Naked-Eye Detection of Water Contaminants Enabled by Engineered Plasmonic Resonance in Hybrid Detector Systems
Abstract
Featured Application
Abstract
1. Introduction
2. Methods and Theory
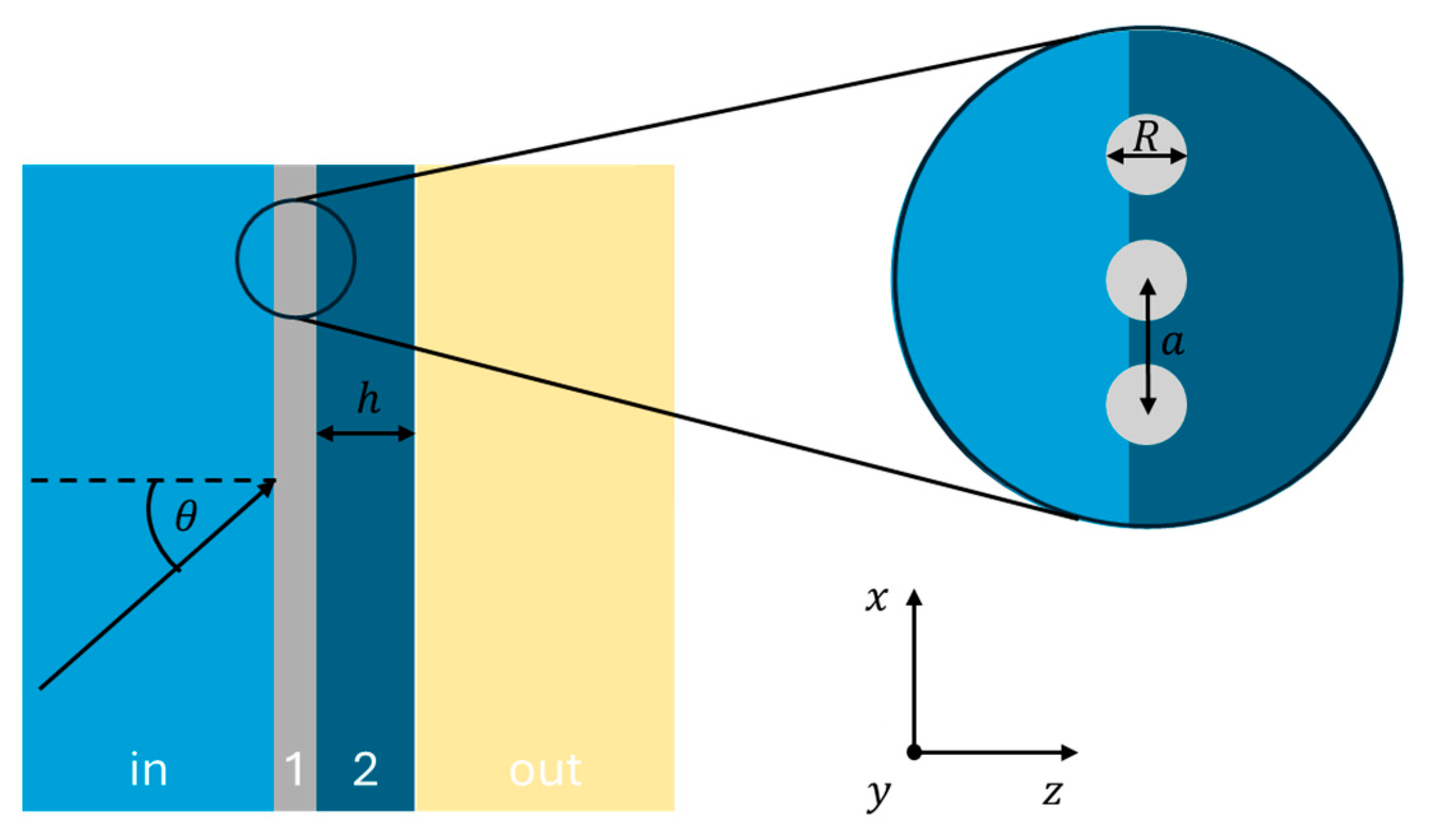
2.1. Optical Model of the Considered System
2.2. Colorimetric Analysis
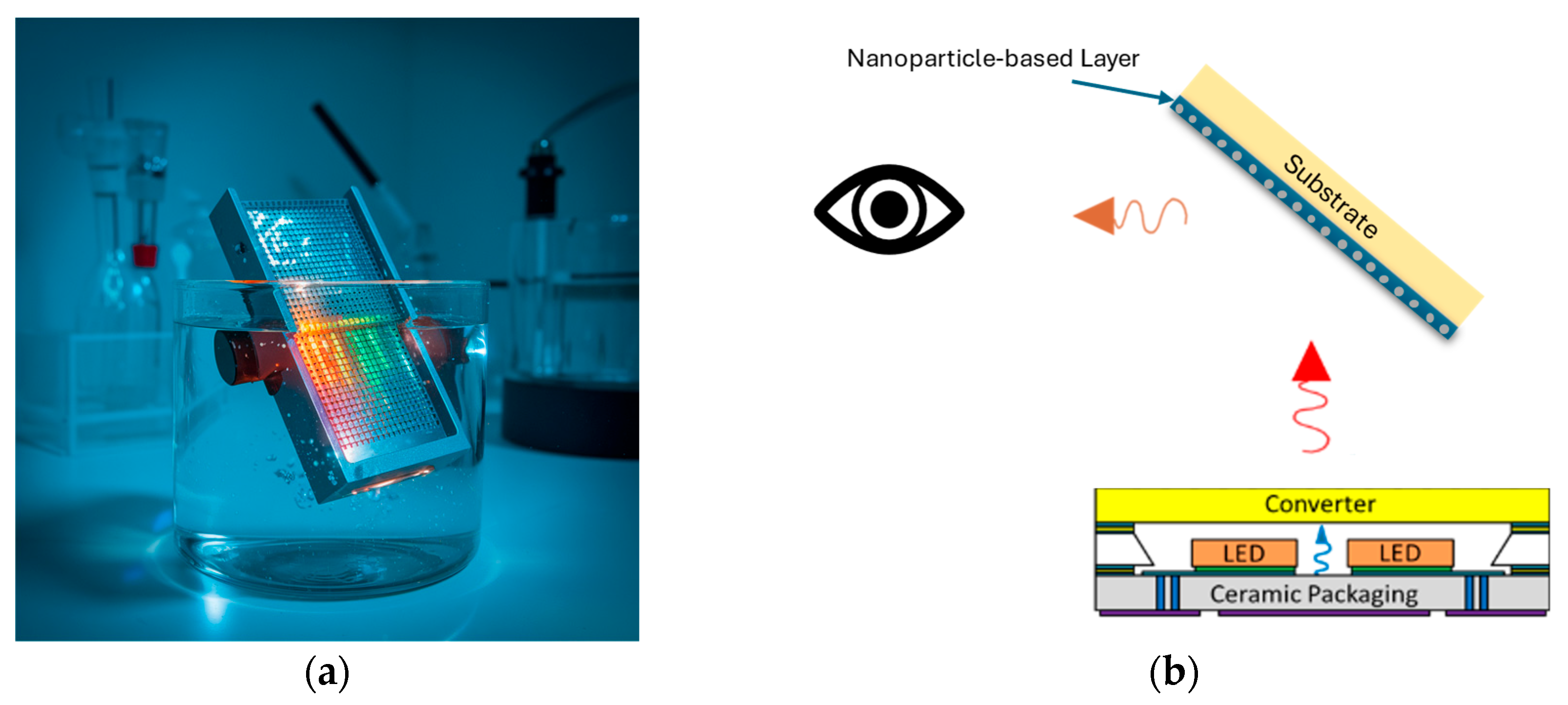
3. The Concept of the Sensing Device
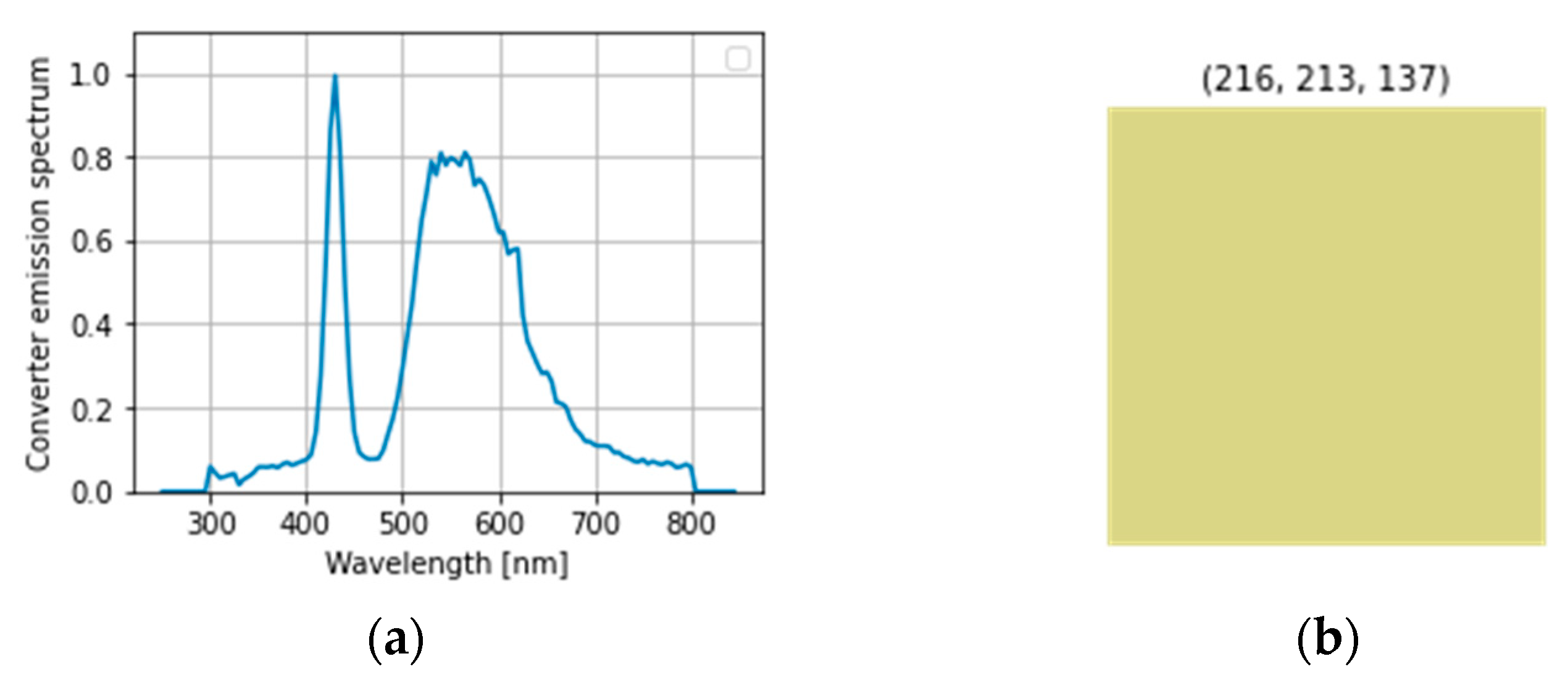
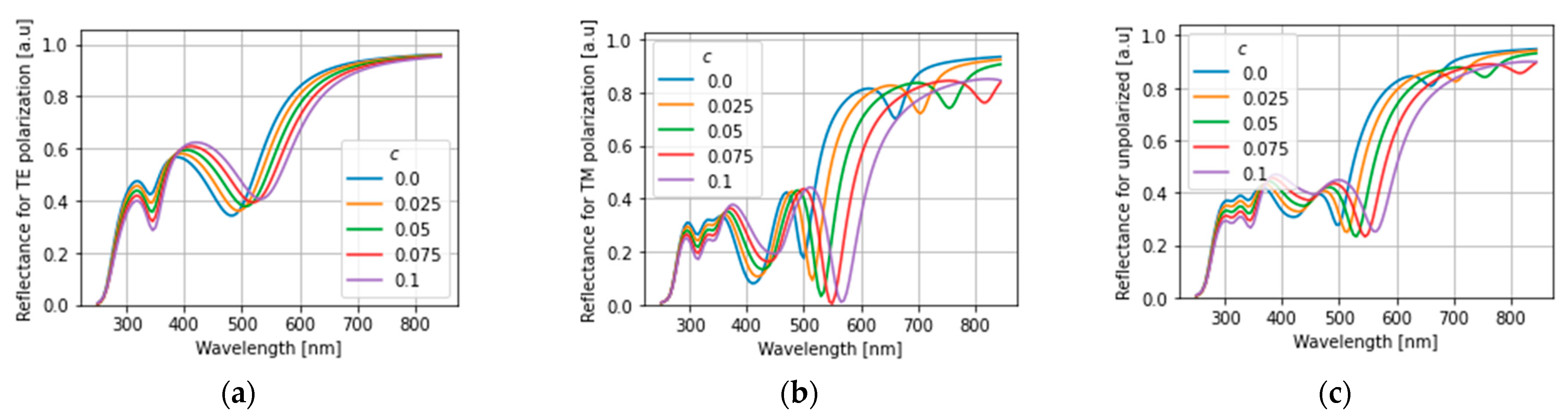
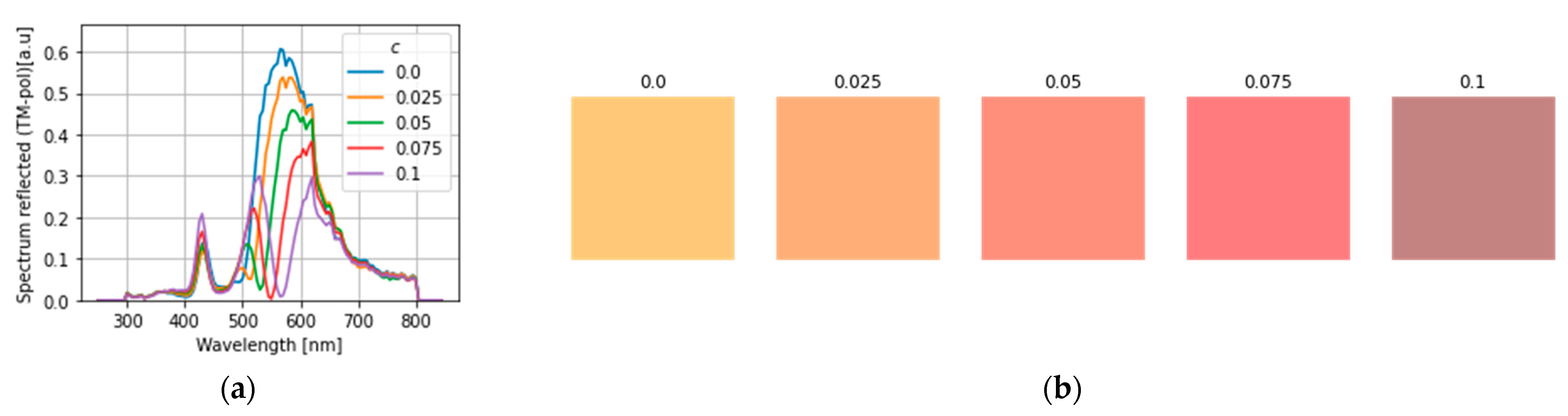
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NP | Nanoparticle |
| LED | Light-emitting diode |
| LPR | Localized plasmon resonance |
| ITO | Indium tin oxide |
Appendix A
Appendix A.1
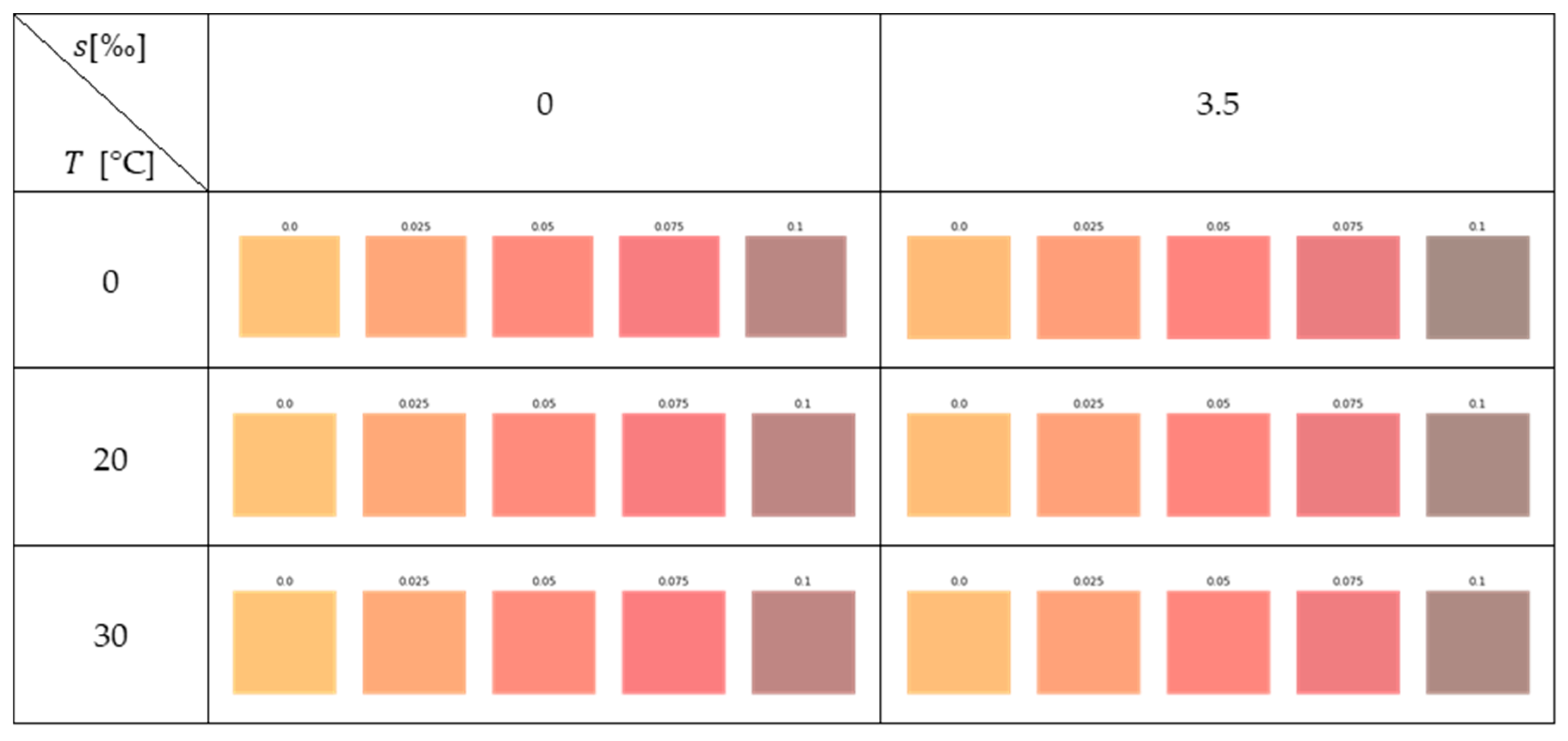

Appendix A.2
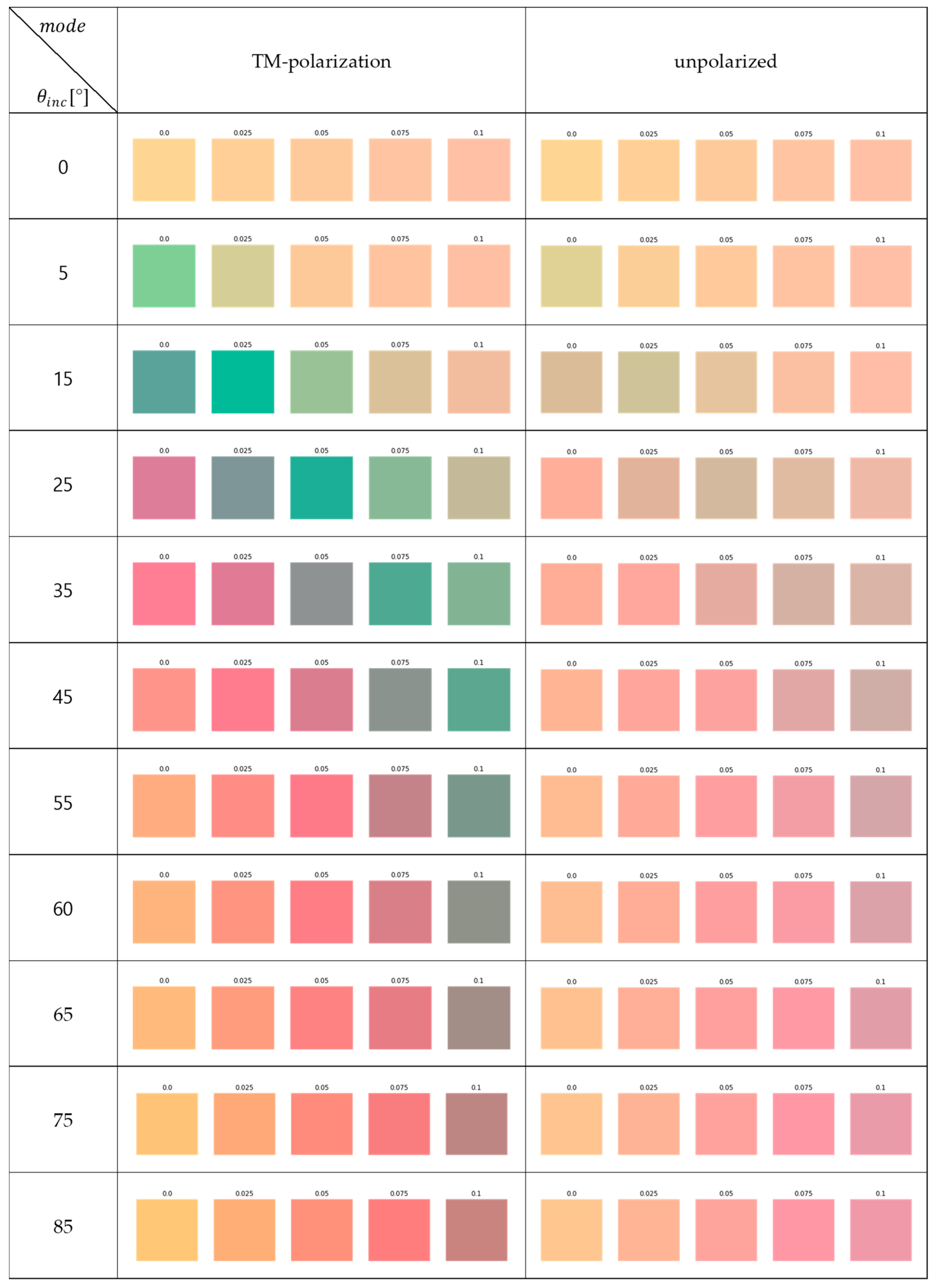
References
- Velasco-Muñoz, J.; Aznar-Sánchez, J.; Belmonte-Ureña, L.; Román-Sánchez, I. Sustainable Water Use in Agriculture: A Review of Worldwide Research. Sustainability 2018, 10, 1084. [Google Scholar] [CrossRef]
- Berga, L. The Role of Hydropower in Climate Change Mitigation and Adaptation: A Review. Engineering 2016, 2, 313–318. [Google Scholar] [CrossRef]
- Ding, G.K.C. Life Cycle Assessment (LCA) of Sustainable Building Materials: An Overview. In Eco-Efficient Construction and Building Materials; Elsevier: Amsterdam, The Netherlands, 2014; pp. 38–62. ISBN 978-0-85709-767-5. [Google Scholar]
- Dutta, D.; Arya, S.; Kumar, S. Industrial Wastewater Treatment: Current Trends, Bottlenecks, and Best Practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef] [PubMed]
- Rosińska, W.; Jurasz, J.; Przestrzelska, K.; Wartalska, K.; Kaźmierczak, B. Climate Change’s Ripple Effect on Water Supply Systems and the Water-Energy Nexus–A Review. Water Resour. Ind. 2024, 32, 100266. [Google Scholar] [CrossRef]
- Gleick, P.H.; Cooley, H. Freshwater Scarcity. Annu. Rev. Environ. Resour. 2021, 46, 319–348. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Ye, R.; Duan, Q. Advances on Water Quality Detection by UV-Vis Spectroscopy. Appl. Sci. 2020, 10, 6874. [Google Scholar] [CrossRef]
- Kumari, V.B.C.; Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Kumar, N.; Sowmya, B.P.; Egbuna, C.; Uche, C.Z.; Patrick-Iwuanyanwu, K.C. Chromatographic Techniques: Types, Principles, and Applications. In Analytical Techniques in Biosciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 73–101. ISBN 978-0-12-822654-4. [Google Scholar]
- Boukhobza, I.; Crans, D.C. Application of HPLC to Measure Vanadium in Environmental, Biological and Clinical Matrices. Arab. J. Chem. 2020, 13, 1198–1228. [Google Scholar] [CrossRef]
- Reinemann, C.; Freiin Von Fritsch, U.; Rudolph, S.; Strehlitz, B. Generation and Characterization of Quinolone-Specific DNA Aptamers Suitable for Water Monitoring. Biosens. Bioelectron. 2016, 77, 1039–1047. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Otero, M.; Esteves, V.I. Monitoring Pharmaceuticals in the Aquatic Environment Using Enzyme-Linked Immunosorbent Assay (ELISA)—A Practical Overview. Anal. Bioanal. Chem. 2020, 412, 3983–4008. [Google Scholar] [CrossRef]
- Kim, K.; Joung, H.-A.; Han, G.-R.; Kim, M.-G. An Immunochromatographic Biosensor Combined with a Water-Swellable Polymer for Automatic Signal Generation or Amplification. Biosens. Bioelectron. 2016, 85, 422–428. [Google Scholar] [CrossRef]
- Pillay, S.J.; Bangira, T.; Sibanda, M.; Kebede Gurmessa, S.; Clulow, A.; Mabhaudhi, T. Assessing Drone-Based Remote Sensing for Monitoring Water Temperature, Suspended Solids and CDOM in Inland Waters: A Global Systematic Review of Challenges and Opportunities. Drones 2024, 8, 733. [Google Scholar] [CrossRef]
- Alharbi, B. Remote Sensing Techniques for Monitoring Algal Blooms in the Area between Jeddah and Rabigh on the Red Sea Coast. Remote Sens. Appl. Soc. Environ. 2023, 30, 100935. [Google Scholar] [CrossRef]
- Ahmed, U.; Mumtaz, R.; Anwar, H.; Mumtaz, S.; Qamar, A.M. Water Quality Monitoring: From Conventional to Emerging Technologies. Water Supply 2020, 20, 28–45. [Google Scholar] [CrossRef]
- AlMashrea, B.A.; Almehdi, A.M.; Damiati, S. Simple Microfluidic Devices for in Situ Detection of Water Contamination: A State-of-Art Review. Front. Bioeng. Biotechnol. 2024, 12, 1355768. [Google Scholar] [CrossRef]
- Krishnan, S.; Syed, Z.U.Q. Colorimetric Visual Sensors for Point-of-Needs Testing. Sens. Actuators Rep. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Yao, C.; Yu, S.; Li, X.; Wu, Z.; Liang, J.; Fu, Q.; Xiao, W.; Jiang, T.; Tang, Y. A Plasmonic ELISA for the Naked-Eye Detection of Chromium Ions in Water Samples. Anal. Bioanal. Chem. 2017, 409, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Hu, Y.; Yu, J.; Gallou, F.; Lipshutz, B.H. Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki–Heck Couplings under Aqueous Micellar Catalysis Conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Salah, N.H. Surface Plasmon Resonance Sensor of Toxic Nanoparticles in Aqueous Systems. Eurasian J. Sci. Eng. 2019, 4, 40–48. [Google Scholar] [CrossRef]
- Śmietana, M.; Burnat, D.; Curda, P.; Janaszek, B.; Kieliszczyk, M.; Sezemsky, P.; Koba, M.; Stranak, V.; Szczepański, P. Electro-Optically Modulated Lossy-Mode Resonance—A New Approach for Label-Free Sensing. ACS Photon. 2024, 11, 2061–2069. [Google Scholar] [CrossRef]
- Mahani, F.F.; Mokhtari, A.; Berini, P. Plasmonic Biosensor on the End-Facet of a Dual-Core Single-Mode Optical Fiber. Biosensors 2023, 13, 558. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Ashikbayeva, Z.; Myrkhiyeva, Z.; Nugmanova, A.; Shaimerdenova, M.; Ayupova, T.; Tosi, D. Label-Free Fiber-Optic Spherical Tip Biosensor to Enable Picomolar-Level Detection of CD44 Protein. Sci. Rep. 2021, 11, 19583. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Chang, Y.-J.; Chuang, Y.-C.; Huang, B.-Z.; Chen, C.-C. A Plasmonic Refractive Index Sensor with an Ultrabroad Dynamic Sensing Range. Sci. Rep. 2019, 9, 5134. [Google Scholar] [CrossRef]
- Chau, Y.-F.C.; Chou Chao, C.-T.; Jumat, S.Z.B.H.; Kooh, M.R.R.; Thotagamuge, R.; Lim, C.M.; Chiang, H.-P. Improved Refractive Index-Sensing Performance of Multimode Fano-Resonance-Based Metal-Insulator-Metal Nanostructures. Nanomaterials 2021, 11, 2097. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Higher-Throughput, Label-Free, Real-Time Molecular Interaction Analysis. Anal. Biochem. 2007, 361, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mullett, W.M.; Lai, E.P.C.; Yeung, J.M. Surface Plasmon Resonance-Based Immunoassays. Methods 2000, 22, 77–91. [Google Scholar] [CrossRef]
- Wo, J.; Wang, G.; Cui, Y.; Sun, Q.; Liang, R.; Shum, P.P.; Liu, D. Refractive Index Sensor Using Microfiber-Based Mach–Zehnder Interferometer. Opt. Lett. 2012, 37, 67. [Google Scholar] [CrossRef]
- Fard, S.T.; Donzella, V.; Schmidt, S.A.; Flueckiger, J.; Grist, S.M.; Talebi Fard, P.; Wu, Y.; Bojko, R.J.; Kwok, E.; Jaeger, N.A.F.; et al. Performance of Ultra-Thin SOI-Based Resonators for Sensing Applications. Opt. Express 2014, 22, 14166. [Google Scholar] [CrossRef]
- Martínez-Ayala, L.; Bornacelli, J.; Ojeda-Misses, M.A.; Arano-Martinez, J.A.; Torres-Torres, C.; Martines-Arano, H. Chaos-Driven Detection of Methylene Blue in Wastewater Using Fractional Calculus and Laser Systems. Meas. Sci. Technol. 2025, 36, 15801. [Google Scholar] [CrossRef]
- Jayaprakash, V.; You, J.B.; Kanike, C.; Liu, J.; McCallum, C.; Zhang, X. Determination of Trace Organic Contaminant Concentration via Machine Classification of Surface-Enhanced Raman Spectra. Environ. Sci. Technol. 2024, 58, 15619–15628. [Google Scholar] [CrossRef]
- Lomae, A.; Chaiyo, S.; Chailapakul, O.; Siangproh, W.; Panchompoo, J. Fully Integrated Colorimetric Sensor Based on Transparency Substrate for Salbutamol Determination. MethodsX 2022, 9, 101913. [Google Scholar] [CrossRef]
- Xu, J.; Gui, M.; Li, Z.; Fang, Y.; Wang, S.; Li, H.; Yu, R. Enhanced Colorimetric Analysis Substrate Based on Graphene Oxide Open-Close Actuator. Sens. Actuators B Chem. 2025, 423, 136748. [Google Scholar] [CrossRef]
- Kornyshev, A.A.; Marinescu, M.; Paget, J.; Urbakh, M. Reflection of Light by Metal Nanoparticles at Electrodes. Phys. Chem. Chem. Phys. 2012, 14, 1850. [Google Scholar] [CrossRef]
- Bagchi, A.; Barrera, R.G.; Fuchs, R. Local-Field Effect in Optical Reflectance from Adsorbed Overlayers. Phys. Rev. B 1982, 25, 7086–7096. [Google Scholar] [CrossRef]
- CIE TC 1-85 International Commission on Illumination (CIE). CIE 015:2018 Colorimetry, 4th ed.; CIE TC 1-85 International Commission on Illumination: Vienna, Austria, 2018. [Google Scholar] [CrossRef]
- Fairman, H.S.; Brill, M.H.; Hemmendinger, H. How the CIE 1931 Color-Matching Functions Were Derived from Wright-Guild Data. Color Res. Appl. 1997, 22, 11–23. [Google Scholar] [CrossRef]
- Kozłowska, A.; Bogucki, O.; Szysiak, A.; Kaczkan, M.; Fetliński, B.; Janaszek, B.; Kieliszczyk, M.; Trihan, R.; Rossignol, F.; Aimable, A.; et al. Luminophore light sources for lighting and sensing applications. In Proceedings of the XIV Scientific Conference for Electron Technology ELTE 2023, Ryn, Poland, 18 April 2023. [Google Scholar]
- Arosa, Y.; De La Fuente, R. Refractive Index Spectroscopy and Material Dispersion in Fused Silica Glass. Opt. Lett. 2020, 45, 4268. [Google Scholar] [CrossRef]
- Blaber, M.G.; Arnold, M.D.; Ford, M.J. Search for the Ideal Plasmonic Nanoshell: The Effects of Surface Scattering and Alternatives to Gold and Silver. J. Phys. Chem. C 2009, 113, 3041–3045. [Google Scholar] [CrossRef]
- Barchiesi, D.; Grosges, T. Fitting the Optical Constants of Gold, Silver, Chromium, Titanium, and Aluminum in the Visible Bandwidth. J. Nanophoton 2014, 8, 083097. [Google Scholar] [CrossRef]
- Wang, B.; Yu, P.; Wang, W.; Zhang, X.; Kuo, H.; Xu, H.; Wang, Z.M. High-Q Plasmonic Resonances: Fundamentals and Applications. Adv. Opt. Mater. 2021, 9, 2001520. [Google Scholar] [CrossRef]
- Trihan, R.; Bogucki, O.; Kozlowska, A.; Ihle, M.; Ziesche, S.; Fetliński, B.; Janaszek, B.; Kieliszczyk, M.; Kaczkan, M.; Rossignol, F.; et al. Hybrid Gold-Silica Nanoparticles for Plasmonic Applications: A Comparison Study of Synthesis Methods for Increasing Gold Coverage. Heliyon 2023, 9, e15977. [Google Scholar] [CrossRef]
- Barad, H.-N.; Kwon, H.; Alarcón-Correa, M.; Fischer, P. Large Area Patterning of Nanoparticles and Nanostructures: Current Status and Future Prospects. ACS Nano 2021, 15, 5861–5875. [Google Scholar] [CrossRef]
- Kadem, L.F.; Lamprecht, C.; Purtov, J.; Selhuber-Unkel, C. Controlled Self-Assembly of Hexagonal Nanoparticle Patterns on Nanotopographies. Langmuir 2015, 31, 9261–9265. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.G.; Gibbs, J.G.; Lee, T.-C.; Fischer, P. Hybrid Nanocolloids with Programmed Three-Dimensional Shape and Material Composition. Nat. Mater. 2013, 12, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Ochs, M.; Andrieu-Brunsen, A.; Vogel, N. Effect of Asymmetry on Plasmon Hybridization and Sensing Capacities of Hole-Disk Arrays. J. Phys. Chem. C 2020, 124, 2609–2618. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver Nanoparticles (AgNPs): Comprehensive Insights into Bio/Synthesis, Key Influencing Factors, Multifaceted Applications, and Toxicity—A 2024 Update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Gupta, V.; Aftenieva, O.; Probst, P.T.; Sarkar, S.; Steiner, A.M.; Vogel, N.; Fery, A.; König, T.A.F. Advanced Colloidal Sensors Enabled by an Out-of-Plane Lattice Resonance. Adv. Photon. Res. 2022, 3, 2200152. [Google Scholar] [CrossRef]
- Chhatre, A.; Solasa, P.; Sakle, S.; Thaokar, R.; Mehra, A. Color and Surface Plasmon Effects in Nanoparticle Systems: Case of Silver Nanoparticles Prepared by Microemulsion Route. Colloids Surf. A Physicochem. Eng. Asp. 2012, 404, 83–92. [Google Scholar] [CrossRef]
- Al-Dossari, M.; Awasthi, S.; Mohamed, A.; Abd El-Gawaad, N.; Sabra, W.; Aly, A. Bio-Alcohol Sensor Based on One-Dimensional Photonic Crystals for Detection of Organic Materials in Wastewater. Materials 2022, 15, 4012. [Google Scholar] [CrossRef]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Review on Anti-Aggregation-Enabled Colorimetric Sensing Applications of Gold and Silver Nanoparticles. Chemosensors 2022, 10, 536. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morey, J.; Duel, P.; Yánez-Jácome, G.S.; Chuisaca-Londa, E.; Guzmán, K.; Caiza, J.; Tapia, M.; Debut, A.; Vizuete, K.; et al. Rapid, Low-Cost Determination of Hg2+, Cu2+, and Fe3+ Using a Cellulose Paper-Based Sensor and UV–Vis Method with Silver Nanoparticles Synthesized with S. mammosum. Sens. Bio-Sens. Res. 2024, 45, 100680. [Google Scholar] [CrossRef]
- Parrish, C. Index of Refraction of Seawater and Freshwater as a Function of Wavelength and Temperature. Parrish Research Group 2020. Available online: https://research.engr.oregonstate.edu/parrish/index-refraction-seawater-and-freshwater-function-wavelength-and-temperature (accessed on 4 September 2025).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janaszek, B.; Kieliszczyk, M.; Fetliński, B.; Kaczkan, M.; Trihan, R.; Rossignol, F.; Aimable, A.; Kozlowska, A.; Bogucki, O.; Ihle, M.; et al. Naked-Eye Detection of Water Contaminants Enabled by Engineered Plasmonic Resonance in Hybrid Detector Systems. Appl. Sci. 2025, 15, 9975. https://doi.org/10.3390/app15189975
Janaszek B, Kieliszczyk M, Fetliński B, Kaczkan M, Trihan R, Rossignol F, Aimable A, Kozlowska A, Bogucki O, Ihle M, et al. Naked-Eye Detection of Water Contaminants Enabled by Engineered Plasmonic Resonance in Hybrid Detector Systems. Applied Sciences. 2025; 15(18):9975. https://doi.org/10.3390/app15189975
Chicago/Turabian StyleJanaszek, Bartosz, Marcin Kieliszczyk, Bartosz Fetliński, Marcin Kaczkan, Romain Trihan, Fabrice Rossignol, Anne Aimable, Anna Kozlowska, Oskar Bogucki, Martin Ihle, and et al. 2025. "Naked-Eye Detection of Water Contaminants Enabled by Engineered Plasmonic Resonance in Hybrid Detector Systems" Applied Sciences 15, no. 18: 9975. https://doi.org/10.3390/app15189975
APA StyleJanaszek, B., Kieliszczyk, M., Fetliński, B., Kaczkan, M., Trihan, R., Rossignol, F., Aimable, A., Kozlowska, A., Bogucki, O., Ihle, M., & Ziesche, S. (2025). Naked-Eye Detection of Water Contaminants Enabled by Engineered Plasmonic Resonance in Hybrid Detector Systems. Applied Sciences, 15(18), 9975. https://doi.org/10.3390/app15189975





