Tissue Characterization by Ultrasound: Linking Envelope Statistics with Spectral Analysis for Simultaneous Attenuation Coefficient and Scatterer Clustering Quantification
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Envelope Statistics Using the Homodyned K-Distribution
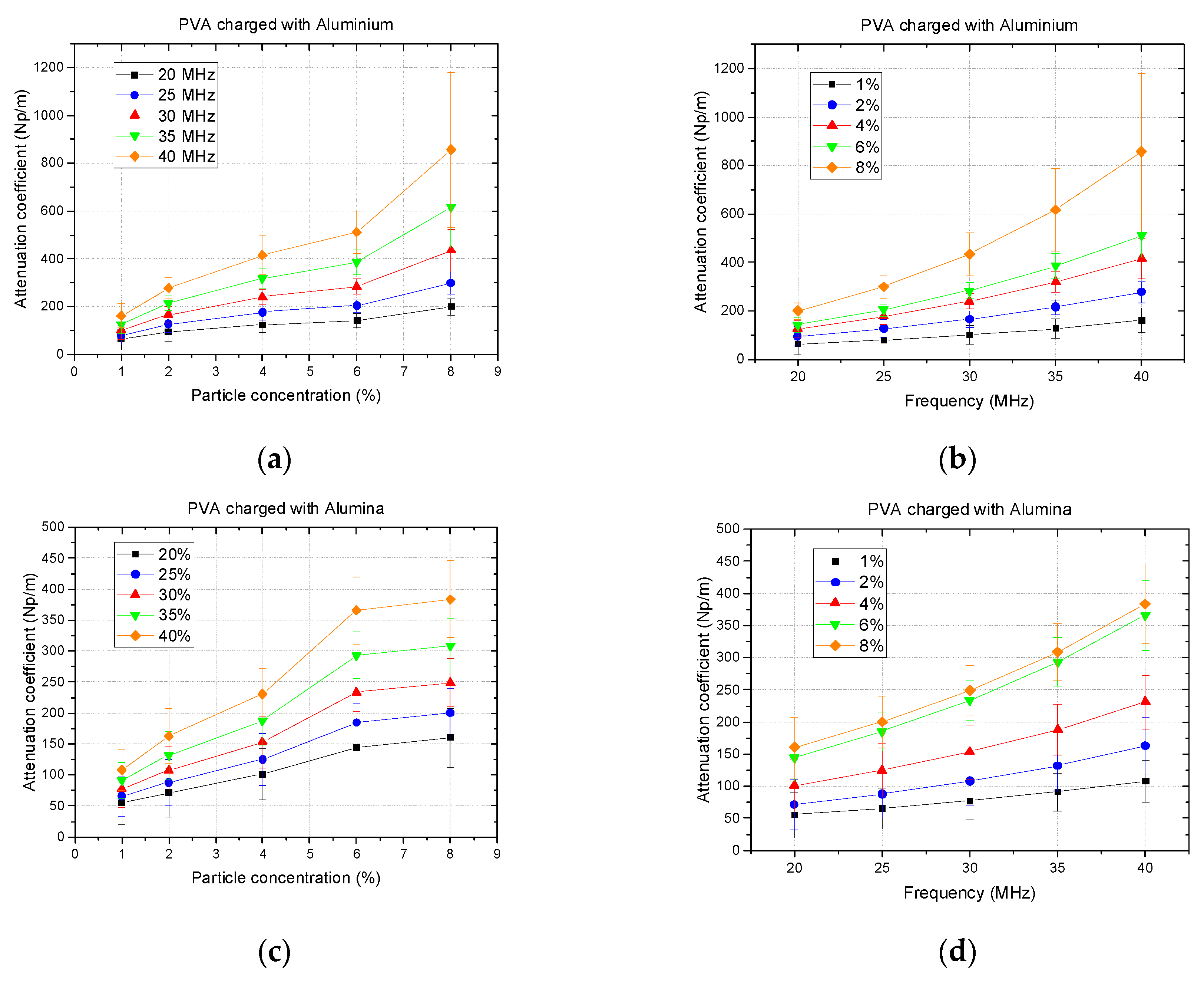
2.2. Attenuation Coefficient
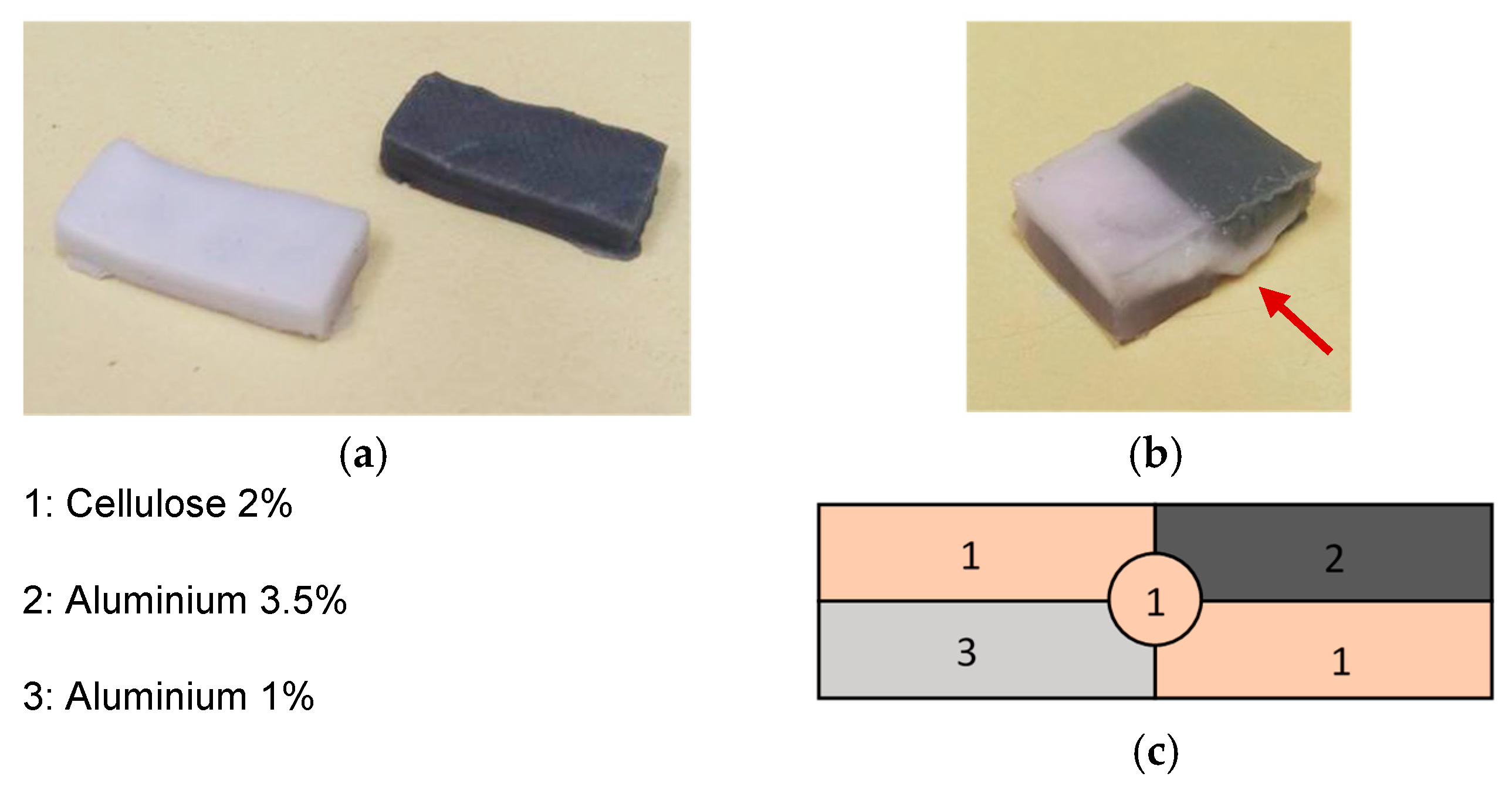
2.3. Experimental Methodology
- (a)
- Narrow bandpass filtering: Acquisitions were subjected to a battery of 10 MHz bandwidth bandpass filters centred at 20 MHz, 25 MHz, 30 MHz, 35 MHz, and 40 MHz, resulting in five sets of signals representative of these frequency bands.
- (b)
- Calculation of the Homodyned K-distribution parameters for each band: For each frequency band, windows with lateral and axial dimensions of 1 mm and 0.75 mm, respectively, were used to extract the envelopes of the signals along the material. The Homodyned K-distribution parameters were then calculated through a two-step process: Initially, a numerical estimation of the Homodyned K-distribution parameters was obtained by solving the system of Equation (4) using the nonlinear equation solver lsqnonlin (MATLAB). This estimation served as the initial value for refining the parameter values on a following step. For this, the same nonlinear equation solver was used, but now envelope data were fitted directly to the probability density function of Equation (1). This two-step process was repeated for all frequencies. Then, a new window was selected with a 0.5 mm lateral displacement for calculating a new set of the Homodyned K-distribution parameters. After completing one row, the procedure continued with the following linear scan until the estimation of the parameters was made for the whole image.
- (c)
- Smoothing of the Homodyned K-distribution parameters: To obtain the attenuation coefficient of the sample, which is based on derivatives with respect to the propagation beam direction, the Homodyned K-distribution parameters were smoothed along the axial direction using splines.
- (d)
- Calculation of the attenuation coefficient for each frequency band: Based on Equation (18), the attenuation coefficient values along the tissue were calculated for each frequency band.
- (e)
- Calculation of the general attenuation coefficient as a function of frequency: Equation (19) was employed to derive the expression of the attenuation coefficient as a function of frequency, utilizing the attenuation coefficients obtained at different frequencies.
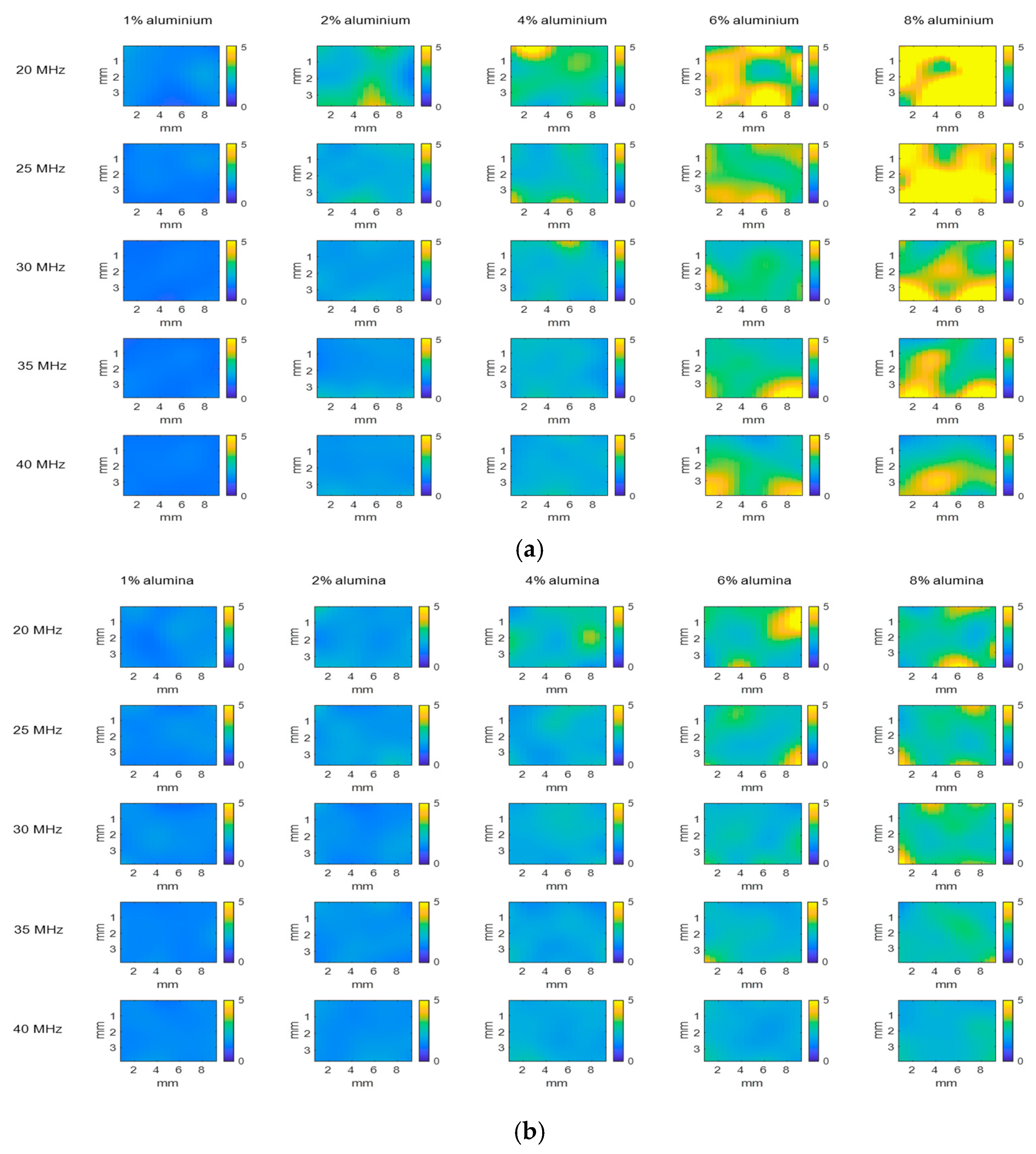
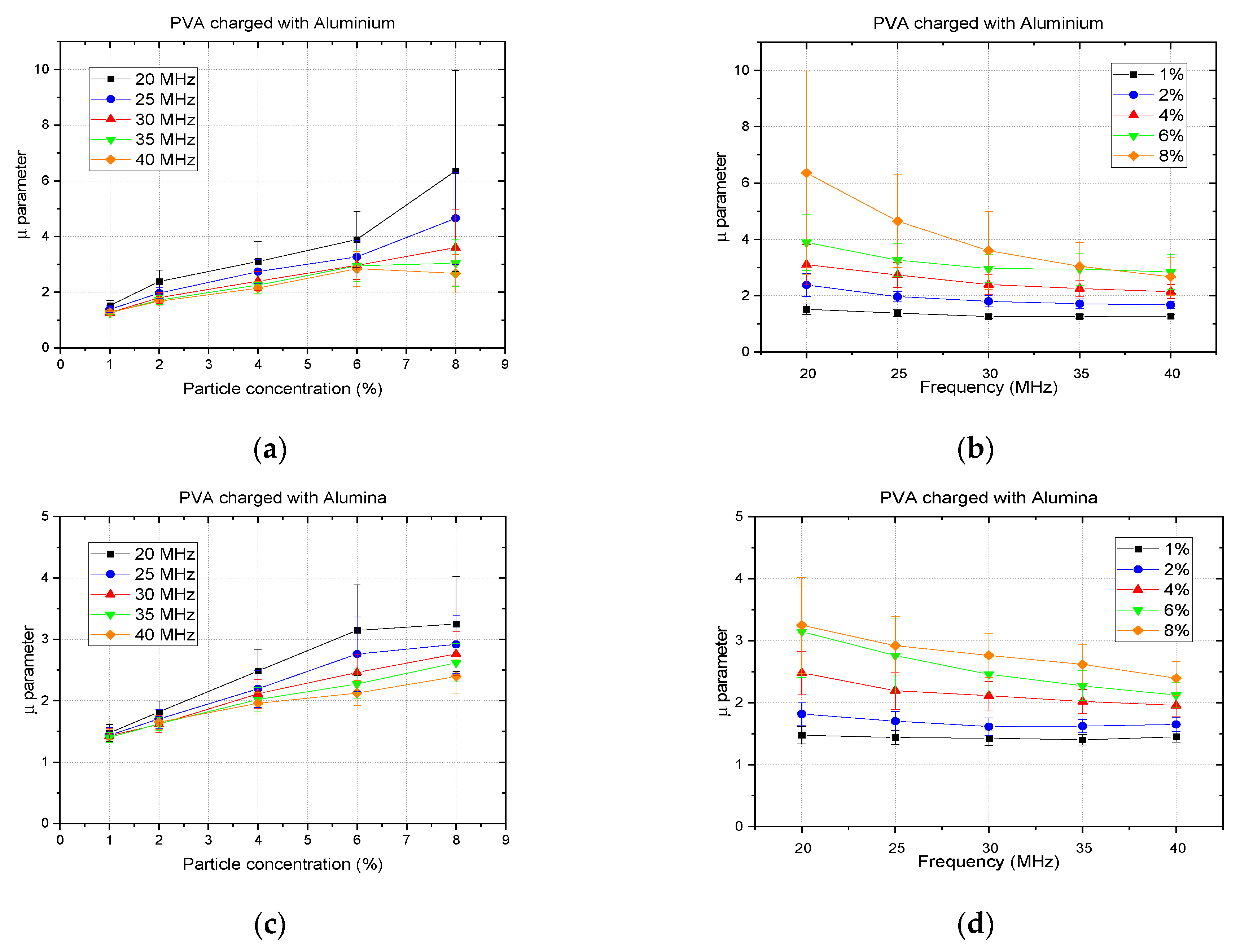
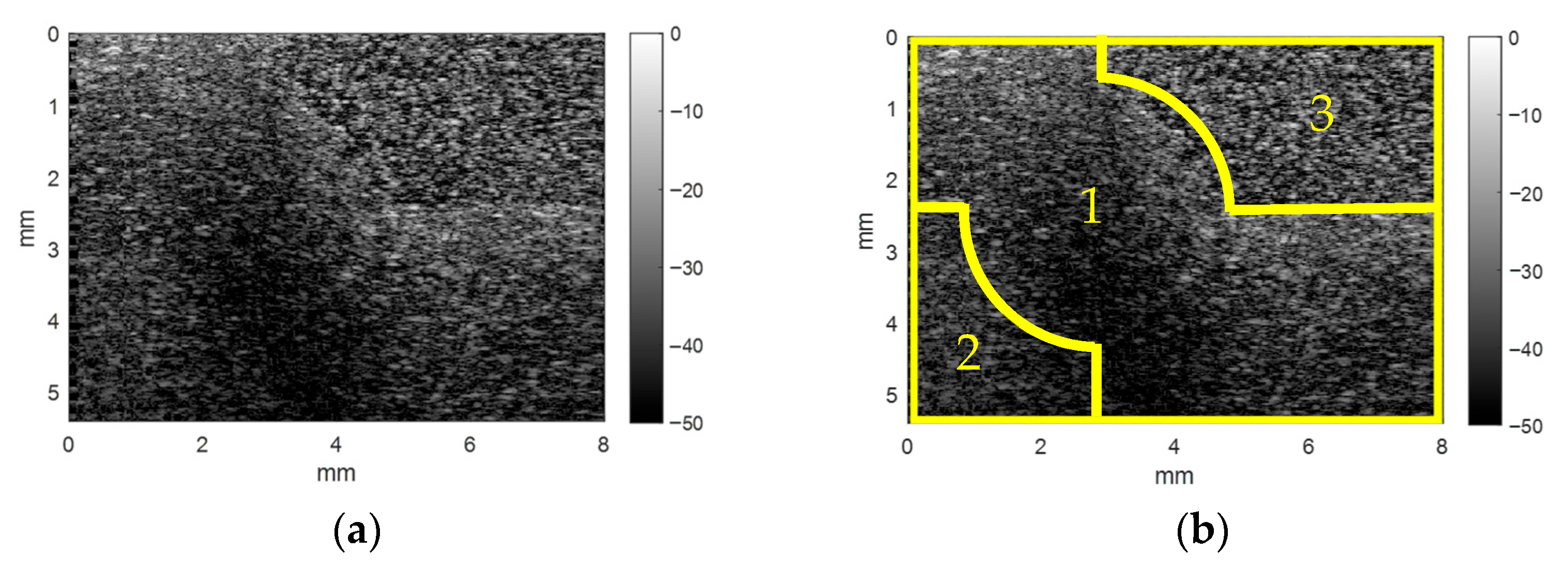
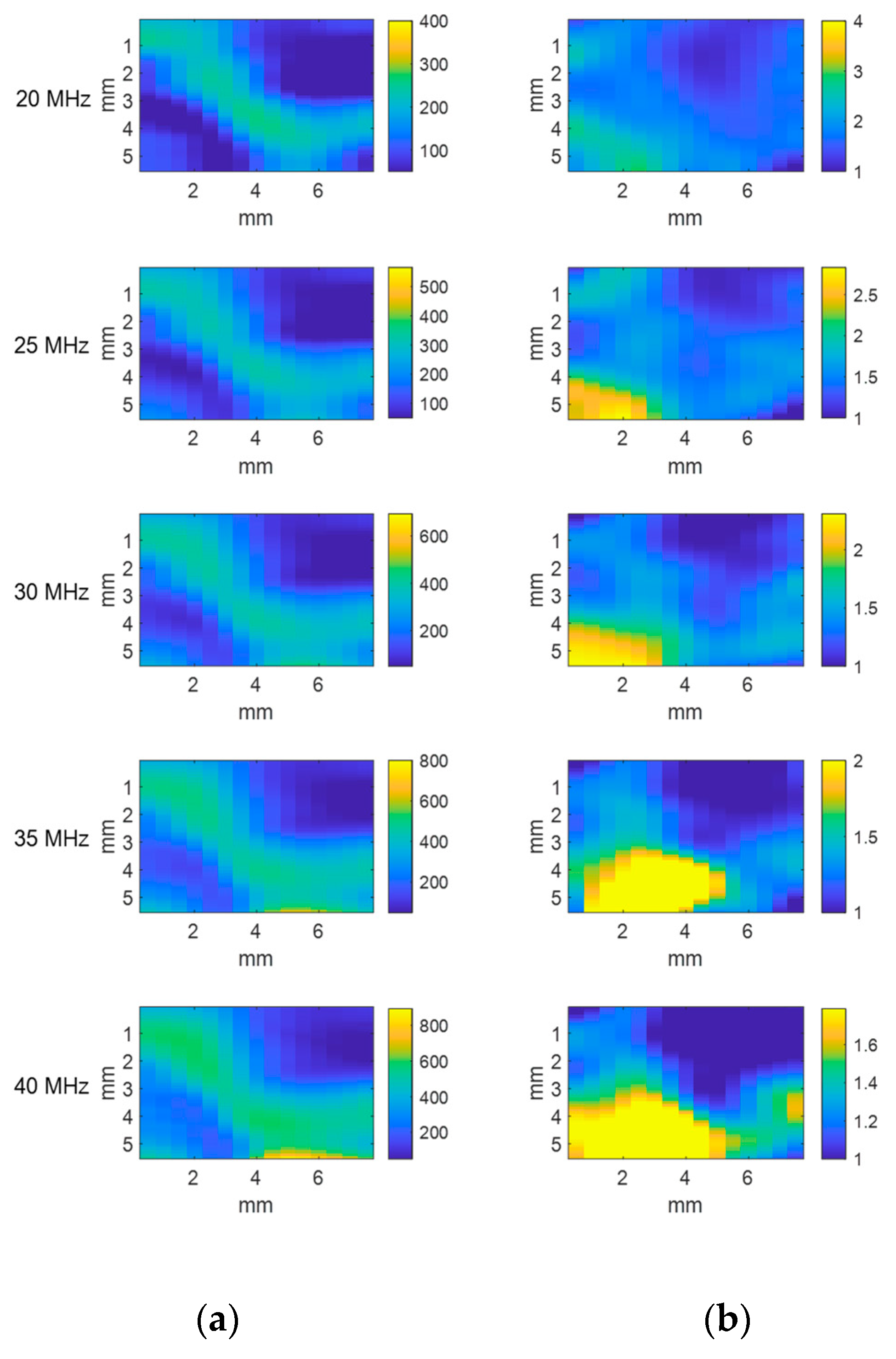
3. Results and Discussion
3.1. Analysis of Single-Material Samples
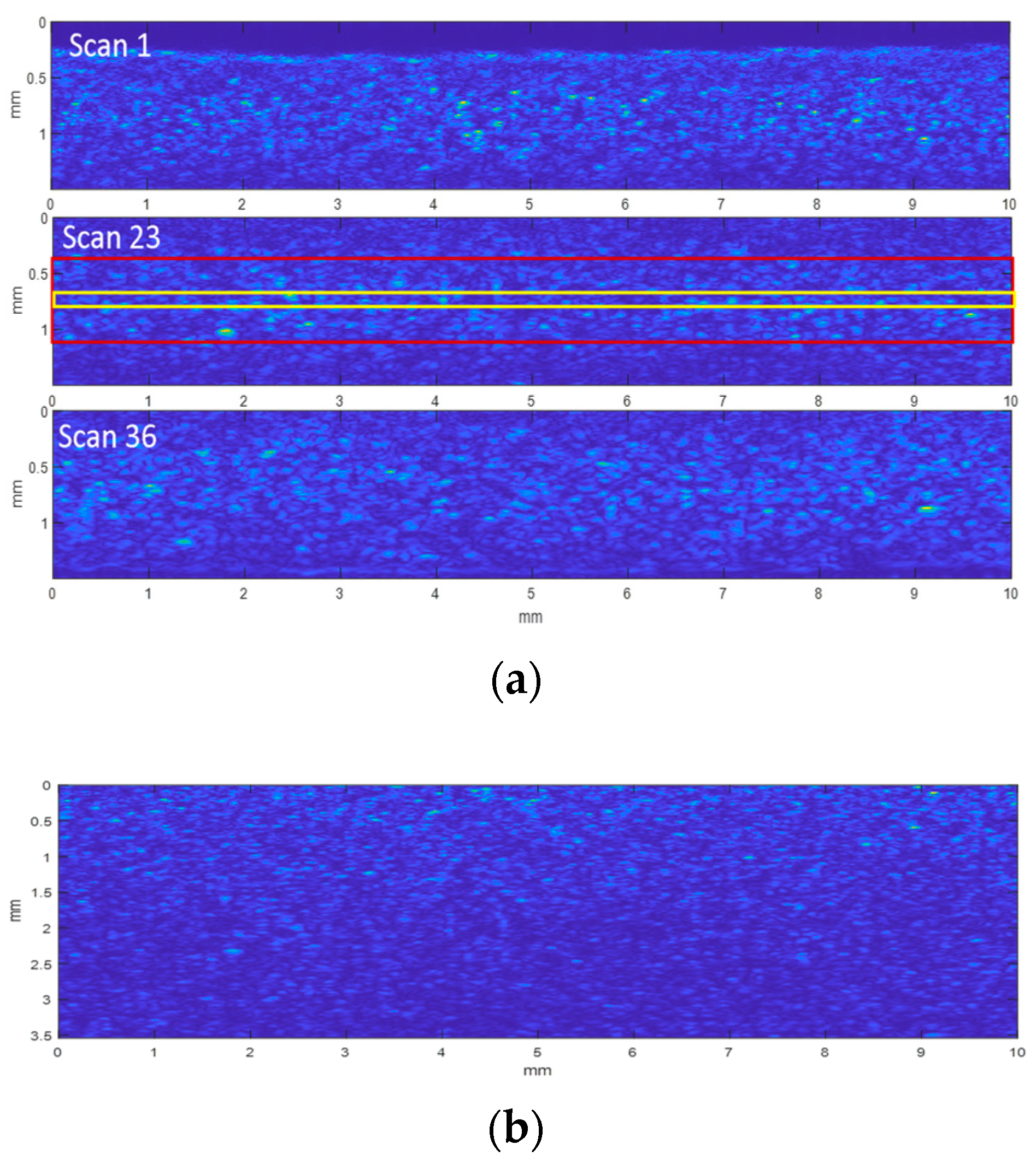
3.1.1. Echo Envelope Images
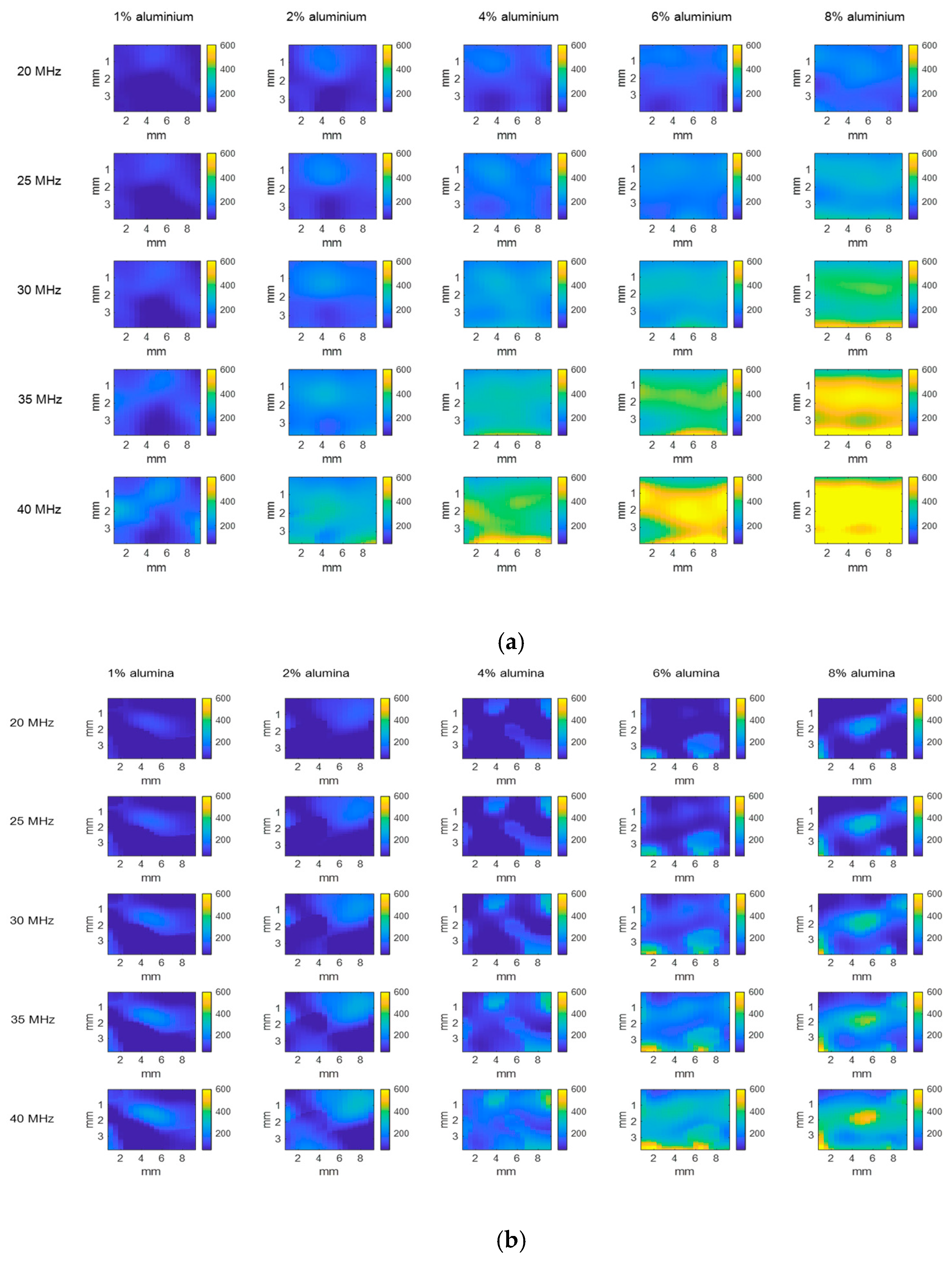
3.1.2. Attenuation Coefficient Images
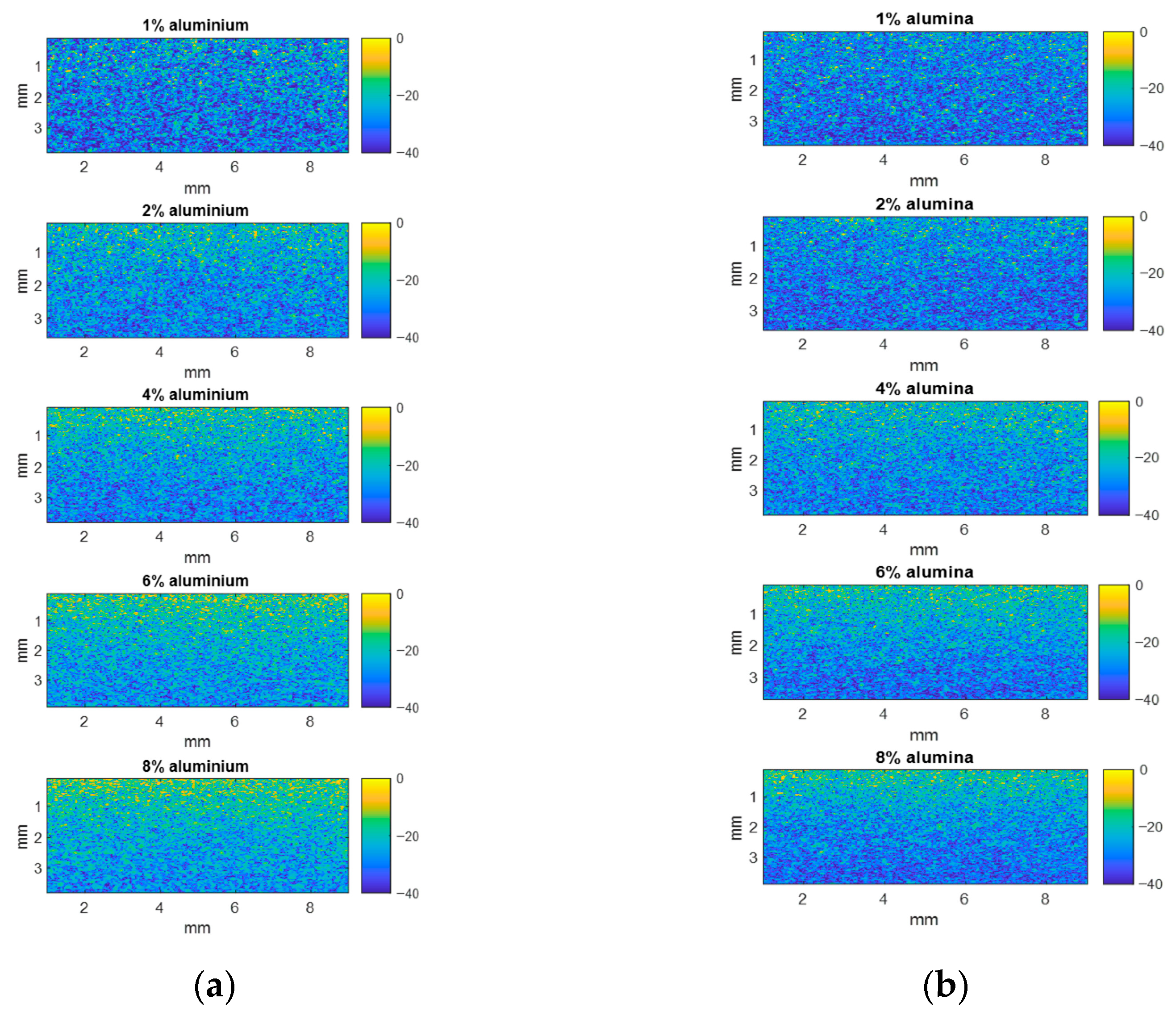
3.1.3. Scatterer Clustering Parameter Images
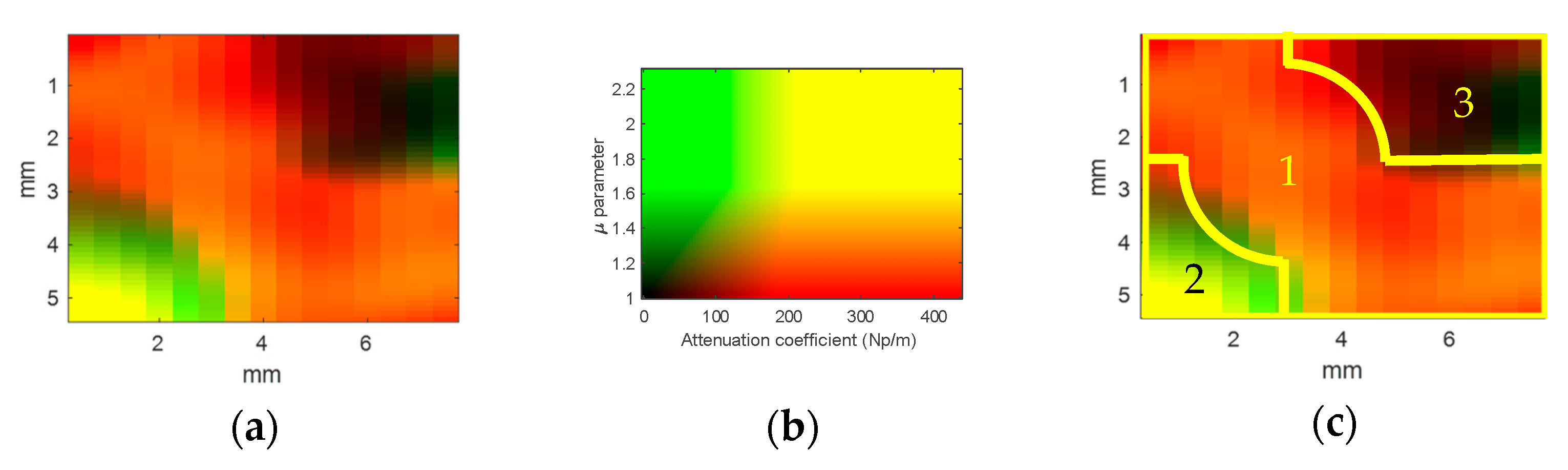
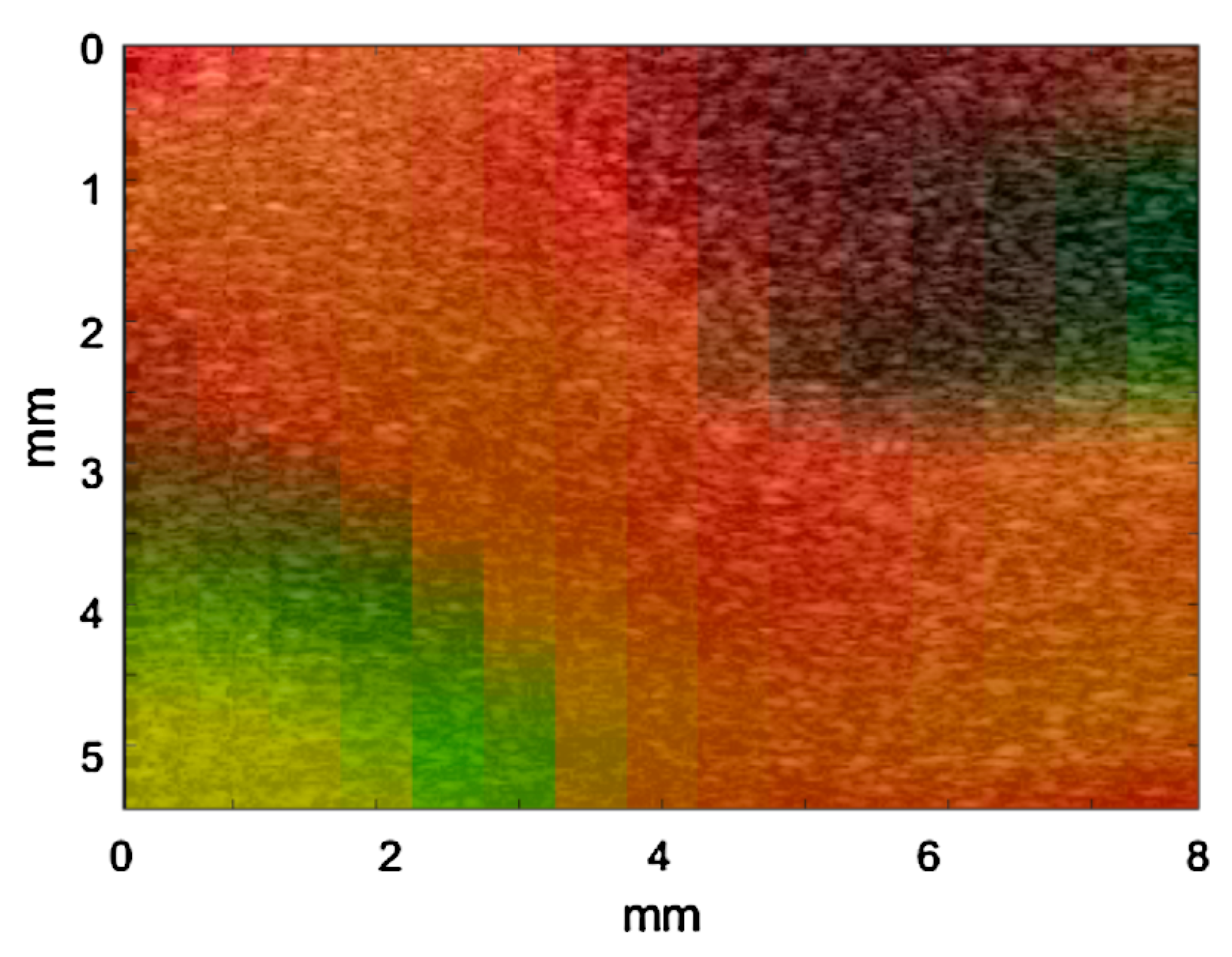
3.2. Multimodal Image of the Composite Phantom
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PVA | poly-vinyl alcohol |
References
- Lizzi, F.; Ostromogilsky, M.; Feleppa, E.; Rorke, M.; Yaremko, M. Relationship of ultrasonic spectral parameters to features of tissue microstructure. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 1987, 34, 319–329. [Google Scholar] [CrossRef]
- Muleki-Seya, P.; Han, A.; Andre, M.P.; Erdman, J.W., Jr.; O’bRien, W.D., Jr. Analysis of two quantitative ultrasound approaches. Ultrason. Imaging 2018, 40, 84–96. [Google Scholar] [CrossRef]
- Labyed, Y.; Bigelow, T.A. Estimating the total ultrasound attenuation along the propagation path by using a reference phantom. J. Acoust. Soc. Am. 2010, 128, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Bigelow, T.A. Estimating the total ultrasound attenuation along the propagation path by applying multiple filters to backscattered echoes from a single spherically focused source. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2010, 57, 900–907. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Varghese, T. Hybrid spectral domain method for attenuation slope estimation. Ultrasound Med. Biol. 2008, 34, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Q.; Wu, W.; Wu, S.; Tsui, P.-H. Hepatic steatosis assessment using quantitative ultrasound parametric imaging based on backscatter envelope statistics. Appl. Sci. 2019, 9, 661. [Google Scholar] [CrossRef]
- Destrempes, F.; Cloutier, G. A Critical Review and Uniformized Representation of Statistical Distributions Modeling the Ultrasound Echo Envelope. Ultrasound Med. Biol. 2010, 36, 1037–1051. [Google Scholar] [CrossRef]
- Piotrzkowska, H.; Litniewski, J.; Lewandowski, M.; Szymanska, E.; Nowicki, A. Use of quantitative ultrasound to measure acoustic properties of human skin. Arch. Acoust. 2009, 34, 471–480. [Google Scholar]
- Omura, M.; Coron, A.; Bridal, S.L.; Yoshida, K.; Yamaguchi, T. Quantitative ultrasound imaging of bacterial infection on skin ulcers by envelope statistics analysis. In Proceedings of the 2016 IEEE International Ultrasonics Symposium (IUS), Tours, France, 18–21 September 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Hachiya, H. Proposal of a parametric imaging method for quantitative diagnosis of liver fibrosis. J. Med. Ultrason. 2010, 37, 155–166. [Google Scholar] [CrossRef]
- Bigelow, T.A.; McFarlin, B.L.; O’bRien, W.D.; Oelze, M.L. In vivo ultrasonic attenuation slope estimates for detecting cervical ripening in rats: Preliminary results. J. Acoust. Soc. Am. 2008, 123, 1794–1800. [Google Scholar] [CrossRef]
- Mamou, J.; Coron, A.; Oelze, M.L.; Saegusa-Beecroft, E.; Hata, M.; Lee, P.; Machi, J.; Yanagihara, E.; Laugier, P.; Feleppa, E.J. Three-Dimensional High-Frequency Backscatter and Envelope Quantification of Cancerous Human Lymph Nodes. Ultrasound Med. Biol. 2011, 37, 345–357. [Google Scholar] [CrossRef]
- Sadeghi-Naini, A.; Papanicolau, N.; Falou, O.; Zubovits, J.; Dent, R.; Verma, S.; Trudeau, M.; Boileau, J.F.; Spayne, J.; Iradji, S.; et al. Quantitative ultrasound evaluation of tumor cell death response in locally advanced breast cancer patients receiving chemotherapy. Clin. Cancer Res. 2013, 19, 2163–2174. [Google Scholar] [CrossRef]
- Rohrbach, D.; Wodlinger, B.; Wen, J.; Mamou, J.; Feleppa, E.J. High-Frequency quantitative ultrasound for imaging prostate cancer using a novel micro-ultrasound scanner. Ultrasound Med. Biol. 2018, 44, 1341–1354. [Google Scholar] [CrossRef]
- Rosado-Mendez, I.M.; Noguchi, K.K.; Castañeda-Martinez, L.; Kirvassilis, G.; Wang, S.H.; Manzella, F.; Swiney, B.S.; Masuoka, K.; Capuano, S.; Brunner, K.G.; et al. Quantitative ultrasound and apoptotic death in the neonatal primate brain. Neurobiol. Dis. 2019, 127, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, G.; Destrempes, F.; Yu, F.; Tang, A. Quantitative ultrasound imaging of soft biological tissues: A primer for radiologists and medical physicists. Insights Imaging 2021, 12, 127. [Google Scholar] [CrossRef]
- Becker, A.S.; Mueller, M.; Stoffel, E.; Marcon, M.; Ghafoor, S.; Boss, A. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: A pilot study. Br. J. Radiol. 2018, 91, 20170576. [Google Scholar] [CrossRef]
- Brattain, L.J.; Telfer, B.A.; Dhyani, M.; Grajo, J.R.; Samir, A.E. Machine learning for medical ultrasound: Status, methods, and future opportunities. Abdom. Radiol. 2018, 43, 786–799. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Yang, X.; Lei, B.; Liu, L.; Li, S.X.; Ni, D.; Wang, T. Deep Learning in Medical Ultrasound Analysis: A Review. Engineering 2019, 5, 261–275. [Google Scholar] [CrossRef]
- Skandha, S.S.; Nicolaides, A.; Gupta, S.K.; Koppula, V.K.; Saba, L.; Johri, A.M.; Kalra, M.S.; Suri, J.S. A hybrid deep learning paradigm for carotid plaque tissue characterization and its validation in multicenter cohorts using a supercomputer framework. Comput. Biol. Med. 2022, 141, 105131. [Google Scholar] [CrossRef] [PubMed]
- Sahashi, Y.; Kawasaki, M.; Okubo, M.; Kawamura, I.; Kawase, Y.; Yoshida, A.; Tanaka, T.; Hattori, A.; Matsuo, H.; Ozaki, Y. Development of 60 MHz integrated backscatter intravascular ultrasound and tissue characterization of attenuated signal coronary plaques that cause myocardial injury after percutaneous coronary intervention. Heart Vessel. 2022, 37, 1689–1700. [Google Scholar] [CrossRef]
- Fernández, A.; Ibáñez, A.; Parrilla, M.; Elvira, L.; Bassat, Q.; Jiménez, J. Estimation of the concentration of particles in suspension based on envelope statistics of ultrasound backscattering. Ultrasonics 2021, 116, 106501. [Google Scholar] [CrossRef]
- Elvira, L.; Fernández, A.; León, L.; Ibáñez, A.; Parrilla, M.; Martínez, Ó.; Jiménez, J. Evaluation of the Cell Concentration in Suspensions of Human Leukocytes by Ultrasound Imaging: The Influence of Size Dispersion and Cell Type. Sensors 2023, 23, 977. [Google Scholar] [CrossRef] [PubMed]
- Destrempes, F.; Cloutier, G. Statistical modeling of ultrasound signals related to the packing factor of wave scattering phenomena for structural characterization. J. Acoust. Soc. Am. 2021, 150, 3544–3556. [Google Scholar] [CrossRef]
- Oelze, M.L.; Mamou, J. Review of Quantitative Ultrasound: Envelope Statistics and Backscatter Coefficient Imaging and Contributions to Diagnostic Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 336–351. [Google Scholar] [CrossRef]
- Destrempes, F.; Cloutier, G. Review of envelope statistics models for quantitative ultrasound imaging and tissue characterization. In Quantitative Ultrasound in Soft Tissues; Mamou, J., Oelze, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 219–274. [Google Scholar] [CrossRef]
- Jakeman, E. On the statistics of K-distributed noise. J. Phys. A Math. Gen. 1980, 13, 31–48. [Google Scholar] [CrossRef]
- Dutt, V.; Greenleaf, J.F. Ultrasound echo envelope analysis using a homodyned K distribution signal model. Ultrason. Imaging 1994, 16, 265–287. [Google Scholar] [CrossRef]
- Bigelow, T.A.; Labyed, Y. Attenuation Compensation and Estimation. In Quantitative Ultrasound in Soft Tissues; Mamou, J., Oelze, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 71–93. [Google Scholar] [CrossRef]
- Szabo, T.L. Causal theories and data for acoustic attenuation obeying a frequency power law. J. Acoust. Soc. Am. 1995, 97, 14–24. [Google Scholar] [CrossRef]
- Elvira, L.; Durán, C.; Higuti, R.T.; Tiago, M.M.; Ibáñez, A.; Parrilla, M.; Valverde, E.; Jiménez, J.; Bassat, Q. Development and Characterization of Medical Phantoms for Ultrasound Imaging Based on Customizable and Mouldable Polyvinyl Alcohol Cryogel–Based Materials and 3-D Printing: Application to High-Frequency Cranial Ultrasonography in Infants. Ultrasound Med. Biol. 2019, 45, 2226–2241. [Google Scholar] [CrossRef]
- Parker, K.J. Scattering and reflection identification in H-scan images. Phys. Med. Biol. 2016, 61, L20–L28. [Google Scholar] [CrossRef] [PubMed]
- Khairalseed, M.; Brown, K.; Parker, K.J.; Hoyt, K. Real-time H-scan ultrasound imaging using a Verasonics research scanner. Ultrasonics 2019, 94, 28–36. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elvira, L.; de León, C.; Durán, C.; Ibáñez, A.; Parrilla, M.; Martínez-Graullera, Ó. Tissue Characterization by Ultrasound: Linking Envelope Statistics with Spectral Analysis for Simultaneous Attenuation Coefficient and Scatterer Clustering Quantification. Appl. Sci. 2025, 15, 9924. https://doi.org/10.3390/app15189924
Elvira L, de León C, Durán C, Ibáñez A, Parrilla M, Martínez-Graullera Ó. Tissue Characterization by Ultrasound: Linking Envelope Statistics with Spectral Analysis for Simultaneous Attenuation Coefficient and Scatterer Clustering Quantification. Applied Sciences. 2025; 15(18):9924. https://doi.org/10.3390/app15189924
Chicago/Turabian StyleElvira, Luis, Carla de León, Carmen Durán, Alberto Ibáñez, Montserrat Parrilla, and Óscar Martínez-Graullera. 2025. "Tissue Characterization by Ultrasound: Linking Envelope Statistics with Spectral Analysis for Simultaneous Attenuation Coefficient and Scatterer Clustering Quantification" Applied Sciences 15, no. 18: 9924. https://doi.org/10.3390/app15189924
APA StyleElvira, L., de León, C., Durán, C., Ibáñez, A., Parrilla, M., & Martínez-Graullera, Ó. (2025). Tissue Characterization by Ultrasound: Linking Envelope Statistics with Spectral Analysis for Simultaneous Attenuation Coefficient and Scatterer Clustering Quantification. Applied Sciences, 15(18), 9924. https://doi.org/10.3390/app15189924





