Solvent-Driven Extraction of Bioactive Compounds from Propolis for Application in Food Industry Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Conditions
2.2. Sample Preparation
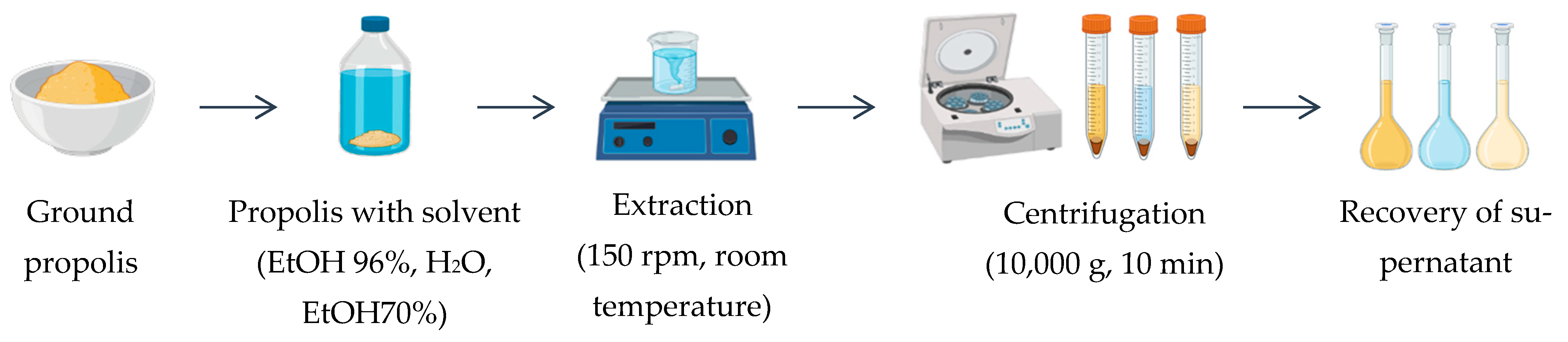
2.3. Extraction of Bioactive Compounds
- 96% ethanol (absolute ethanol, EtOH 96%);
- 70% ethanol (hydroethanol, EtOH 70%);
- Ultrapure water (Milli-Q grade).
2.4. Physicochemical Characterization
2.5. Extraction Yield
2.6. Determination of Total Phenolic Content (TPC)
2.7. Determination of Total Flavonoid Content (TFC)
2.8. Antioxidant Activity Assays
2.9. Tyrosinase Inhibition Assay
2.10. Elastase Inhibition Assay
2.11. Background Absorbance Correctio N
2.12. Statistical Analysis
3. Results
3.1. Proximate Composition Analysis
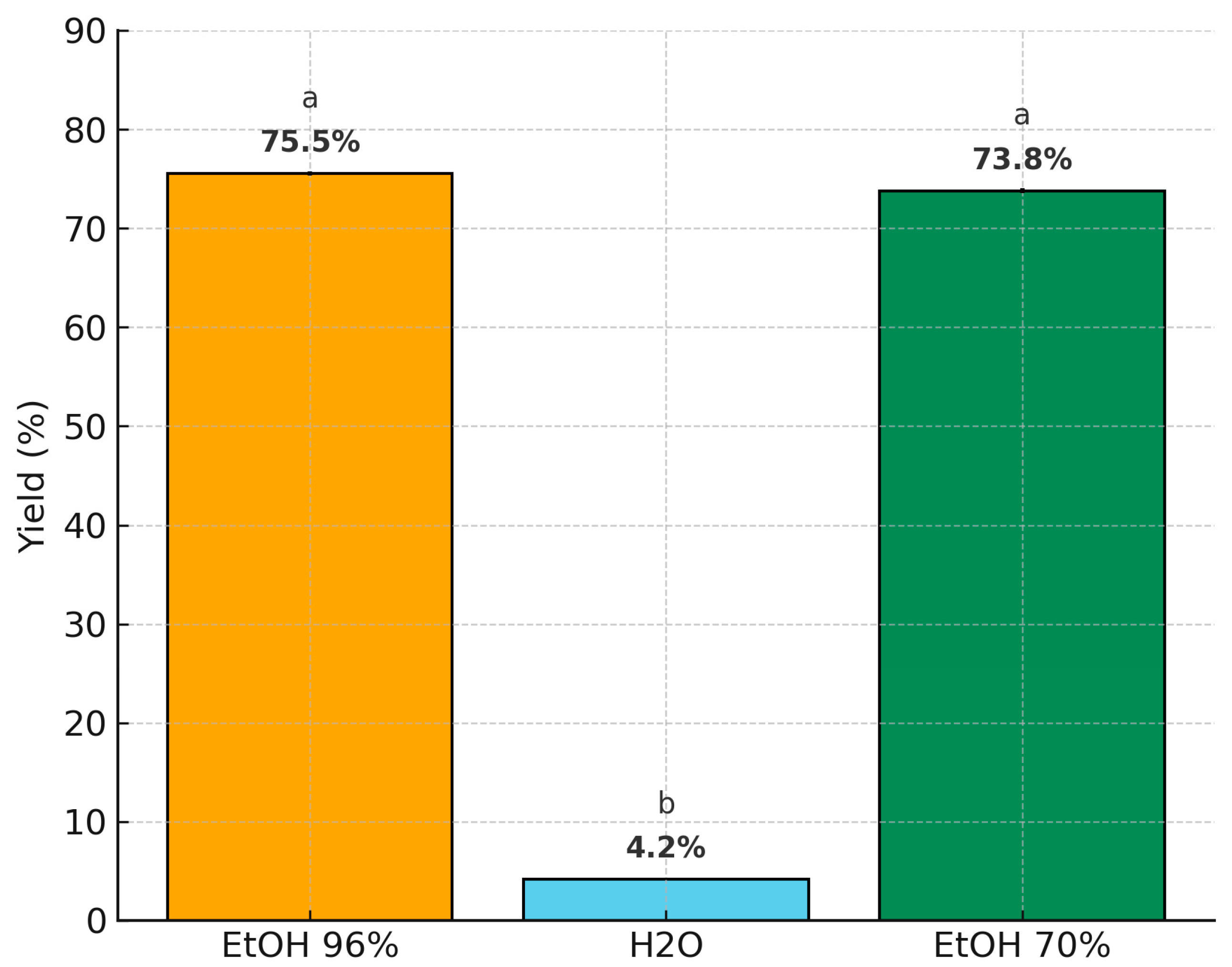
3.2. Efficiency of Extraction
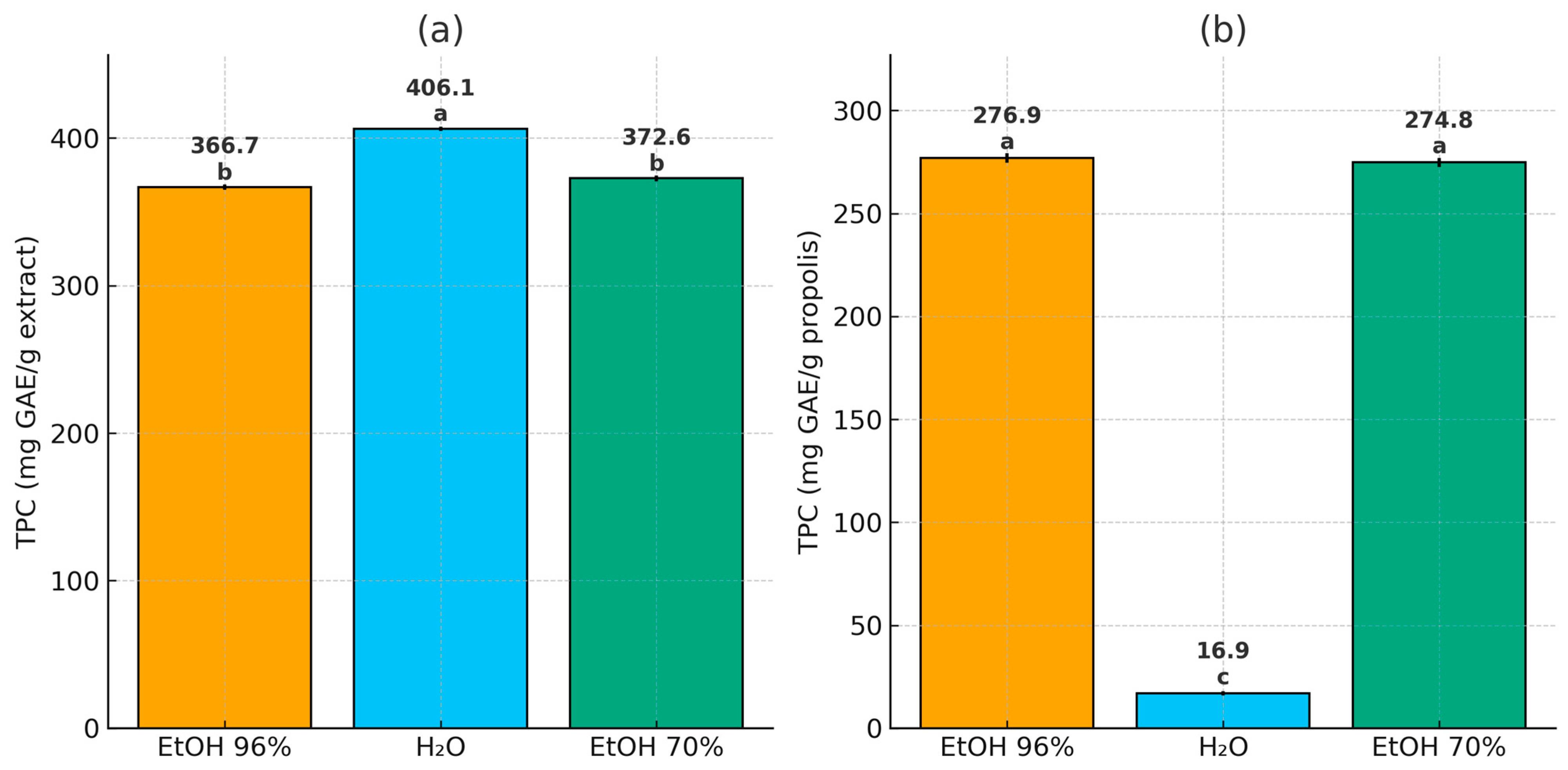
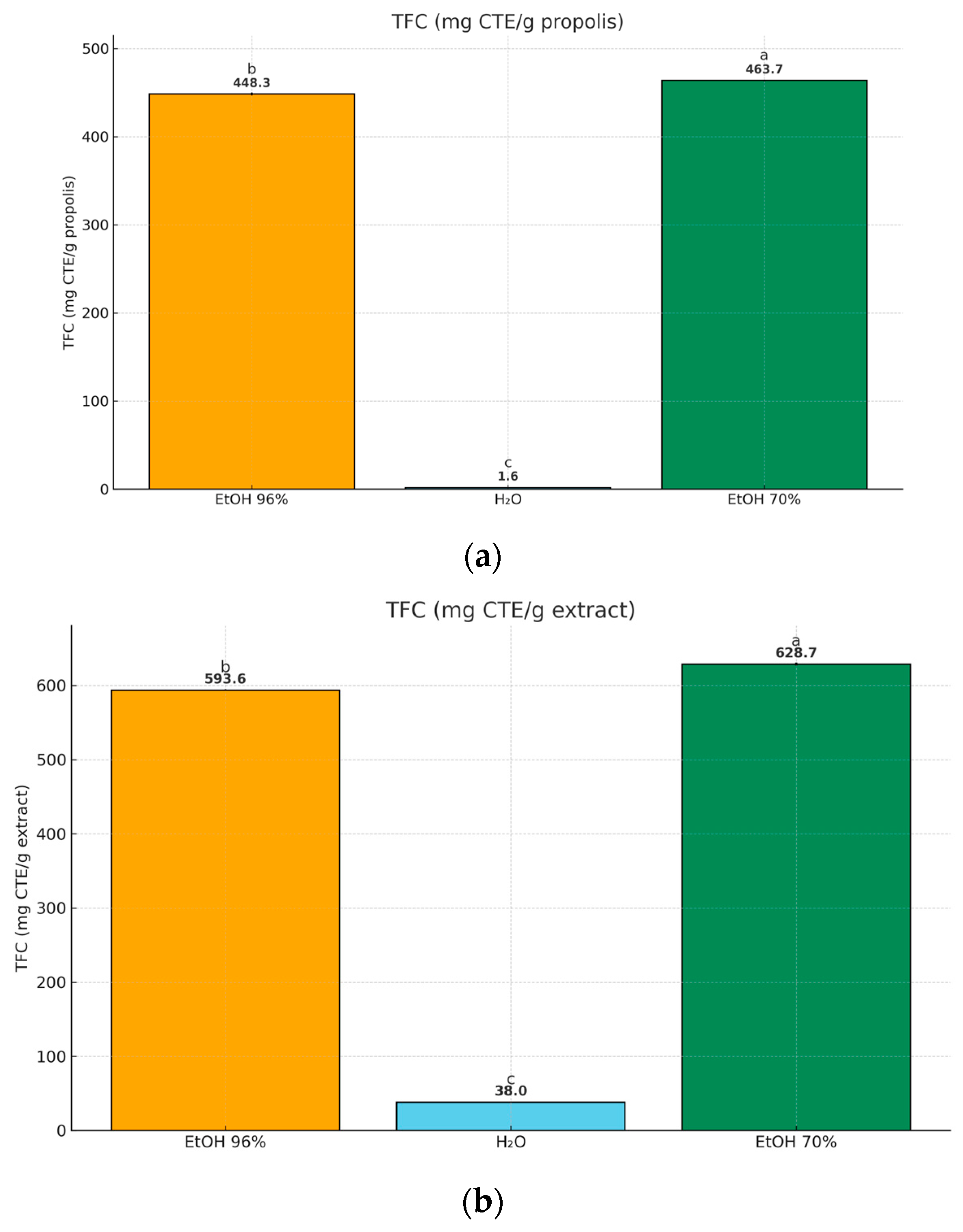
3.3. Phenolic Composition of Propolis
3.4. Anti-Aging Activity of Propolis
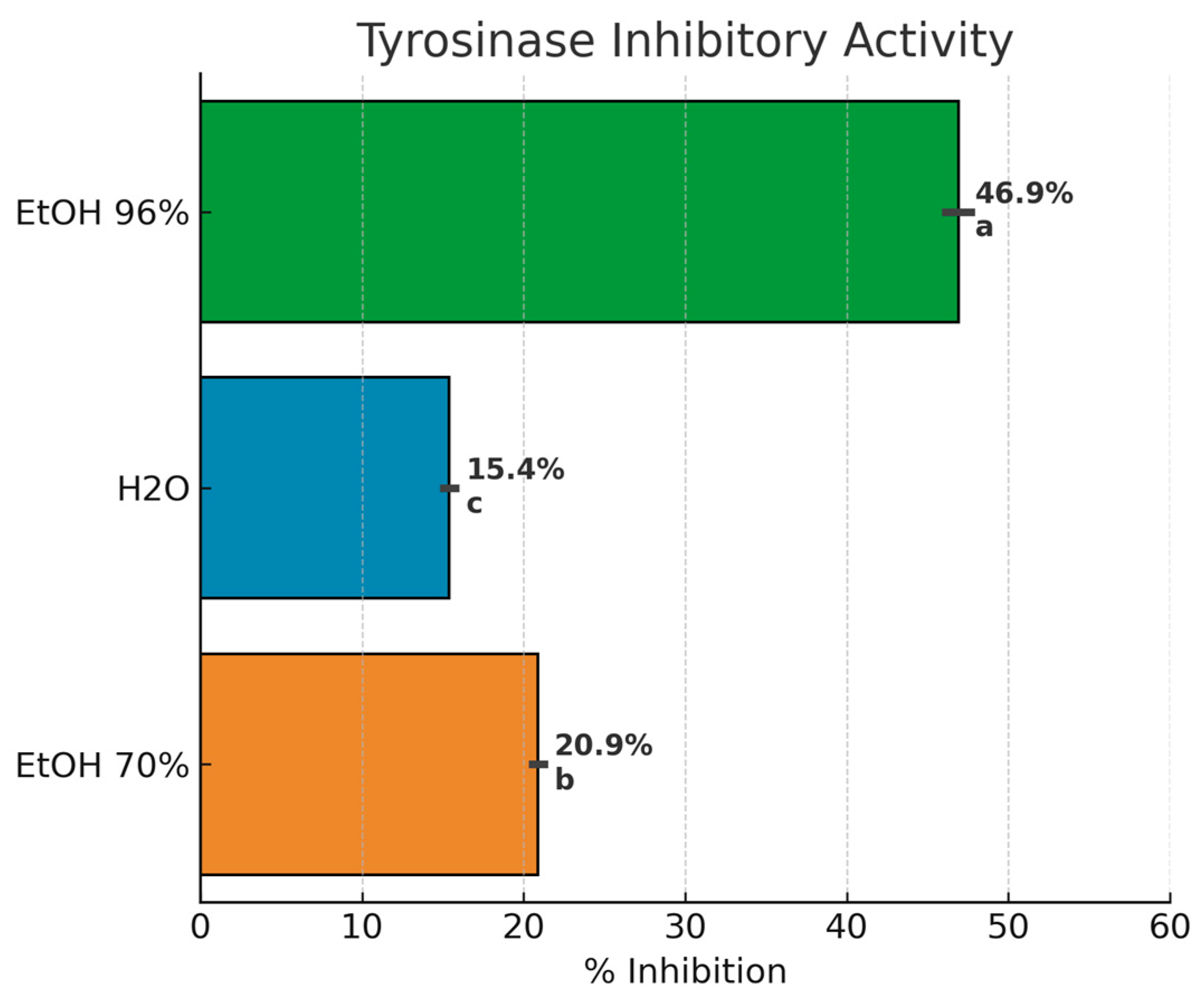
3.4.1. Tyrosinase Inhibition Assay
3.4.2. Elastase Inhibition Assay
3.5. Overall Interpretation of Bioactivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef]
- Bankova, V.S.; Popova, M.; Trusheva, B. Propolis: Recent advances in chemistry and plant origin. Apidologie 2020, 52, 68–82. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; Mohamed, S.A.A. Applications of propolis in food industries and packaging. Biomass Conv. Bioref. 2024, 14, 13731–13746. [Google Scholar] [CrossRef]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Ivanov, I.; Todorova, M.; Apostolova, S.; Tzoneva, R.; Nikolova, K. Phenolic content, antioxidant activity and in vitro anti-inflammatory and antitumor potential of selected Bulgarian propolis samples. Biomedicines 2025, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Celeiro, S.P.; Barbosa-Matos, C.; Freitas, A.S.; Cardoso, S.M.; Viana-Pereira, M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese propolis antitumoral activity in melanoma involves ROS production and induction of apoptosis. Molecules 2022, 27, 3533. [Google Scholar] [CrossRef]
- Justino, I.A.; Furlan, J.P.R.; Ferreira, I.R.S.; Marincek, A.; Aldana-Mejía, J.A.; Tucci, L.F.F.; Bastos, J.K.; Stehling, E.G.; Marzocchi-Machado, C.M.; Marcato, P.D. Antimicrobial, antioxidant, and anticancer effects of nanoencapsulated Brazilian red propolis extract: Applications in cancer therapy. Processes 2024, 12, 2856. [Google Scholar] [CrossRef]
- Shin, S.-B.; Lee, J.-K.; Ko, M.-J. Enhanced extraction of bioactive compounds from propolis (Apis mellifera L.) using subcritical water. Sci. Rep. 2023, 13, 15038. [Google Scholar] [CrossRef]
- Segueni, N.; Boutaghane, N.; Asma, S.T.; Tas, N.; Acaroz, U.; Arslan-Acaroz, D.; Shah, S.R.A.; Abdellatieff, H.A.; Akkal, S.; Peñalver, R.; et al. Review on Propolis Applications in Food Preservation and Active Packaging. Plants 2023, 12, 1654. [Google Scholar] [CrossRef]
- Maicelo Quintana, J.L.; Reyna Gonzales, K.; Balcázar Zumaeta, C.R.; Auquinivin Silva, E.A.; Castro Alayo, E.M.; Medina Mendoza, M. Potential application of bee products in the food industry. Heliyon 2024, 10, e24056. [Google Scholar] [CrossRef]
- Santiago, K.B.; Rodrigues, J.C.Z.; Conte, F.L.; Tasca, K.I.; Romagnoli, G.G.; Aldana Mejía, J.A.; Bastos, J.K.; Sforcin, J.M. Brazilian red propolis exerts selective cytotoxic activity against prostate cancer cells and modulates human monocyte functions. Phytother. Res. 2022, 36, 399–409. [Google Scholar] [CrossRef]
- Aboulghazi, A.; Bakour, M.; Fadil, M.; Lyoussi, B. Simultaneous optimization of extraction yield, phenolic compounds and antioxidant activity of Moroccan propolis extracts: Improvement of ultrasound-assisted technique using response surface methodology. Processes 2022, 10, 297. [Google Scholar] [CrossRef]
- Bhadange, Y.A.; Carpenter, J.; Saharan, V.K. A comprehensive review on advanced extraction techniques for natural products. ACS Omega 2024, 9, 7150–7165. [Google Scholar] [CrossRef]
- Barreto, G.A.; Viana, L.C.; Costa, R.V.; Rocha, J.C.; Justino, I.A. Evaluation of Brazilian red propolis extracts by supercritical CO2 extraction. Appl. Sci. 2022, 12, 11741. [Google Scholar] [CrossRef]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.M.; Salazar, G.; Ramírez, J.; Quintero, L.; Díaz, F.; Marín, M.L. Supercritical fluid extraction of red propolis: Optimization and antioxidant potential. Molecules 2024, 29, 284. [Google Scholar] [CrossRef]
- Prgomet, I.; Gonçalves, B.; Domínguez-Perles, R.; Santos, R.; Saavedra, M.J.; Aires, A.; Pascual-Seva, N.; Barros, A. Irrigation deficit turns almond by-products into a valuable source of antimicrobial (poly) phenols. Ind. Crops Prod. 2019, 132, 186–196. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Nascimento, A.P.S.; Carvalho, A.J.D.B.A.; Lima, M.D.S.; Barros, S.L.; Ribeiro, S.; Pasqualli, M.; Lisboa, H.M.; Barros, A.N. Enhancing antioxidant retention through varied wall material combinations in grape spray drying and storage. Antioxidants 2023, 12, 1745. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Freire, C.; Fernandes, E.; Cunha, A.; Almeida-Aguiar, C. Phenolic Profiling of Portuguese Propolis by LC-MS Spectrometry: Uncommon Propolis Rich in Flavonoid Glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef]
- Breda, C.; Catarino, M.D.; Ferreira, R.B.; Silva, A.M. Phenolic composition and antioxidant activity of edible flowers: Insights from synergistic effects and multivariate analysis. Antioxidants 2025, 14, 282. [Google Scholar] [CrossRef]
- Buitrago, D.M.; Perdomo, S.J.; Silva, F.A.; Cely-Veloza, W.; Lafaurie, G.I. Physicochemical characterization, antioxidant, and proliferative activity of Colombian propolis extracts: A comparative study. Molecules 2024, 29, 1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, Y.; Qiao, J.; El-Seedi, H.R.; Kong, L.; Zhang, H. Inhibitory effects of aqueous ethanol extracts of poplar-type propolis on advanced glycation end products and protein oxidation. Foods 2024, 13, 3022. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.; Abdul Munaim, M.S.; Veloo Kutty, R. Effects of Different Ethanol Concentrations on Total Phenolic and Flavonoid Contents in Malaysian Propolis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012033. [Google Scholar] [CrossRef]
- Park, Y.K.; Ikegaki, M. Preparation of Water and Ethanolic Extracts of Propolis and Evaluation of Their Antioxidant Activities. Biosci. Biotechnol. Biochem. 1998, 62, 2230–2232. [Google Scholar] [CrossRef]
- Miłek, M.; Bonikowski, R.; Dżugan, M. Effect of extraction conditions on the chemical profile of raw poplar-type propolis extract. Chem. Pap. 2024, 78, 6709–6720. [Google Scholar] [CrossRef]
- Balasubramaniam, A.K.; Elangovan, A.; Abdul Rahman, M.; Nayak, S.; Swain, D.; Parasur Babu, H.; Narasimhan, A.; Monga, V. Propolis: A comprehensive review on nature’s polyphenolic wonder. Fitoterapia 2025, 183, 106526. [Google Scholar] [CrossRef]
- Pereira, L.; Cunha, A.; Almeida-Aguiar, C. Portuguese propolis from Caramulo as a biocontrol agent of apple blue mold. Food Control. 2022, 139, 109071. [Google Scholar] [CrossRef]
- Kubiliene, L.; Laugaliene, V.; Pavilonis, A.; Maruska, A.; Majiene, D.; Barcauskaite, K.; Kubilius, R.; Kasparaviciene, G.; Savickas, A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complement. Altern. Med. 2015, 15, 156. [Google Scholar] [CrossRef]
- Ma, S.; Ma, H.; Pan, Z.; Luo, L.; Weng, L. Antioxidant activities of propolis extracts by different solvents in vitro. Zhongguo Shipin Xuebao 2016, 16, 53–58. [Google Scholar]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Abang Zaidel, D.N.; Che Yunus, M.A.; Md Salleh, L. Valorisation of plant seeds as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, U. Classical single factor optimisation of parameters for phenolic antioxidant extraction from tamarind seed (Tamarindus indica). Plant Sci. Today 2016, 3, 258–266. Available online: https://horizonepublishing.com/journals/index.php/PST/article/view/242 (accessed on 7 June 2025). [CrossRef]
- Niazmand, R.; Shahidi Noghabi, M.; Niazmand, A. Optimization of subcritical water extraction of phenolic compounds from Ziziphus jujuba using response surface methodology: Evaluation of thermal stability and antioxidant activity. Chem. Biol. Technol. Agric. 2021, 8, 6. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- De Aguiar, S.C.; Cottica, S.M.; dos Santos, S.T.; da Fonseca, J.M.; da Silva Leite, L.; da Silva, M.L. Antioxidant activity, phenolic acid, and flavonoid composition of an antiseptic ointment based on aloe and green propolis and its potential for preventing mastitis in dairy cows. Vet. Sci. 2025, 12, 248. [Google Scholar] [CrossRef]
- Pobiega, K.; Kot, A.M.; Przybył, J.L.; Synowiec, A.; Gniewosz, M. Comparison of the chemical composition and antioxidant properties of propolis from urban apiaries. Molecules 2023, 28, 6744. [Google Scholar] [CrossRef]
- Silva, A.M.; Rocha, B.; Moreira, M.M.; Delerue-Matos, C.; das Neves, J.; Rodrigues, F. Biological Activity and Chemical Composition of Propolis Extracts with Potential Use in Vulvovaginal Candidiasis Management. Int. J. Mol. Sci. 2024, 25, 2478. [Google Scholar] [CrossRef]
- Ruiz Ruiz, J.C.; Pacheco López, N.A.; Rejón Méndez, E.G.; Samos López, F.A.; Medina Medina, L.; Quezada-Euán, J.J.G. Phenolic Content and Bioactivity as Geographical Classifiers of Propolis from Stingless Bees in Southeastern Mexico. Foods 2023, 12, 1434. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Karagecili, H.; Yılmaz, M.A.; Ertürk, A.; Kiziltas, H.; Güven, L.; Alwasel, S.H.; Gulcin, İ. Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects. Molecules 2023, 28, 1739. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Kara, Y.; Pokajewicz, K.; Ben Hammouda, I.; Wieczorek, P.P.; Marciniak, D.; Balwierz, R.; Kłósek, M.; Czuba, Z.P.; Kolaylı, S. Phenolic Content, Volatile Compounds and Antioxidant Activity in Propolis Samples Collected from Turkey and Poland. Eur. Food Res. Technol. 2025, 251, 2199–2210. [Google Scholar] [CrossRef]
- Izol, E.; Turhan, M. Detailed Phytochemical Profile by LC-MS/MS, Mineral Content by ICP-MS, and In Vitro Antioxidant, Antidiabetic, Antiepileptic, Anticholinergic and Antiglaucoma Properties of Bitlis Propolis. Life 2024, 14, 1389. [Google Scholar] [CrossRef]
- Fathi Hafshejani, S.; Lotfi, S.; Rezvannejad, E.; Mortazavi, M.; Riahi-Madvar, A. Correlation between Total Phenolic and Flavonoid Contents and Biological Activities of 12 Ethanolic Extracts of Iranian Propolis. Food Sci. Nutr. 2023, 11, 4308–4325. [Google Scholar] [CrossRef]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic Constituents, Antioxidant and Antimicrobial Activity and Clustering Analysis of Propolis Samples Based on PCA from Different Regions of Anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An Update on Its Chemistry and Pharmacological Applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Rendueles, E.; Mauriz, E.; Sanz-Gómez, J.; González Paramás, A.M.; Vallejo Pascual, M.-E.; Adanero Jorge, F.; García Fernández, C. Biochemical Profile and Antioxidant Properties of Propolis from Northern Spain. Foods 2023, 12, 4337. [Google Scholar] [CrossRef]
- Woods, D.C.; Olsson, M.A.; Heard, T.A.; Wallace, H.M.; Tran, T.D. Quality Assessment and Chemical Diversity of Australian Propolis from Tetragonula carbonaria and Tetragonula hockingsi Stingless Bees. Sci. Rep. 2025, 15, 17928. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase Inhibitors from Natural and Synthetic Sources: Structure, Inhibition Mechanism and Perspective for the Future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Choi, H.-J.; Chung, T.-W.; Kim, C.-H.; Jeong, H.-S.; Ha, K.-T. Caffeic Acid Phenethyl Ester Inhibits Alpha-Melanocyte Stimulating Hormone-Induced Melanin Synthesis through Suppressing Transactivation Activity of Microphthalmia-Associated Transcription Factor. J. Nat. Prod. 2013, 76, 1399–1405. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-Radical Scavenging Capacity and Reducing Power of Wild Edible Mushrooms from Northeast Portugal: Individual Cap and Stipe Activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Acito, M.; Varfaj, I.; Brighenti, V.; Cengiz, E.C.; Rondini, T.; Fatigoni, C.; Russo, C.; Pietrella, D.; Pellati, F.; Bartolini, D.; et al. A Novel Black Poplar Propolis Extract with Promising Health Promoting Properties: Focus on Its Chemical Composition, Antioxidant, Anti-Inflammatory, and Antigenotoxic Activities. Food Funct. 2024, 15, 4983–4999. [Google Scholar] [CrossRef]
- Mohtar, L.G.; Messina, G.A.; Bertolino, F.A.; Pereira, S.V.; Raba, J.; Nazareno, M.A. Comparative Study of Different Methodologies for the Determination of the Antioxidant Activity of Venezuelan Propolis. Microchem. J. 2020, 158, 105244. [Google Scholar] [CrossRef]
- Can, Z.; Kara, Y.; Kolayli, S.; Çakmak, I. Antioxidant Activity and Phenolic Composition of Propolis from Marmara Region, Turkey. J. Apic. Res. 2022, 61, 542–548. [Google Scholar] [CrossRef]
- Xie, L.-P.; Chen, Q.-X.; Huang, H.; Wang, H.-Z.; Zhang, R.-Q. Inhibitory Effects of Some Flavonoids on the Activity of Mushroom Tyrosinase. Biochemistry 2003, 68, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of Dietary Flavonoid Structure with Its Tyrosinase Inhibitory Activity and Affinity. LWT 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Stavropoulou, M.-I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR Metabolic Profiling of Greek Propolis Samples: Comparative Evaluation of Their Phytochemical Compositions and Investigation of Their Anti-Ageing and Antioxidant Properties. J. Pharm. Biomed. Anal. 2021, 194, 113814. [Google Scholar] [CrossRef] [PubMed]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-Acetylcholinesterase, Antidiabetic, Anti-Inflammatory, Antityrosinase and Antixanthine Oxidase Activities of Moroccan Propolis. Int. J. Food Sci. Technol. 2016, 51, 1762–1773. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Orhan, I.E.; Duman, H. Profiling Cosmeceutical Effects of Various Herbal Extracts through Elastase, Collagenase, Tyrosinase Inhibitory and Antioxidant Assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Kim, D.; Park, J.; Kim, J.; Han, C.; Yoon, J.; Kim, N.; Seo, J.; Lee, C. Flavonoids as Mushroom Tyrosinase Inhibitors: A Fluorescence Quenching Study. J. Agric. Food Chem. 2006, 54, 935–941. [Google Scholar] [CrossRef]
- Majdi, F.; Alizadeh Behbahani, B.; Barzegar, H.; Mehrnia, M.A. Active packaging coating based on Lepidium sativum seed mucilage and propolis extract: Preparation, characterization, and application for buffalo meat preservation. PLoS ONE 2024, 19, e0311802. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef] [PubMed]


| Sample | Ash (%) | Moisture (%) |
|---|---|---|
| Sample 1 (this study) | 0.64 ± 0.01 | 5.90 ± 0.17 |
| Sample 2 (literature) | 1.90 ± 0.02 | 3.60 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, S.; Nascimento, A.P.S.; Vicente, C.; Barros, A.N. Solvent-Driven Extraction of Bioactive Compounds from Propolis for Application in Food Industry Matrices. Appl. Sci. 2025, 15, 9928. https://doi.org/10.3390/app15189928
Peixoto S, Nascimento APS, Vicente C, Barros AN. Solvent-Driven Extraction of Bioactive Compounds from Propolis for Application in Food Industry Matrices. Applied Sciences. 2025; 15(18):9928. https://doi.org/10.3390/app15189928
Chicago/Turabian StylePeixoto, Sara, Amanda Priscila Silva Nascimento, Cristina Vicente, and Ana Novo Barros. 2025. "Solvent-Driven Extraction of Bioactive Compounds from Propolis for Application in Food Industry Matrices" Applied Sciences 15, no. 18: 9928. https://doi.org/10.3390/app15189928
APA StylePeixoto, S., Nascimento, A. P. S., Vicente, C., & Barros, A. N. (2025). Solvent-Driven Extraction of Bioactive Compounds from Propolis for Application in Food Industry Matrices. Applied Sciences, 15(18), 9928. https://doi.org/10.3390/app15189928









