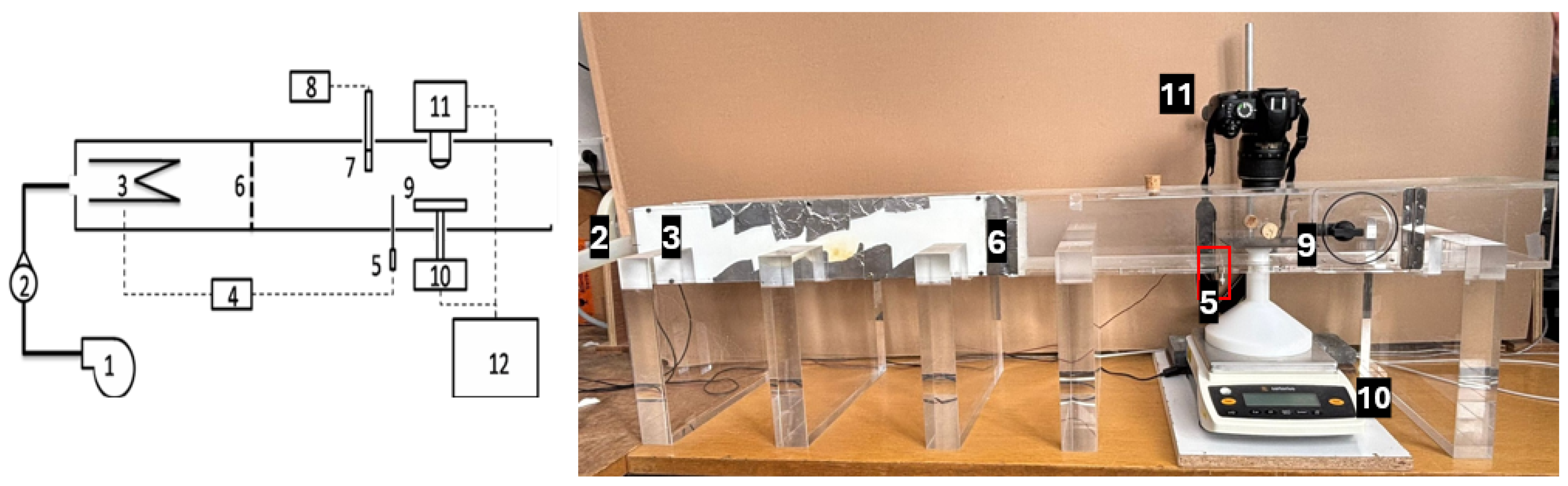
1. Introduction
Tofu is a traditional Chinese food product made from curdled soy milk. Its consumption has become widespread due to its nutritional value, health benefits, and suitability as an alternative to animal protein [
1,
2,
3,
4]. Tofu exists in a wide variety of forms, differentiated by water content. Silken tofu, also known as soft tofu, has a water content of about 87–90%, while firm tofu is pressed to reduce its moisture content, resulting in a more compact matrix with a water content of about 67–81% [
5]. Finally, leisure-dried tofu is a reprocessed form of tofu that is dried before marinating to enhance its flavor [
6]. Its production usually involves an extended drying phase of three hours at an elevated temperature of around 85 °C [
7].
Drying is, therefore, a possible transformation process in the production and consumption of tofu. It occurs both in domestic cooking processes, like oven cooking, where it is one of the key processes [
8], and in industrial settings like the production of leisure-dried tofu [
7]. In particular, leisure-dried tofu has become increasingly popular on the market due to its taste, ready-to-eat attributes, and nutritious advantages [
6,
7,
9]. Drying tofu changes its flavor and texture, creating a product that is crispy yet tender, and extends storage time by reducing the risk of spoilage [
10]. Therefore, studying the drying kinetics of tofu has direct industrial applications.
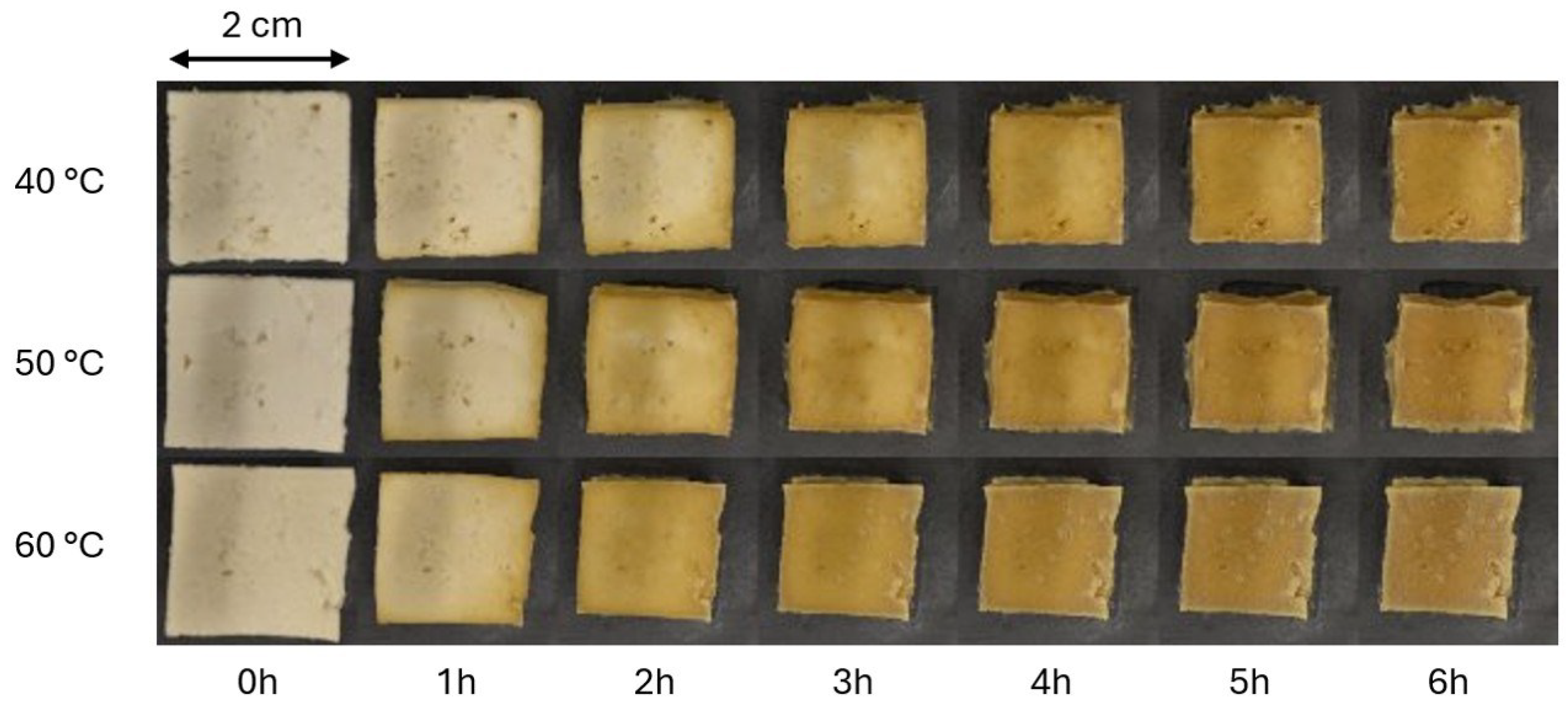
While water content is a key factor in tofu texture, it also influences other physical properties, such as color and shape [
11]. Significant water loss inevitably leads to shrinkage and impacts the product’s coloration [
12,
13]. Since color and shape influence consumer acceptance [
12], further examination of their relationship to the drying process is necessary.
Baik and Mittal [
14] investigated the thermal properties of tofu as a function of water content, but the drying process itself has not been studied. The drying kinetics of soybean grains [
15] and okara (a byproduct of soy milk and tofu production) [
16,
17,
18] have been studied and characterized. Several empirical models, such as Page, Henderson, and Thompson, have been tested for soybean drying and have produced satisfactory results [
15]. For okara, both empirical models [
16] and physical models (heat and mass transfer [
18]) have been successfully tested. While the drying kinetics of other soy-related products have been studied, this has not been the case for tofu itself.
In this study, the experimental results were compared to three types of models: simple empirical models [
19], a shrinking-core model (SK) [
20], and a newly developed phenomenological model for oven-cooking processes (the cooking model). Mathematical modeling of tofu drying kinetics serves multiple purposes. First, it helps us understand and predict the physical mechanisms involved in the drying process. Second, the two physics-based models examined here provide meaningful parameters that can inform process design [
20,
21]. Finally, this study evaluates a newly developed model for oven cooking. By linking drying dynamics to cooking processes through mathematical modeling, this study sheds light on the key mechanisms that influence cooking performance [
22].
Therefore, the objective of this work is to characterize the drying kinetics of tofu at different temperatures using experimental analysis and modeling. Changes in shape and color during the drying process were also examined.
4. Discussion
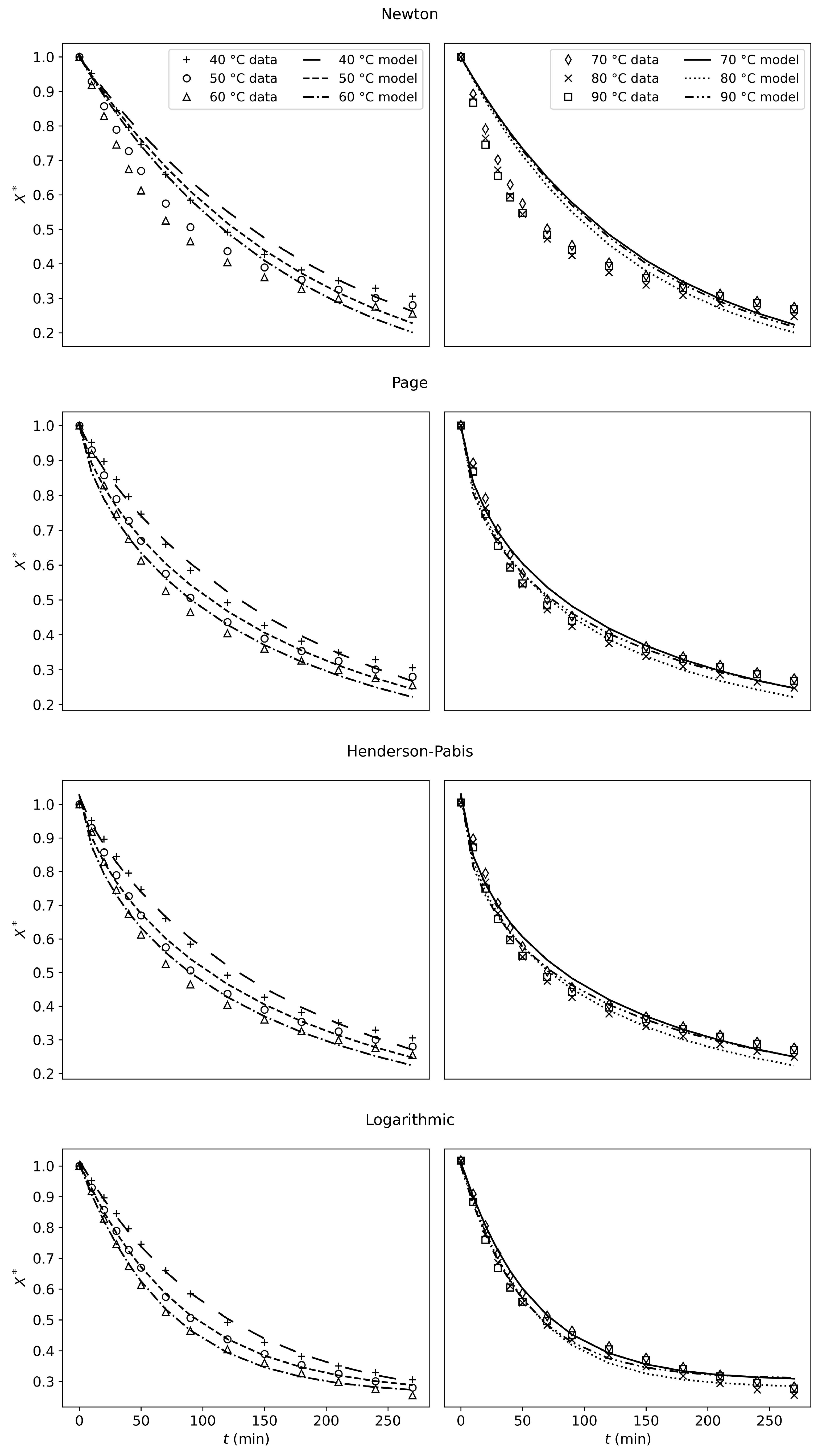
The modeling results show that the Newton model can only capture one linear drying mechanism, which is insufficient for describing the drying kinetics of tofu. This model lacks physical validity and should not be used to describe tofu-drying kinetics.
The Page, Henderson–Pabis, and Logarithmic models show good results and fit the experimental data well.
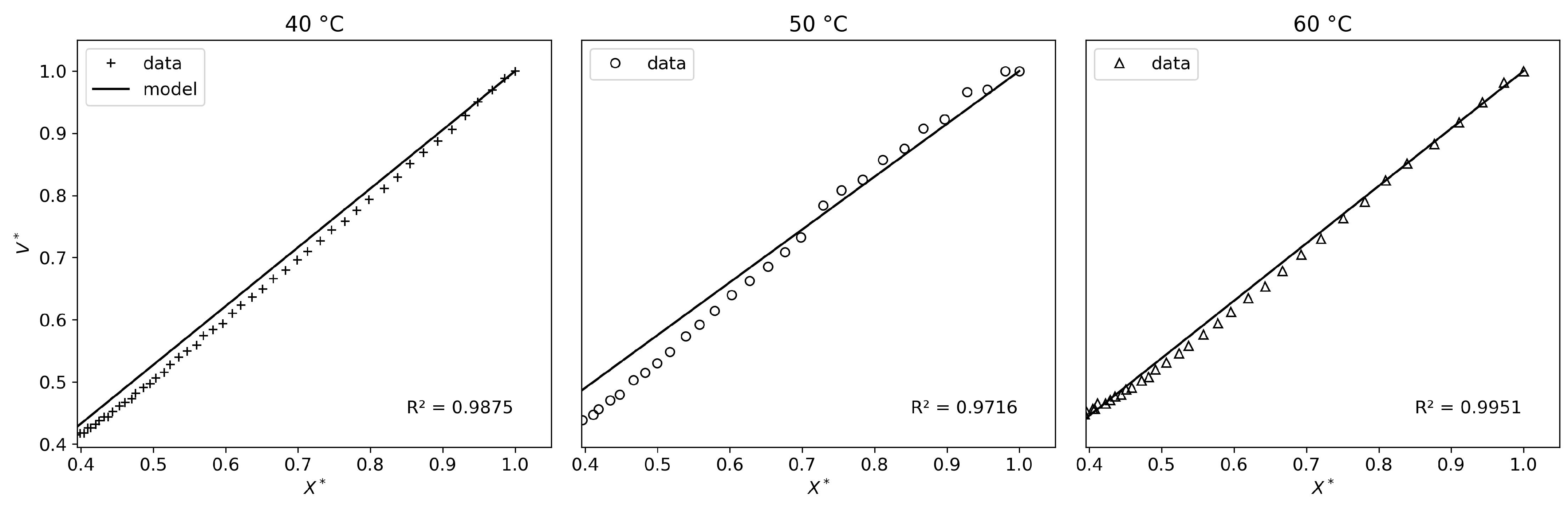
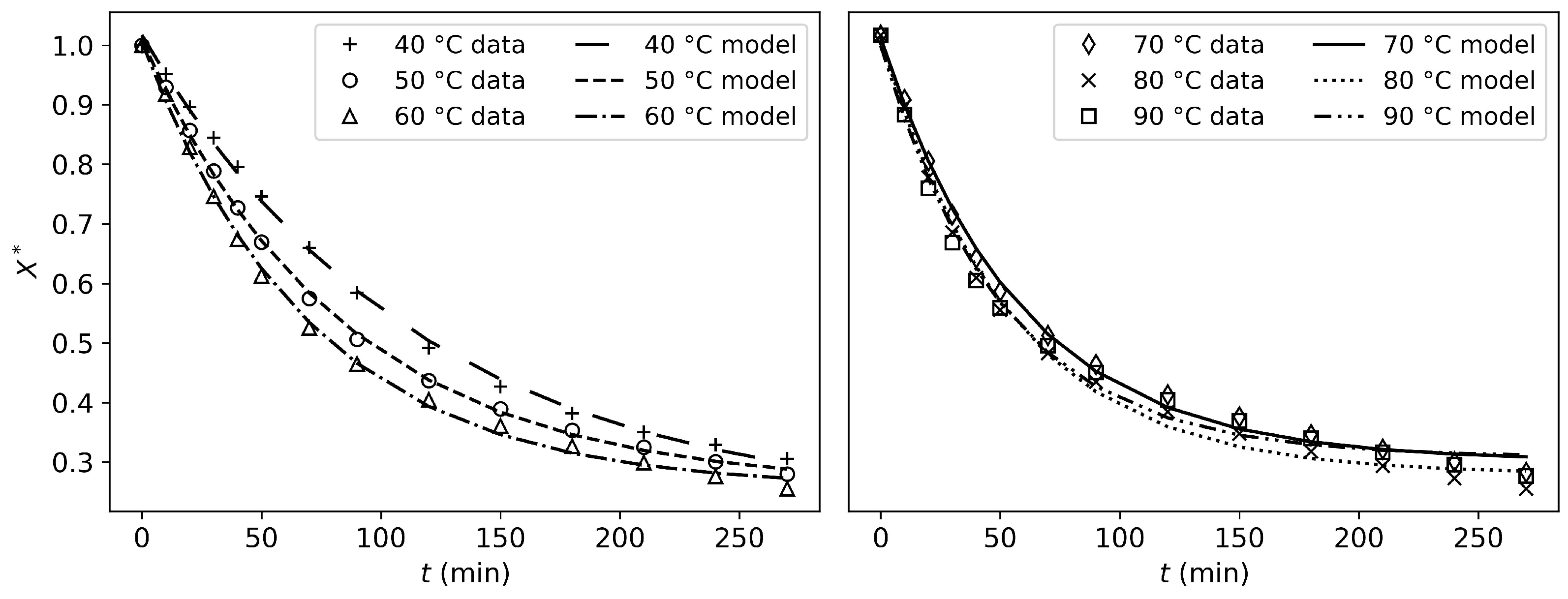
As shown in
Figure 8, the shrinking-core model presents good results. Although the mean criteria values (R
2 and RMSE) are no better than those of the Newton model, the drying mechanisms are captured much more accurately. This makes sense, as the model is capable of predicting two distinct drying periods (constant-rate and falling-rate). However, while both distinct periods are captured, the fitting accuracy is not satisfactory for all experimental conditions. Specifically, from 70 °C to 90 °C, the model poorly describes the falling-rate period, compensating by prematurely halting evaporation (i.e., the drying rate
j suddenly drops to zero, which has no physical validity). Therefore, this model could be used for control and optimization if the parameters were adapted to each experimental condition. However, it has no predictive or explanatory power, as fitting the parameters to all experimental conditions simultaneously does not produce satisfactory results for each condition, despite an overall satisfactory R
2.
The obtained value for
kSK is of the same order of magnitude as the value obtained for
kHMT using literature correlations (see
Table 2 and
Table 3). In this model, porosity and tortuosity are considered constant, meaning that shrinkage is not taken into account. Porosity was calculated for the initial volume of 2 cm
3 and was found to be
, which is quite high but still within the range observed for other porous food products in the literature [
37]. High porosity was also observed through visual examination of the sample, which means that tofu is a product with low structure. This explains why the product collapses significantly. From the calculated porosity and the fitted
, tortuosity could be deduced, which was equal to
. This value is quite high, but it is within the range of tortuosity determined for bread [
38]. Finally, the effective diffusivity
Deff calculated from the fitting results for the shrinking-core model ranged from
to
. This is near the expected range for food [
36]. Overall, the fitted parameters obtained for the shrinking core model are consistent with literature data.
The cooking model results in the best fit to the experimental data
j. This is confirmed by the good results of the R
2 and RMSE values and by
Figure 8, where it can be seen that the drying kinetics are captured very well. Compared to the shrinking-core model, this model’s particularity is that it can predict a complex, multi-phasic drying process without breaking the model down into two periods.
Although the model is physics-based, some expressions remain empirical. The fitted parameters
A,
B, and
Z have no physical meaning. However, these parameters allow us to calculate the effective diffusivity
Deff. The calculated values of
Deff fall outside the expected range for food products at the beginning of the drying process, but progressively approach the expected range as the process continues (see
Appendix G) [
36]. This is due to the capillary term that is taken into account in the expression of
Deff (Equation (
18)). Initially, water transport is heavily influenced by capillary transport, which progressively diminishes throughout the process. Next,
KBET and
XBET have physical interpretations.
KBET reflects the strength of the interaction between the adsorbate and the surface. The fitted value for this constant is within the expected range for food, with a value of 26.76 [
31].
XBET is the monolayer capacity. The fitted value (
) is about an order of magnitude higher than values reported in the literature for other food products [
31]. Therefore, the suitability of the BET model to estimate the water content in the cooking model could be discussed, and other models could be used to replace this relationship. For example, the GAB model [
31] could be used instead. Finally, the value obtained for the thermal conductivity of the dry product (
W/m/K) allows for the calculation of the thermal conductivity of the product before drying (
at
); the obtained value is
, which is consistent with values reported in the literature for other food products [
39].
These fitting results demonstrate that the empirical, the shrinking-core, and the cooking models each have their own strengths and weaknesses.
Except for the Newton model, the empirical models fit the experimental data well. However, they lack physical meaning, and they have no predictive or explanatory power. Unlike the physical models, these models cannot fit the drying rate j.
Although the shrinking-core model shows weaker fitting results, it has only two fitted parameters. This is a significant advantage compared to the cooking model, which has six fitted parameters.
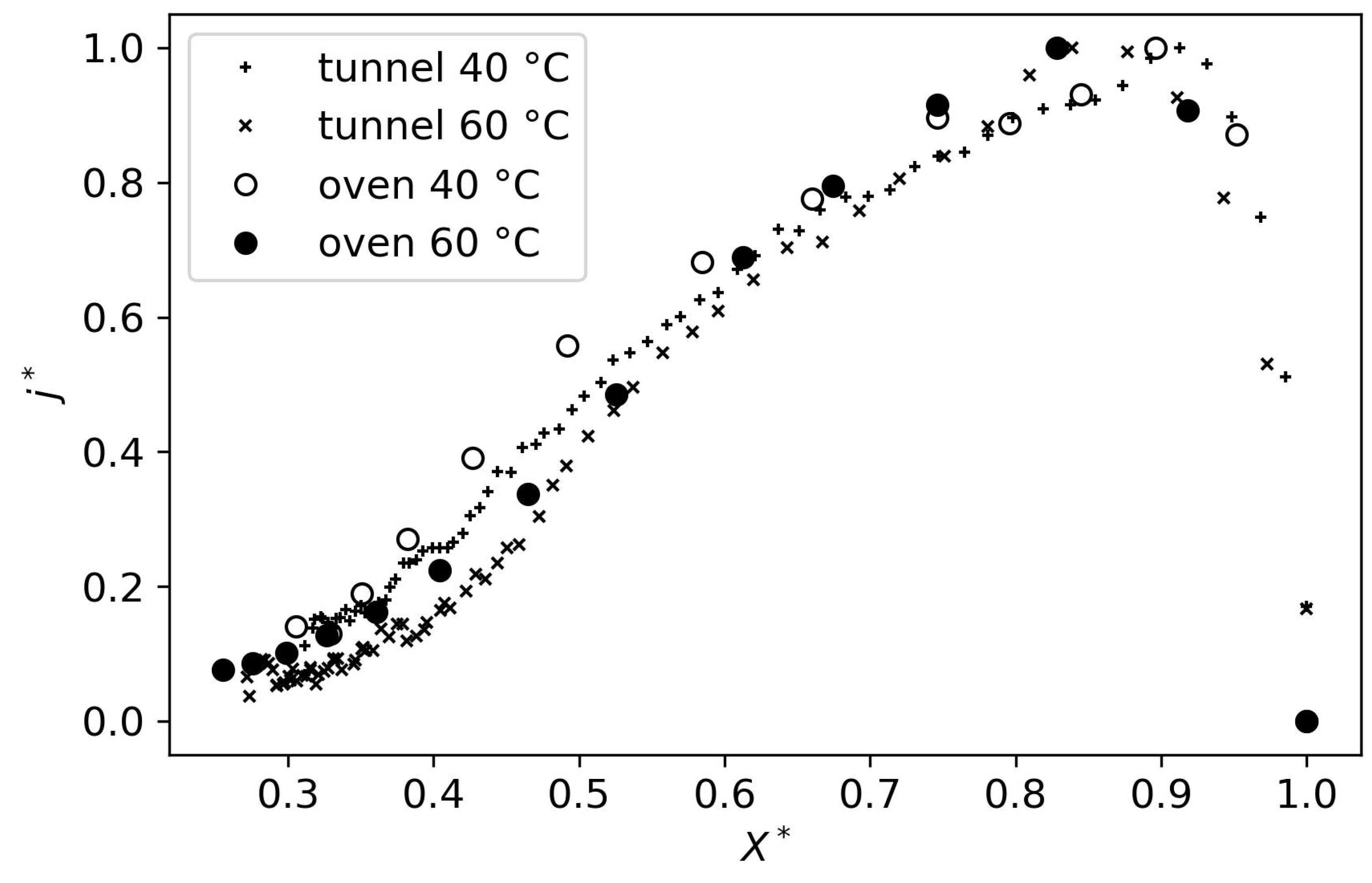
It is important to note that both physical models underwent a stringent fitting procedure. Only one set of parameters was adjusted to fit all experimental conditions. Moreover, the experimental data used for the fitting procedure consisted of the drying rate values
j, to ensure that all the drying mechanisms were well captured. Even though fitting on
X would have yielded better results, some physical phenomena, such as the distinct drying periods, are less pronounced in this data. This is why it was chosen to fit on
j. Even with this strict fitting procedure, the physical models can compete with the results of the empirical models (
Table 4).
Shrinkage is not taken into account in either of the models. Regarding experimental results, it would be a considerable improvement to take it into account for simulations.
5. Conclusions
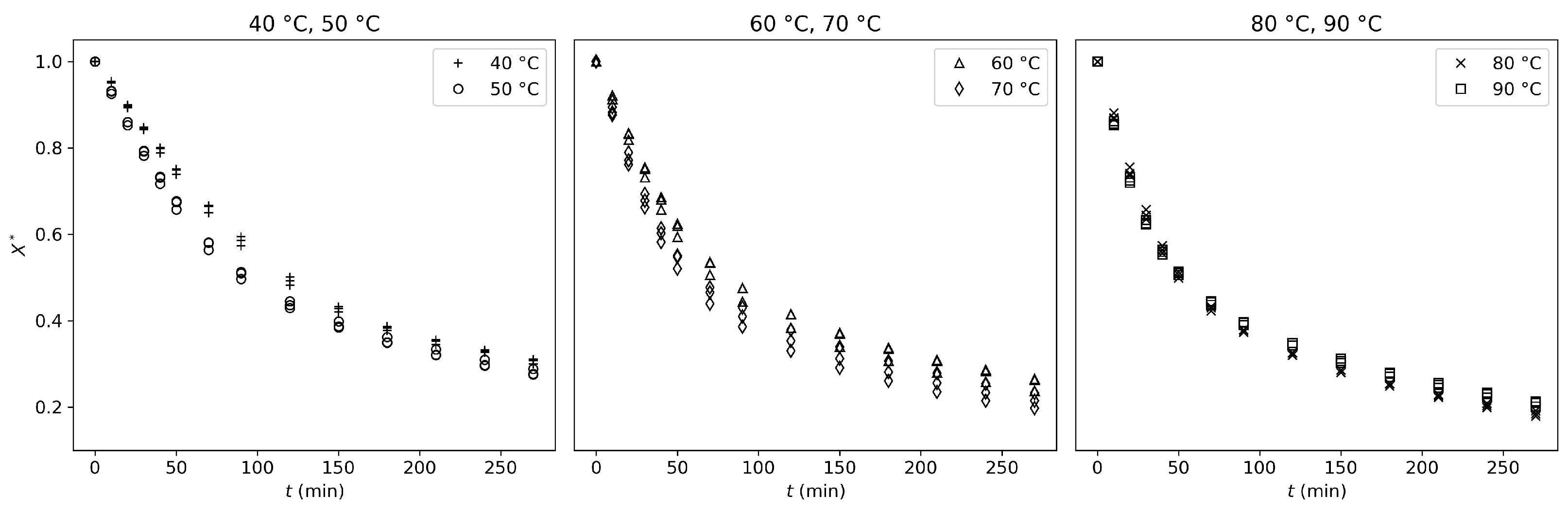
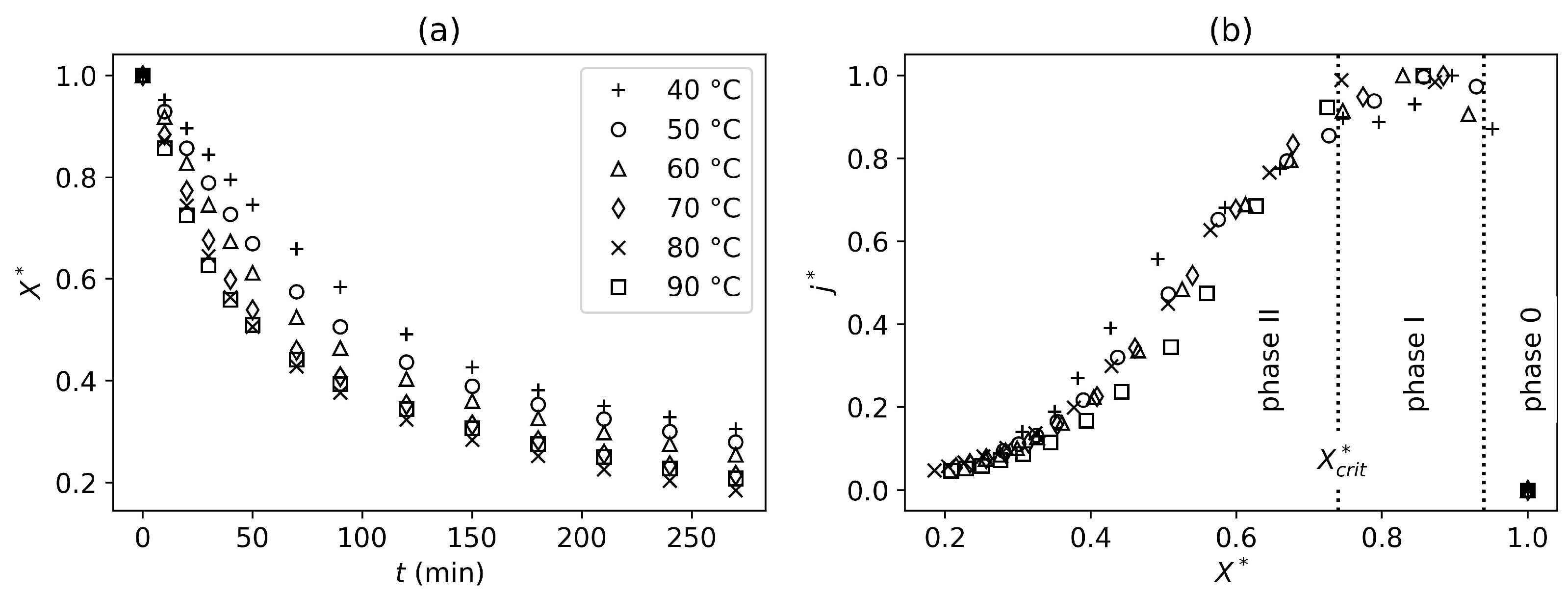
In this study, the convective drying behavior of tofu samples was investigated across a range of drying temperatures (40–90 °C), using both an oven and a tunnel dryer. Experimental analysis of the tofu-drying process showed that it can be described as a sequence of different mechanisms (drying phases I and II separated by
). These mechanisms are consistent across all studied temperatures, with higher temperatures resulting in faster drying.
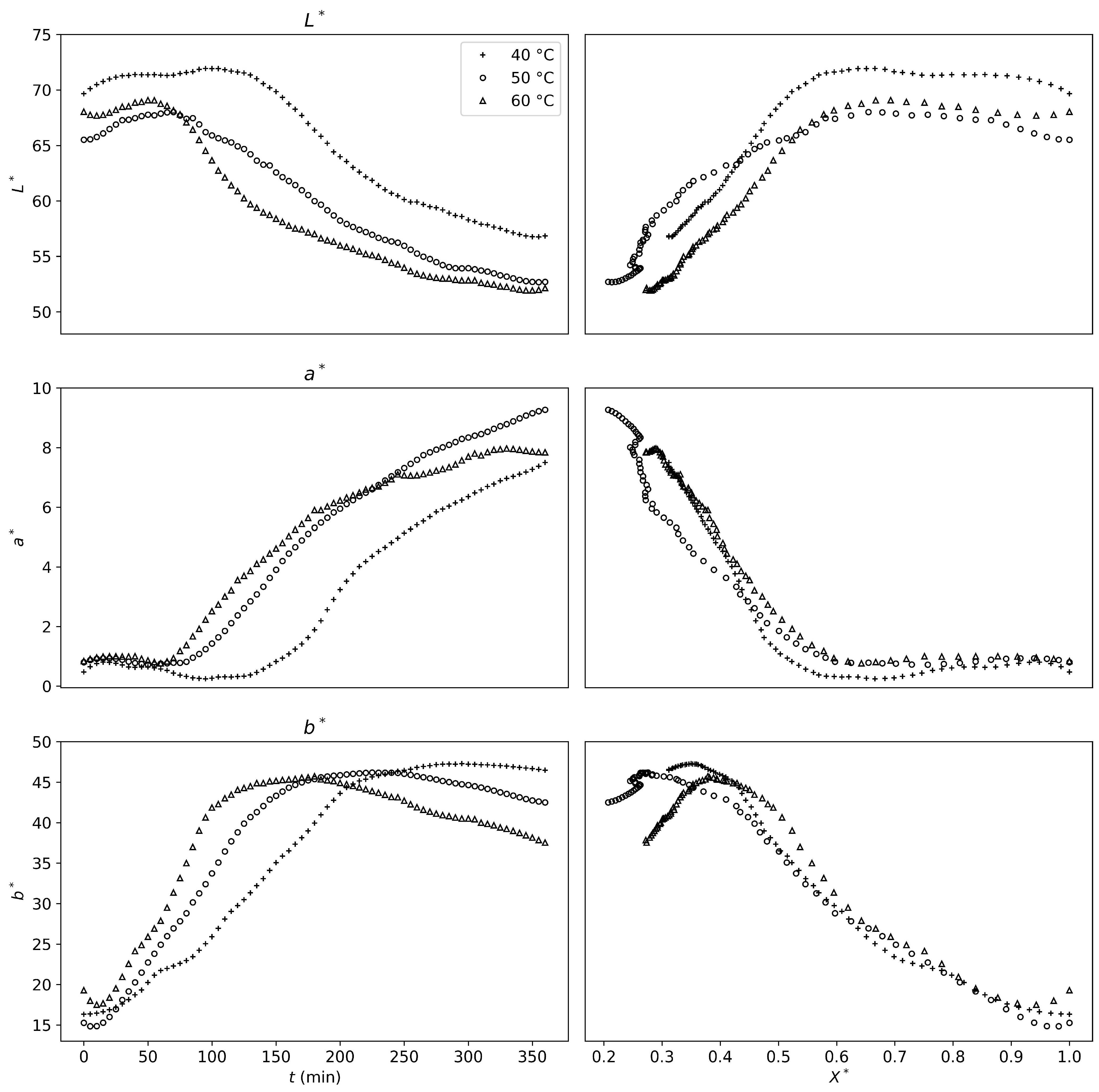
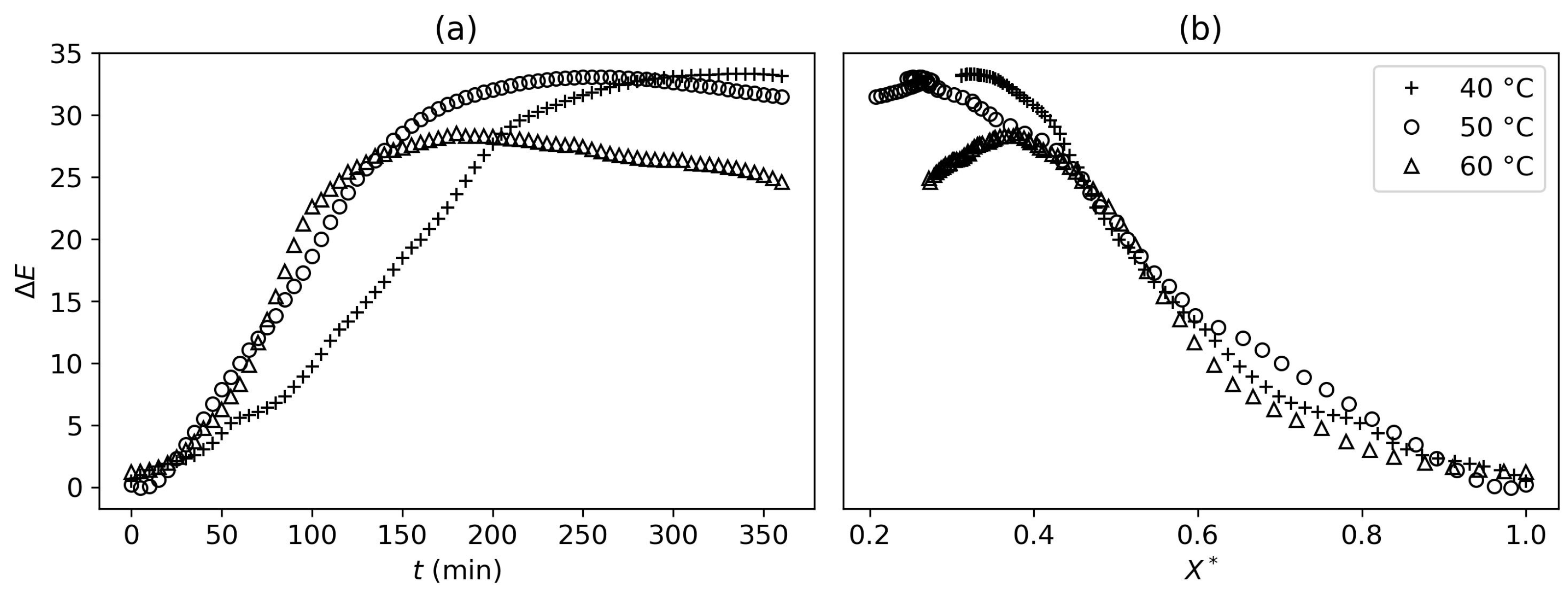
Drying further, a second threshold could be defined at
, based on product shrinkage. Until that point, the shrinkage mechanisms were not temperature-dependent. However, once this threshold was reached, shrinkage became temperature-dependent and was more significant for lower drying temperatures (
Figure 5). Similar observations were made for non-enzymatic browning at a very similar threshold (
Figure 6).
It can be concluded that a faster drying process will reduce physical alterations of the product if the desired final water content is below
. Therefore, higher drying temperatures should be prioritized to avoid these alterations.
Six models were assessed against the experimental data. The Newton model failed to adequately represent the drying behavior of tofu. The Logarithmic, Henderson–Pabis, and Page models showed good fitting results but lack physical validity. In contrast, both the shrinking core and the cooking models showed good capacity to predict the drying rate, while capturing the physical mechanisms occurring during the drying process. While the shrinking-core model requires separate fits for each experimental condition to properly capture the drying processes, it remains useful due to its simplicity, as it relies on only two fitting parameters. The newly developed cooking model delivered more accurate predictions (). It successfully captured the various drying periods without segmenting the process into distinct rate expressions. This offers a more comprehensive and adaptable approach despite its reliance on six adjustable parameters.
Future research should evaluate the accuracy of the cooking model for larger-scale drying processes. Additionally, since the model was accurate for oven-drying processes, it should be tested at higher temperatures to replicate cooking conditions. Taking physical variations, especially shrinkage, into account would also be a considerable improvement. Further study of the nutritional and structural changes in tofu during drying is also required.