Nutritional Analysis of Bottarga and Pilot Study Protocol for Bottarga Supplementation in Individuals with Prediabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Lipid Analysis and Nutritional Profile of a Commercial Bottarga Sample
2.1.1. Lipid Extraction
2.1.2. NMR Analysis
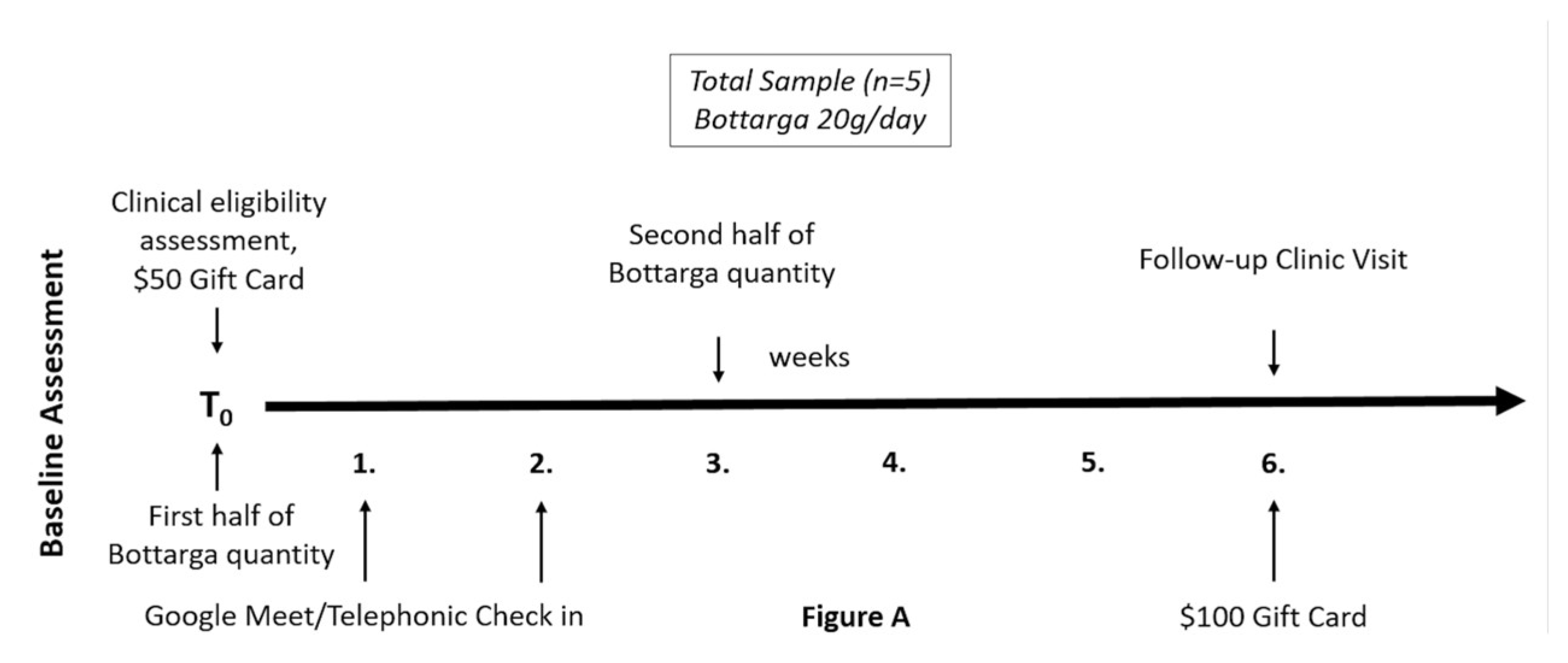
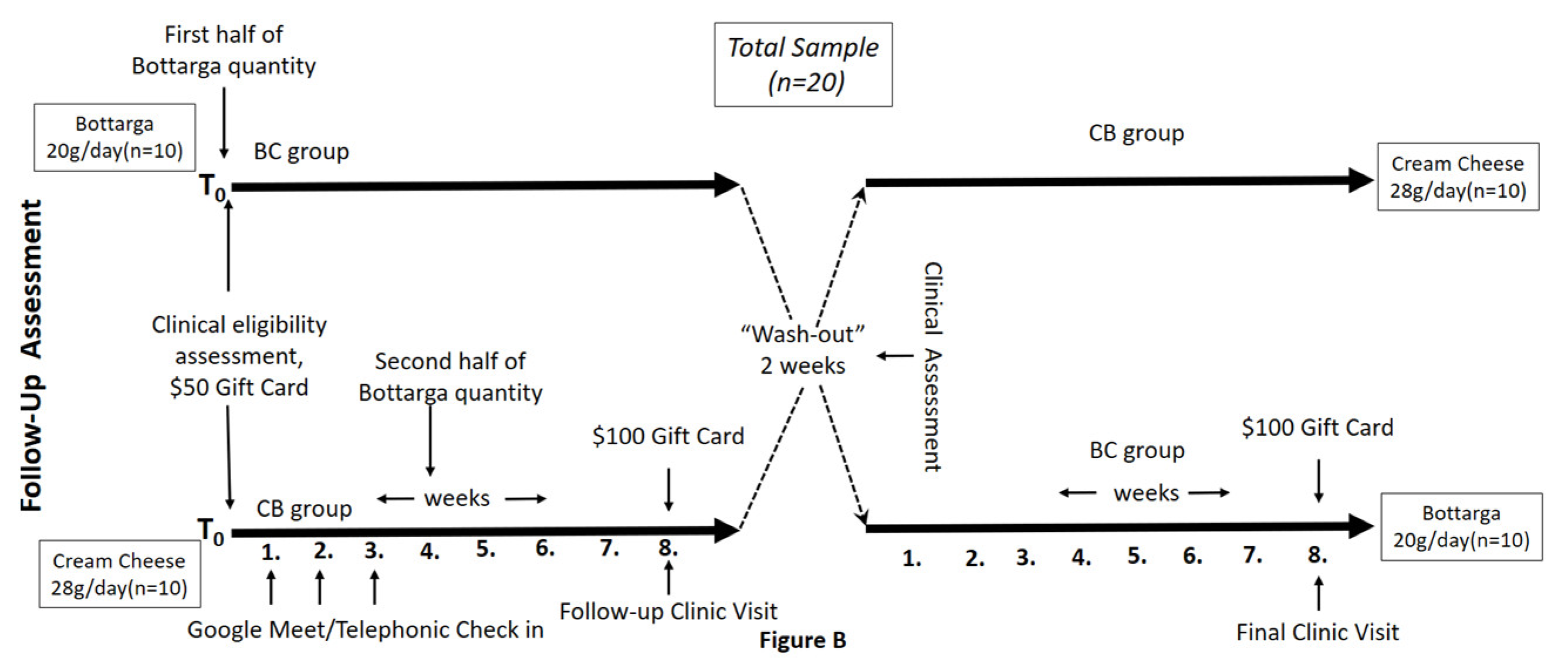
2.2. Pilot Study Sample
2.3. Study Screening Assessment
2.4. Baseline Clinical Eligibility Assessment
2.5. Bottarga Supplementation
2.6. Study Measurements
2.6.1. Sociodemographic Data and Mediterranean Diet Adherence Screener Score (MEDAS)
2.6.2. Fasting Blood Draws
2.6.3. Blood Pressure and Heart Rate
2.6.4. Anthropometrics/Body Composition
2.6.5. Waist (WC) and Hip Circumference (HC)
2.7. Safety of the Study Supplements
- Food storage at study site: the bottarga and cream cheese will be stored in a dedicated refrigerator at a temperature of 4 °C (39 °F) or below, in sealed, air-tight containers to maintain freshness and quality, in compliance with food safety guidelines.
- Packaging for distribution:
- -
- Both bottarga and cream cheese will be provided in pre-packaged, sealed portions to minimize handling and ensure product safety. They will be distributed to participants in insulated cooler bags containing ice packs to maintain a temperature of 4 °C (39 °F) or below during transport.
- Transport and Storage by Participants:
- -
- Participants will be instructed to refrigerate both the bottarga and cream cheese immediately upon arriving home, maintaining storage at or below 4 °C (39 °F).
- -
- Participants will also receive instructions to consume the products within the specified use-by period and to report any issues with product condition promptly.
- Additional Food Safety Considerations:
- -
- Given the short travel time expected for most participants and the use of insulated cooler bags with icepacks, there is minimal concern about food safety during transport.
- -
- Participants will receive written guidelines on proper handling and storage of both Bottarga and cream cheese, in alignment with food safety protocols.
2.8. Study Incentives
2.9. Statistical Analysis
3. Results
3.1. Nutritional Profile of a Commercial Bottarga Sample
3.2. Lipid Analysis of Bottarga
3.3. Anticipated Outcomes of the Pilot Study
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasco, L.; Gai, F.; Maricchiolo, G.; Parisi, G.; Terova, G.; Rotolo, L.; Lussiana, C.; Antonopoulou, E.; Mente, E. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Crippa, M.; Solazzo, E.; Guizzardi, D.; Monforti-Ferrario, F.; Tubiello, F.N.; Leip, A. Food systems are responsible for a third of global anthropogenic GHG emissions. Nat. Food 2021, 2, 198–209. [Google Scholar] [CrossRef]
- Parker, R.W.R.; Blanchard, J.L.; Gardner, C.; Green, B.S.; Hartmann, K.; Tyedmers, P.H.; Watson, R.A. Fuel use and greenhouse gas emissions of world fisheries. Nat. Clim. Chang. 2018, 8, 333–337. [Google Scholar] [CrossRef]
- Leape, J.; von Braun, J.; Afsana, K.; Fresco, L.O.; Hassan, M.H.A. Science and Innovations for Food Systems Transformation. In Science and Innovations for Food Systems Transformation; Springer: Cham, Switzerland, 2023; pp. 401–419. [Google Scholar] [CrossRef]
- Crona, B.I.; Wassénius, E.; Jonell, M.; Troell, M.; Deutsch, L.; MacNeil, M.A.; Klinger, D.H.; Vermeer, D.; Campbell, L.M.; Rönnbäck, P.; et al. Four ways blue foods can help achieve food system ambitions across nations. Nature 2023, 616, 104–112. [Google Scholar] [CrossRef]
- Gephart, J.A.; Henriksson, P.J.G.; Parker, R.W.R.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef] [PubMed]
- The Sustainable Development Goals Report Special Edition. 2023. Available online: https://unstats.un.org/sdgs/report/2023/ (accessed on 15 June 2025).
- Food and Agriculture Organization of the United Nations. 2011. Available online: http://www.fao.org/docrep/014/mb060e/mb060e00.pdf (accessed on 15 June 2025).
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, S.; Fayyaz-ul-Hassan; Qadir, G.; Hayat, R.; Shaheen, F.A.; Raza, M.A. Innovative Processes and Technologies for Nutrient Recovery from Wastes: A Comprehensive Review. Sustainability 2019, 11, 4938. [Google Scholar] [CrossRef]
- Saleh, N.E.; Wassef, E.A.; Abdel-Mohsen, H.H. Sustainable fish and seafood production and processing. In Sustainable Fish Production and Processing; Academic Press: Cambridge, MA, USA, 2022; pp. 259–291. [Google Scholar] [CrossRef]
- Vallainc, D.; Mura, L.; Cossu, P.; Scarpa, F.; Cau, A.; Meloni, M.; Coroneo, V.; Cannas, R.; Addis, P. Producing flat-head grey mullet Mugil cephalus (Linnaeus, 1758) fries in captivity from sexually mature adults collected in Sardinian lagoons. Aquac. Rep. 2021, 21, 100844. [Google Scholar] [CrossRef]
- Cossu, P.; Mura, L.; Scarpa, F.; Cau, A.; Meloni, M.; Coroneo, V.; Cannas, R.; Addis, P. Genetic patterns in Mugil cephalus and implications for fisheries and aquaculture management. Sci. Rep. 2021, 11, 2887. [Google Scholar] [CrossRef]
- Ben Khemis, I.; Hamza, N.; Sadok, S. Nutritional quality of the fresh and processed grey mullet (Mugilidae) products: A short review including data concerning fish from freshwater. Aquat. Living Resour. 2019, 32, 2. [Google Scholar] [CrossRef]
- Georgiou, D.; Tzika, P.; Charisis, A.; Kalogianni, E.P. Effect of extraction method on the yield and quality of mullet roe oil. Eur. J. Lipid Sci. Technol. 2023, 125, 2300008. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Nomikos, T.; Chiou, A.; Fragopoulou, E.; Antonopoulou, S. Chemical composition of greek avgotaracho prepared from mullet (Mugil cephalus): Nutritional and health benefits. J. Agric. Food Chem. 2008, 56, 5916–5925. [Google Scholar] [CrossRef]
- Torres-Castillo, N.; Campos-Perez, W.; Gonzalez-Becerra, K.; Hernandez-Cañaveral, I.; Vizmanos, B.; Muñoz-Valle, J.; Martinez-Lopez, E. Waist Circumference Is an Anthropometric Parameter That Identifies Women with Metabolically Unhealthy Phenotypes. Nutrients 2018, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 Fatty Acids in Obesity and Metabolic Syndrome: A Mechanistic Update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Atzeri, A.; Putzu, D.; Scano, P. A diet enriched with Mugil cephalus processed roes modulates the tissue lipid profile in healthy rats: A biochemical and chemometric assessment. Food Funct. 2016, 7, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Scano, P.; Atzeri, A.; Deiana, M.; Falchi, A.M. Potential anti-tumor effects of Mugil cephalus processed roe extracts on colon cancer cells. Food Chem. Toxicol. 2013, 60, 471–478. [Google Scholar] [CrossRef]
- Rosa, A.; Atzeri, A.; Deiana, M.; Melis, M.P.; Loru, D.; Incani, A.; Cabboi, B.; Dessì, M.A. Effect of aqueous and lipophilic mullet (Mugil cephalus) Bottarga extracts on the growth and lipid profile of intestinal Caco-2 cells. J. Agric. Food Chem. 2011, 59, 1658–1666. [Google Scholar] [CrossRef]
- Rosa, A.; Piras, A.; Nieddu, M.; Putzu, D.; Cesare Marincola, F.; Falchi, A.M. Mugil cephalus roe oil obtained by supercritical fluid extraction affects the lipid profile and viability in cancer HeLa and B16F10 cells. Food Funct. 2016, 7, 4092–4103. [Google Scholar] [CrossRef]
- Fatahi, S.; Sohouli, M.H.; da Silva Magalhães, E.I.; da Cruz Silveira, V.N.; Zanghelini, F.; Rahmani, P.; Kord-Varkaneh, H.; Sharifi-Zahabi, E.; Shidfar, F. Comparing the effects of docosahexaenoic and eicosapentaenoic acids on cardiovascular risk factors: Pairwise and network meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 11–21. [Google Scholar] [CrossRef]
- Zhang, X.; Ritonja, J.A.; Zhou, N.; Chen, B.E.; Li, X. Omega-3 polyunsaturated fatty acids intake and blood pressure: A dose-response meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2022, 11, e025071. [Google Scholar] [CrossRef]
- Xie, S.; Galimberti, F.; Olmastroni, E.; Luscher, T.F.; Carugo, S.; Catapano, A.L.; Casula, M.; META-LIPID Group. Effect of lipid-lowering therapies on C-reactive protein levels: A comprehensive meta-analysis of randomized controlled trials. Cardiovasc. Res. 2024, 120, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Talebi, S.; Sadeghi, O.; Hassanizadeh, S.; Askari, G.; Bagherniya, M.; Sahebkar, A. Effects of marine-derived n-3 PUFA supplementation on soluble adhesion molecules: A systematic review and dose-response meta-analysis of randomized controlled trials. Pharmacol. Res. 2023, 197, 106963. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2023; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2023. Available online: https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 10 July 2025).
- Folch, M.; Lees, G.H.; Sloane-Stanley. A simple method for the isolation and purification of total lipid from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Scano, P.; Rosa, A.; Locci, E.; Dessì, M.A.; Lai, A. NMR study of the lipid profile of mullet raw roe and bottarga. Eur. J. Lipid Sci. Technol. 2009, 111, 505–512. [Google Scholar] [CrossRef]
- Williamson, K.; Hatzakis, E. NMR spectroscopy as a robust tool for the rapid evaluation of the lipid profile of fish oil supplements. J. Vis. Exp. 2017, 123, e55547. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Tzika, P.; Georgiou, D.; Charisis, A.; Hans, S.; Lordan, R.; Zabetakis, I.; Kalogianni, E.P. Oil from mullet roe byproducts: Effect of oil extraction method on human erythrocytes and platelets. Foods 2023, 13, 79. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Bonesi, M.; Tundis, R.; Menichini, F.; Picci, N.; Loizzo, M.R. Evaluation of fatty acids and biogenic amines profiles in mullet and tuna roe during six months of storage at 4 °C. J. Food Compos. Anal. 2015, 40, 52–60. [Google Scholar] [CrossRef]
- Caredda, M.; Addis, M.; Pes, M.; Fois, N.; Sanna, G.; Piredda, G.; Sanna, G. Physico-chemical, colorimetric, rheological parameters and chemometric discrimination of the origin of Mugil cephalus roes during the manufacturing process of bottarga. Food Res. Int. 2018, 108, 128–135. [Google Scholar] [CrossRef]
- Sanna, M.; Carta, S.; Murgia, M.A.; Chessa, M.; Nudda, A.; Mangia, N.P. Microbiological Control and Nutritional and Sensorial Characterization of Bottarga by Mugil cephalus Produced in Sardinia (Italy). Appl. Sci. 2025, 15, 1714. [Google Scholar] [CrossRef]
- Scano, P.; Rosa, A.; Cesare Marincola, F.; Locci, E.; Melis, M.P.; Dessì, M.A.; Lai, A. 13C NMR, GC and HPLC characterization of lipid components of the salted and dried mullet (Mugil cephalus) roe “bottarga”. Chem. Phys. Lipids 2008, 151, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, R.; Bolzacchini, E.; Galliani, G.; Gugliersi, F.; Rindone, B.; Rindone, M.; Tacconi, M.T.; Terraneo, A. Determination of the content of wax esters in some seafoods and their molecular composition: A comparison with ω-3 enriched wax esters. LWT–Food Sci. Technol. 2007, 40, 569–573. [Google Scholar] [CrossRef]
- Schots, P.C.; Pedersen, A.M.; Eilertsen, K.E.; Olsen, R.L.; Larsen, T.S. Possible health effects of a wax ester rich marine oil. Front. Pharmacol. 2020, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Höper, A.C.; Salma, W.; Sollie, S.J.; Hafstad, A.D.; Lund, J.; Khalid, A.M.; Raa, J.; Aasum, E.; Larsen, T.S. Wax esters from the marine copepod Calanus finmarchicus reduce diet-induced obesity and obesity-related metabolic disorders in mice. J. Nutr. 2014, 144, 164–169. [Google Scholar] [CrossRef]
- Wasserfurth, P.; Nebl, J.; Boßlau, T.K.; Krüger, K.; Hahn, A.; Schuchardt, J.P. Intake of Calanus finmarchicus oil for 12 weeks improves omega-3 index in healthy older subjects engaging in an exercise programme. Br. J. Nutr. 2021, 125, 432–439. [Google Scholar] [CrossRef]
- Osipova, D.; Kokoreva, K.; Lazebnik, L.; Golovanova, E.; Pavlov, C.; Dukhanin, A.; Orlova, S.; Starostin, K. Regression of liver steatosis following phosphatidylcholine administration: A review of molecular and metabolic pathways involved. Front. Pharmacol. 2022, 13, 797923. [Google Scholar] [CrossRef]
| Nutrition Facts | |
|---|---|
| Cream cheese (28 g) | |
| Calories (kcal) | 70 |
| Fat (g) | 6.5 |
| of which saturated | 3.6 |
| of which monounsaturated | 1.6 |
| of which polyunsaturated | 0.3 |
| Trans fat (g) | 0 |
| Carbohydrates (g) | 1 |
| of which sugars | 1 |
| Dietary fibers (g) | 0 |
| Protein (g) | 2 |
| Salt (mg) | 300 |
| Sodium (mg) | 120 |
| Cholesterol (mg) | 20 |
| Measures and Timing | Study Phase | |||||
|---|---|---|---|---|---|---|
| Phase 1 (Dose-Confirming Study) | Phase 2 (Randomized, Controlled, Cross-Over Pilot Study) | |||||
| Baseline | 6 Weeks | Baseline | 8 weeks | Washout Period | 8 Weeks | |
| Dietary questionnaires to evaluate n-3 fatty acids and olive oil intake [Mediterranean Diet Adherence Screener (MEDAS)] | X | X | X | X | X | X |
| Anthropometrics/Body composition weight, height, waist and hip circumference, bioelectrical impedance analysis (BIA) | X | X | X | X | X | X |
| Resting blood pressure and heart rate | X | X | X | X | X | X |
| Biochemical measures (fasting glucose, liver function tests, hemoglobin A1C, hs-CRP, lipid profile) | X | X | X | X | X | X |
| Nutrition Facts | |||
|---|---|---|---|
| Per 100 g | Per 20 g | ||
| Energy | 338/1411 | 68/282.2 | kcal/Kj |
| Protein | 34.8 | 6.96 | g |
| Lipids | 20.9 | 4.18 | g |
| Saturated fatty acids | 6.5 | 1.3 | g |
| Monounsaturated fatty acids | 9.7 | 1.94 | g |
| Polyunsaturated fatty acids | 4.5 | 0.9 | g |
| Trans fatty acids | 0.1 | 0.02 | g |
| Carbohydrates | 2.6 | 0.52 | g |
| of which sugars | <0.5 | <0.1 | g |
| Dietary fibers | <0.5 | <0.1 | g |
| Cholesterol | 484.9 | 96.98 | mg |
| Ash | 2.8 | 0.56 | g |
| Macrominerals | |||
| Sodium | 725 | 145 | mg |
| Phosphorus | 327 | 65.4 | mg |
| Potassium | 234.2 | 46.84 | mg |
| Calcium | 65.3 | 13.06 | mg |
| Trace minerals | |||
| Iodine | 256 | 51.2 | μg |
| Chromium | 20 | 4 | μg |
| Iron | 10.5 | 2.1 | mg |
| Zinc | 4.05 | 0.81 | mg |
| Lipid-soluble vitamins | |||
| Vitamin A | 1040 | 208 | μg |
| Vitamin Κ | 21.9 | 4.38 | μg |
| Water-soluble vitamins | |||
| Vitamin Β5 | |||
| (Pantothenic acid) | 78.4 | 15.68 | mg |
| Vitamin Β7 (biotin) | 21.9 | 4.38 | μg |
| Vitamin Β12 | 6.4 | 1.28 | μg |
| Type of Compound | mol% |
|---|---|
| Wax esters (WEs) | 72.9 a |
| n-3 fatty chains | 12.6 b |
| DHA | 4.9 b |
| EPA | 3.1 b |
| Triacylglycerols (TAGs) | 8.8 a |
| Free fatty acids (FFAs) | 8.2 a |
| Phosphatidyl choline (PC) | 6.2 a |
| Cholesterol (Ch) | 3.8 a |
| Cholesterol esters (CEs) | 0.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lidoriki, I.; Magiatis, P.; Melliou, E.; Georgakopoulos, S.; Kales, S.N. Nutritional Analysis of Bottarga and Pilot Study Protocol for Bottarga Supplementation in Individuals with Prediabetes. Appl. Sci. 2025, 15, 9877. https://doi.org/10.3390/app15189877
Lidoriki I, Magiatis P, Melliou E, Georgakopoulos S, Kales SN. Nutritional Analysis of Bottarga and Pilot Study Protocol for Bottarga Supplementation in Individuals with Prediabetes. Applied Sciences. 2025; 15(18):9877. https://doi.org/10.3390/app15189877
Chicago/Turabian StyleLidoriki, Irene, Prokopios Magiatis, Eleni Melliou, Spyridon Georgakopoulos, and Stefanos N. Kales. 2025. "Nutritional Analysis of Bottarga and Pilot Study Protocol for Bottarga Supplementation in Individuals with Prediabetes" Applied Sciences 15, no. 18: 9877. https://doi.org/10.3390/app15189877
APA StyleLidoriki, I., Magiatis, P., Melliou, E., Georgakopoulos, S., & Kales, S. N. (2025). Nutritional Analysis of Bottarga and Pilot Study Protocol for Bottarga Supplementation in Individuals with Prediabetes. Applied Sciences, 15(18), 9877. https://doi.org/10.3390/app15189877








