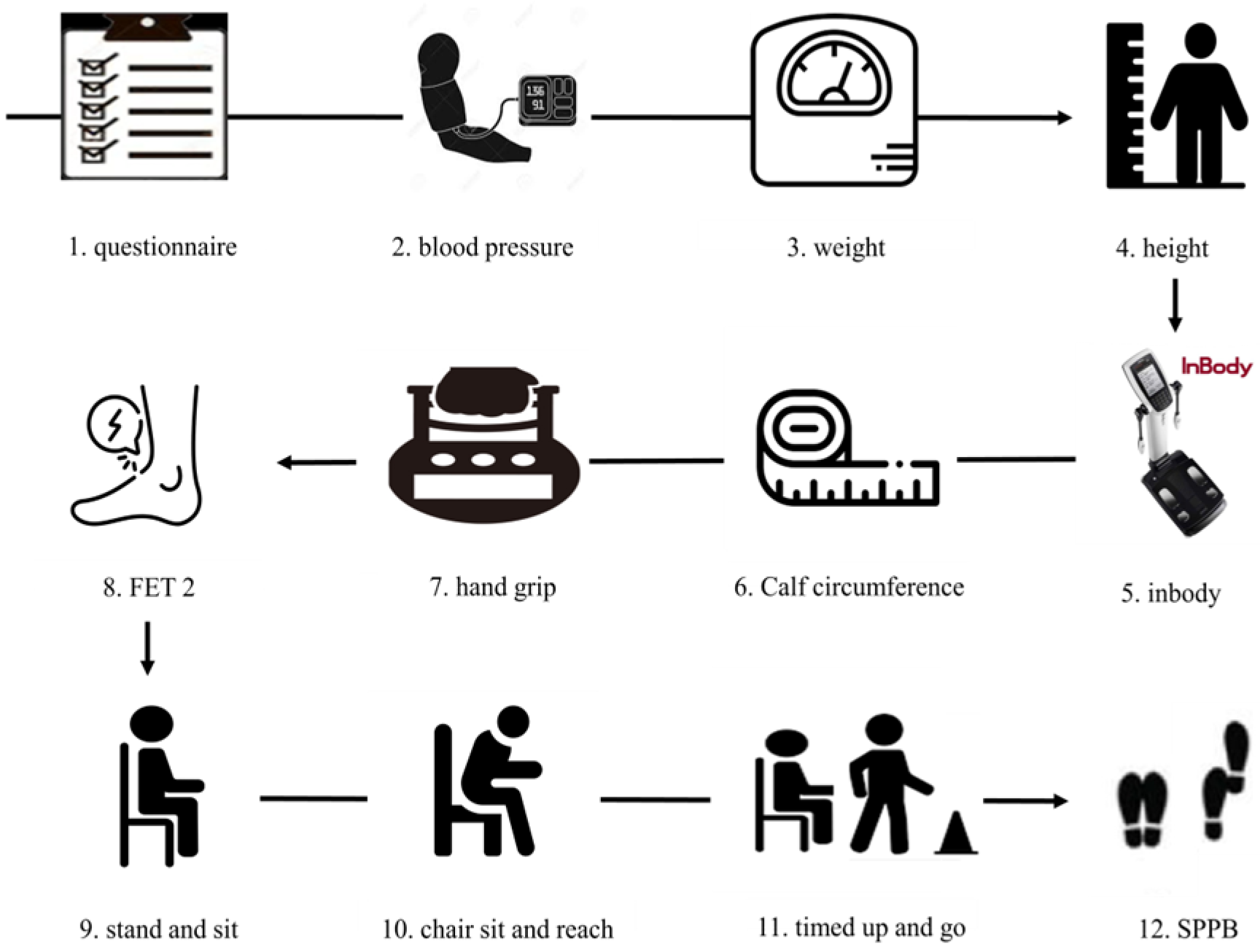
2.2.3. Measurement of Body Composition and Physical Fitness Factors
- Hand grip strength (HG): A hand dynamometer (Takei Scientific Instruments Co. Ltd., Tokyo, Japan) was used for HG measurement. Participants received a brief demonstration and verbal instructions for the test. Adjustments were made as needed based on participants’ hand size. Measurements were taken with the participant standing with arms at their sides and elbows fully extended. Measurements were taken twice alternately without an intermediate rest period. Participants were instructed to exert maximum force for 3 s during each measurement. The maximum value was utilized for data analysis [15,16].
- Plantar and Dorsi flexion: Isometric muscle strength measurement of the ankle was performed using a modified method reported by Li et al. [17]. The microFET®2 (Hoggan Scientific, LLC, Salt Lake City, UT, USA) was placed in close contact with the sole or top of the foot. Participants then applied force in the sole or top direction for approximately 5 s upon the start signal. Resistance was applied by the examiner to prevent knee flexion or rebound.
- Stand and sit: Participants sit on a chair without armrests (approximately 43 cm/17 inches high) and secure themselves against the wall for stability. Arms were crossed and held in front of the chest, maintaining a straight back. Using the arms to stand up was counted as “0 times.” The number of times the movement was performed correctly was counted. The participant repeated the action of sitting in the chair for 30 s and then standing up completely, and the number of times they stood up completely was measured. Additionally, even if the participant was only partially standing by the end of the 30 s, it was counted as one attempt [15].
- Timed up and go (TUG): Participants were instructed to stand up from an armless chair (height 46 cm), walk 3 m, turn direction at a cone-shaped marker, then walk back and sit down. They were told to walk at their normal pace, regardless of whether they wore shoes. Time was measured from when the participant’s buttocks left the chair upon standing until they returned to a seated position and their buttocks touched the seat. The test was performed consecutively twice, and the average of the millisecond scores was used for further analysis [20].
- Short Physical Performance Battery (SPPB): The SPPB consists of three tests: the standing balance test, the 4 m walk test, and the 5-time chair stand test. In the standing balance test, participants received 4 points if they could maintain each of the following positions for 10 s: feet together (1 point), semi-tandem (1 point), and tandem (2 points). For the 4 m walk test, participants were allowed two attempts to walk 4 m at their normal pace, with the shorter time recorded as their score. The use of assistive devices was permitted during the walking test. The five-time chair rise test scored the time taken to rise five times as quickly as possible with arms crossed over the chest. The total SPPB score ranged from 0 to 12 points [21,22].
2.3. Statistical Analysis
3. Results
3.1. SarQoL Quatiles and Participant Characteristics
3.2. Determinants of SarQoL
3.3. Physical Fitness and SarQoL
3.4. SarQoL Stages and Physical Fitness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SarQoL | Sarcopenia & quality of life |
| HRQoL | Health-related quality of life |
| PCA | Principal component analysis |
| SPPB | Short Physical Performance Battery |
| microFET 2 | Force Evaluation and Testing (FET) |
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 4, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Demonceau, C.; Reginster, J.Y.; Locquet, M.; Cesari, M.; Cruz Jentoft, A.J.; Bruyère, O. Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Fonfría-Vivas, R.; Pérez-Ros, P.; Barrachina-Igual, J.; Pablos-Monzó, A.; Martínez-Arnau, F.M. Assessing quality of life with SarQol is useful in screening for sarcopenia and sarcopenic obesity in older women. Aging Clin. Exp. Res. 2023, 35, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Kull, M.; Kallikorm, R.; Lember, M. Impact of a new sarco-osteopenia definition on health-related quality of life in a population-based cohort in northern Europe. J. Clin. Densitom. 2012, 15, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Reginster, J.Y.; Amuthavalli, T.J.; Bautmans, I.; Bauer, J.; Burlet, N.; Cesari, M.; Cherubini, A.; Cooper, C.; Cruz-Jentoft, A.J.; et al. Measuring health-related quality of life in sarcopenia: Summary of the SarQoL psychometric properties. Aging Clin. Exp. Res. 2023, 35, 1581–1593. [Google Scholar] [CrossRef]
- Beaudart, C.; Biver, E.; Reginster, J.Y.; Rizzoli, R.; Rolland, Y.; Bautmans, I.; Petermans, J.; Gillain, S.; Buckinx, F.; Dardenne, N.; et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for sarcopenia. J. Cachexia Sarcopenia Muscle 2017, 8, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, M.V.; Sandoval-Hernández, I.; Galán-Mercant, A.; Gonzalez-Sanchez, M.; Martínez-Cal, J.; Molina-Torres, G. Analysis of structural characteristics and psychometric properties of the SarQoL® questionnaire in different languages: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 4561. [Google Scholar] [CrossRef] [PubMed]
- Geerinck, A.; Dawson-Hughes, B.; Beaudart, C.; Locquet, M.; Reginster, J.Y.; Bruyère, O. Assessment of the performance of the SarQoL® questionnaire in screening for sarcopenia in older people. Aging Clin. Exp. Res. 2021, 33, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Guillamón-Escudero, C.; Diago-Galmés, A.; Zuazua Rico, D.; Maestro-González, A.; Tenías-Burillo, J.M.; Soriano, J.M.; Fernández-Garrido, J.J. SarQoL questionnaire in community-dwelling older adults under EWGSOP2 sarcopenia diagnosis algorithm: A new screening method? Int. J. Environ. Res. Public Health 2022, 19, 8473. [Google Scholar] [CrossRef]
- Bahat, G.; Yilmaz, O.; Kılıç, C.; Oren, M.M.; Karan, M.A. Performance of SARC-F in regard to sarcopenia definitions, muscle mass and functional measures. J. Nutr. Health Aging 2018, 22, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Ha, Y.C.; Kim, M.; Seo, S.H.; Kim, M.J.; Lee, G.Y.; Seo, Y.M.; Sung, C.; Park, K.S. Translation and validation of the Korean version of the sarcopenia Quality of Life (SarQoL-K®) questionnaire and applicability with the SARC-F screening tool. Qual. Life Res. 2021, 30, 603–611. [Google Scholar] [CrossRef]
- National Physical Strength Awards. Available online: https://nfa.kspo.or.kr/reserve/4/selectMeasureItemListByAgeSe.kspo (accessed on 15 March 2024).
- Trajković, N.; Rančić, D.; Ilić, T.; Herodek, R.; Korobeynikov, G.; Pekas, D. Measuring handgrip strength in school children: Inter-instrument reliability between Takei and Jamar. Sci. Rep. 2024, 14, 1074. [Google Scholar] [CrossRef]
- Li, R.C.; Jasiewicz, J.M.; Middleton, J.; Condie, P.; Barriskill, A.; Hebnes, H.; Purcell, B. The development, validity, and reliability of a manual muscle testing device with integrated limb position sensors. Arch. Phys. Med. Rehabil. 2006, 87, 411–417. [Google Scholar] [CrossRef]
- Jones, C.J.; Rikli, R.E.; Max, J.; Noffal, G. The reliability and validity of a chair sit-and-reach test as a measure of hamstring flexibility in older adults. Res. Q. Exerc. Sport 1998, 69, 338–343. [Google Scholar] [CrossRef]
- Bhattacharya, P.K.; Deka, K.; Roy, A.G. Assessment of inter-rater variability of the Senior Fitness Test in the geriatric population: A community based study. Int. J. Biomed. Adv. Res. 2016, 7, 208–212. [Google Scholar] [CrossRef]
- Ibrahim, A.; Singh, D.K.A.; Shahar, S. ‘Timed Up and Go’ test: Age, gender and cognitive impairment stratified normative values of older adults. PLoS ONE 2017, 12, e0185641. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.T.; Song, C.Y. Reliability and minimal detectable change of the Short Physical Performance Battery in older adults with mild cognitive impairment. Geriatr. Nurs. 2024, 57, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Hamer, M.; Molloy, G.J. Association of C-reactive protein and muscle strength in the English Longitudinal Study of Ageing. AGE 2009, 31, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Goss, A.M.; Locher, J.L.; Ard, J.D.; Gower, B.A. Physical function and strength in relation to inflammation in older adults with obesity and increased cardiometabolic risk. J. Nutr. Health Aging 2019, 23, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor necrosis factor-alpha: Role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Pinkney, J.H.; Stehouwer, C.D.; Coppack, S.W.; Yudkin, J.S. Endothelial dysfunction: Cause of the insulin resistance syndrome. Diabetes 1997, 46 (Suppl. S2), S9–S13. [Google Scholar] [CrossRef]
- Cooper, A.; Lamb, M.; Sharp, S.J.; Simmons, R.K.; Griffin, S.J. Bidirectional association between physical activity and muscular strength in older adults: Results from the UK Biobank study. Int. J. Epidemiol. 2017, 46, 141–148. [Google Scholar] [CrossRef]
- Go, S.W.; Cha, Y.H.; Lee, J.A.; Park, H.S. Association between sarcopenia, bone density, and health-related quality of life in Korean men. Korean J. Fam. Med. 2013, 34, 281–288. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Kutty, V.R.; Lanas, F.; Hui, C.; Quanyong, X.; Zhenzhen, Q.; Jinhua, T.; Noorhassim, I.; et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: A prospective urban rural epidemiologic (PURE) study. J. Cachexia Sarcopenia Muscle 2016, 7, 535–546. [Google Scholar] [CrossRef]
- Sreedhar, A.; Zhao, Y. Uncoupling protein 2 and metabolic diseases. Mitochondrion 2017, 34, 135–140. [Google Scholar] [CrossRef]
| Variables | 1 (n = 44) | 2 (n = 193) | 3 (n = 211) | 4 (n = 103) | Total (n = 551) | F | p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ±or % | Mean | ±or % | Mean | ±or % | Mean | ±or % | Mean | ±or % | |||||
| Age (years) | 82.27 | 5.78 | 78.85 | 7.78 | 74.55 | 7.26 | 72.76 | 7.77 | 76.35 | 7.98 | 28.574 | <0.001 *** | ||
| Sex | Male | 7 | 15.9 | 32 | 16.6 | 56 | 26.5 | 34 | 33.0 | 129 | 23.4 | 4.036 | 0.001 *** | |
| Female | 37 | 84.1 | 161 | 83.4 | 155 | 73.5 | 69 | 67.0 | 422 | 76.6 | ||||
| Height (cm) | 150.28 | 8.30 | 152.68 | 7.46 | 156.29 | 8.07 | 158.39 | 7.74 | 154.94 | 8.20 | 19.198 | <0.001 *** | ||
| Weight (kg) | 56.26 | 9.30 | 58.23 | 9.69 | 58.91 | 9.13 | 60.39 | 9.80 | 58.74 | 9.50 | 2.224 | 0.008 ** | ||
| Appendicular Skeletal Muscle (kg) | 6.09 | 1.43 | 6.34 | 1.34 | 6.66 | 1.38 | 6.89 | 1.49 | 6.54 | 1.41 | 5.410 | <0.001 *** | ||
| Body fat mass (%) | 20.66 | 7.01 | 21.66 | 7.26 | 19.43 | 6.06 | 19.01 | 6.02 | 20.21 | 6.64 | 5.140 | <0.001 *** | ||
| Body Mass Index (kg/m2) | 25.16 | 4.26 | 25.15 | 4.16 | 24.12 | 3.13 | 23.98 | 3.16 | 24.54 | 3.65 | 4.003 | 0.001 *** | ||
| Diabetes | Yes | 16 | 36.4 | 60 | 31.1 | 51 | 24.2 | 18 | 17.5 | 145 | 26.3 | 3.246 | 0.002 ** | |
| No | 28 | 63.6 | 133 | 68.9 | 160 | 75.8 | 85 | 82.5 | 406 | 73.7 | ||||
| Hyperlipidemia | Yes | 11 | 25.0 | 66 | 34.2 | 76 | 36.0 | 32 | 31.1 | 185 | 33.5 | 0.695 | 0.056 | |
| No | 33 | 75.0 | 127 | 65.8 | 135 | 64.0 | 71 | 68.9 | 366 | 66.5 | ||||
| Hypertension | Yes | 25 | 56.8 | 104 | 53.9 | 108 | 51.2 | 40 | 38.8 | 277 | 50.2 | 2.481 | 0.006 ** | |
| No | 19 | 43.2 | 89 | 46.1 | 103 | 48.8 | 63 | 61.2 | 274 | 49.8 | ||||
| Sleep disorder | Yes | 19 | 43.2 | 81 | 42.0 | 75 | 35.5 | 19 | 18.4 | 194 | 35.2 | 6.070 | <0.001 *** | |
| No | 25 | 56.8 | 112 | 58.0 | 136 | 64.5 | 84 | 81.6 | 357 | 64.8 | ||||
| Sarcopenia | Yes | 40 | 90.9 | 138 | 71.5 | 93 | 43.1 | 30 | 29.1 | 344 | 60.6 | 38.838 | <0.001 *** | |
| No | 4 | 9.1 | 55 | 28.5 | 118 | 55.9 | 73 | 70.9 | 207 | 39.4 | ||||
| SarQoL | S Total | 31.33 | 4.15 | 48.99 | 6.40 | 69.08 | 5.80 | 86.97 | 4.84 | 62.37 | 17.42 | 1501.662 | <0.001 *** | |
| Independent Variable | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | B | β | t | p | ||
| A | (Constant) | 5.111 | 12.896 | <0.001 *** | 4.536 | 10.953 | <0.001 *** | ||
| Sex | 0.200 | 0.098 | 2.233 | 0.026 * | 0.098 | 0.048 | 1.090 | 0.276 | |
| Age | −0.031 | −0.296 | −6.574 | <0.001 *** | −0.020 | −0.185 | −3.746 | <0.001 *** | |
| Education level | 0.110 | 0.144 | 3.067 | 0.002 ** | 0.095 | 0.125 | 2.729 | 0.007 ** | |
| RPA | −0.258 | −0.146 | −3.532 | <0.001 *** | −0.220 | −0.125 | −3.110 | 0.002 ** | |
| Diabetes | −0.208 | −0.110 | −2.717 | 0.007 ** | |||||
| Hyperlipidemia | 0.034 | 0.019 | 0.451 | 0.652 | |||||
| Hypertension | −0.027 | −0.016 | −0.380 | 0.704 | |||||
| Sleep disorder | −0.235 | −0.133 | −3.327 | 0.001 *** | |||||
| Sarcopenia | −0.179 | −0.216 | −4.519 | <0.001 *** | |||||
| F (p) | 29.245 (<0.001 ***) | 19.061 (<0.001 ***) | |||||||
| R2 | 0.194 | 0.263 | |||||||
| adjR2 | 0.188 | 0.249 | |||||||
| Independent Variable | Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | B | β | t | p | B | β | t | p | ||
| A | (Constant) | 4.762 | 11.508 | <0.001 *** | 6.485 | 3.109 | 0.002 ** | 4.114 | 2.011 | 0.045 * | |||
| Sex | 0.147 | 0.074 | 1.647 | 0.100 | −0.059 | −0.03 | −0.458 | 0.647 | 0.131 | 0.065 | 1.004 | 0.316 | |
| Age | −0.022 | −0.216 | −4.269 | <0.001 *** | −0.018 | −0.175 | −3.283 | 0.001 *** | −0.011 | −0.104 | −1.973 | 0.049 * | |
| Education level | 0.076 | 0.102 | 2.211 | 0.028 * | 0.048 | 0.065 | 1.373 | 0.170 | 0.009 | 0.012 | 0.260 | 0.795 | |
| RPA | −0.191 | −0.109 | −2.658 | 0.008 ** | −0.162 | −0.092 | −2.263 | 0.024 * | −0.122 | −0.070 | −1.758 | 0.079 | |
| Diabetes | −0.219 | −0.118 | −2.936 | 0.003 ** | −0.208 | −0.112 | −2.76 | 0.006 ** | −0.182 | −0.099 | −2.509 | 0.012 * | |
| Sleep disorder | −0.245 | −0.141 | −3.473 | 0.001 *** | −0.218 | −0.125 | −3.084 | 0.002 ** | −0.196 | −0.113 | −2.891 | 0.004 ** | |
| Sarcopenia | −0.168 | −0.204 | −4.210 | <0.001 *** | −0.175 | −0.213 | −4.071 | <0.001 *** | −0.086 | −0.105 | −1.983 | 0.048 * | |
| Height | −0.016 | −0.152 | −1.196 | 0.232 | −0.005 | −0.049 | −0.392 | 0.695 | |||||
| Weight | 0.033 | 0.381 | 2.020 | 0.044 * | 0.014 | 0.163 | 0.879 | 0.380 | |||||
| Body Fat Mass | −0.023 | −0.177 | −1.788 | 0.074 | −0.010 | −0.082 | −0.844 | 0.399 | |||||
| Body Mass Index | −0.064 | −0.278 | −1.654 | 0.099 | −0.034 | −0.147 | −0.899 | 0.369 | |||||
| Calf Circumference | 0.015 | 0.056 | 0.787 | 0.431 | 0.018 | 0.067 | 0.976 | 0.330 | |||||
| Timed up and go | −0.022 | −0.155 | −3.214 | 0.001 *** | |||||||||
| Chair sit and reach | 0.009 | 0.136 | 2.888 | 0.004 ** | |||||||||
| Plantar flexion | 0.019 | 0.118 | 2.307 | 0.022 * | |||||||||
| F (p) | 23.591 (<0.001 ***) | 15.235 (<0.001 ***) | 15.890 (<0.001 ***) | ||||||||||
| R2 | 0.264 | 0.287 | 0.345 | ||||||||||
| adjR2 | 0.253 | 0.268 | 0.324 | ||||||||||
| Variables Category | Exp(B) | OR (95% CI) | p | ||
|---|---|---|---|---|---|
| Hand grip | 1 | Reference | |||
| 2 | 0.996 | 0.923 | 1.075 | 0.918 | |
| 3 | 1.046 | 0.964 | 1.134 | 0.279 | |
| 4 | 1.093 | 1.002 | 1.193 | 0.045 * | |
| Dorsal flexion | 1 | Reference | |||
| 2 | 1.06 | 0.936 | 1.201 | 0.359 | |
| 3 | 1.154 | 1.012 | 1.316 | 0.032 * | |
| 4 | 1.212 | 1.053 | 1.396 | 0.007 ** | |
| Stand and sit | 1 | Reference | |||
| 2 | 0.992 | 0.917 | 1.073 | 0.843 | |
| 3 | 1.051 | 0.956 | 1.155 | 0.305 | |
| 4 | 1.034 | 0.916 | 1.166 | 0.591 | |
| Chair sit and reach | 1 | Reference | |||
| 2 | 1.004 | 0.972 | 1.037 | 0.808 | |
| 3 | 1.014 | 0.979 | 1.05 | 0.444 | |
| 4 | 1.041 | 1.001 | 1.083 | 0.045 * | |
| Timed up and go | 1 | Reference | |||
| 2 | 1.006 | 0.949 | 1.066 | 0.844 | |
| 3 | 0.943 | 0.865 | 1.029 | 0.444 | |
| 4 | 0.873 | 0.757 | 1.007 | 0.062 | |
| SPPB | 1 | Reference | |||
| 2 | 1.330 | 1.143 | 1.549 | <0.001 *** | |
| 3 | 1.609 | 1.32 | 1.961 | <0.001 *** | |
| 4 | 1.671 | 1.259 | 2.217 | <0.001 *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.-Y.; Roh, S.-Y.; Lee, M.J.; Kim, J. Determinants of Quality of Life in Older Adults: The Role of Sarcopenia, Physical Fitness, and Lifestyle Factors. Appl. Sci. 2025, 15, 10423. https://doi.org/10.3390/app151910423
Sung J-Y, Roh S-Y, Lee MJ, Kim J. Determinants of Quality of Life in Older Adults: The Role of Sarcopenia, Physical Fitness, and Lifestyle Factors. Applied Sciences. 2025; 15(19):10423. https://doi.org/10.3390/app151910423
Chicago/Turabian StyleSung, Jun-Young, Su-Yeon Roh, Moon Jin Lee, and Jiyoun Kim. 2025. "Determinants of Quality of Life in Older Adults: The Role of Sarcopenia, Physical Fitness, and Lifestyle Factors" Applied Sciences 15, no. 19: 10423. https://doi.org/10.3390/app151910423
APA StyleSung, J.-Y., Roh, S.-Y., Lee, M. J., & Kim, J. (2025). Determinants of Quality of Life in Older Adults: The Role of Sarcopenia, Physical Fitness, and Lifestyle Factors. Applied Sciences, 15(19), 10423. https://doi.org/10.3390/app151910423






