High-Pressure Green Technologies for the Recovery and Functionalization of Bioactive Compounds from Petiveria alliacea
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Chemicals
2.2. High-Pressure Extraction
2.3. Characterization of the Extracts
2.3.1. Extraction Yields
2.3.2. Total Phenolic Content
2.3.3. Antioxidant Activity
2.3.4. Determination of the Antimicrobial Activity of the Extract
2.4. Impregnation at High Pressure
2.5. Statistical Analysis
3. Results and Discussion
3.1. Enhanced Solvent Extraction
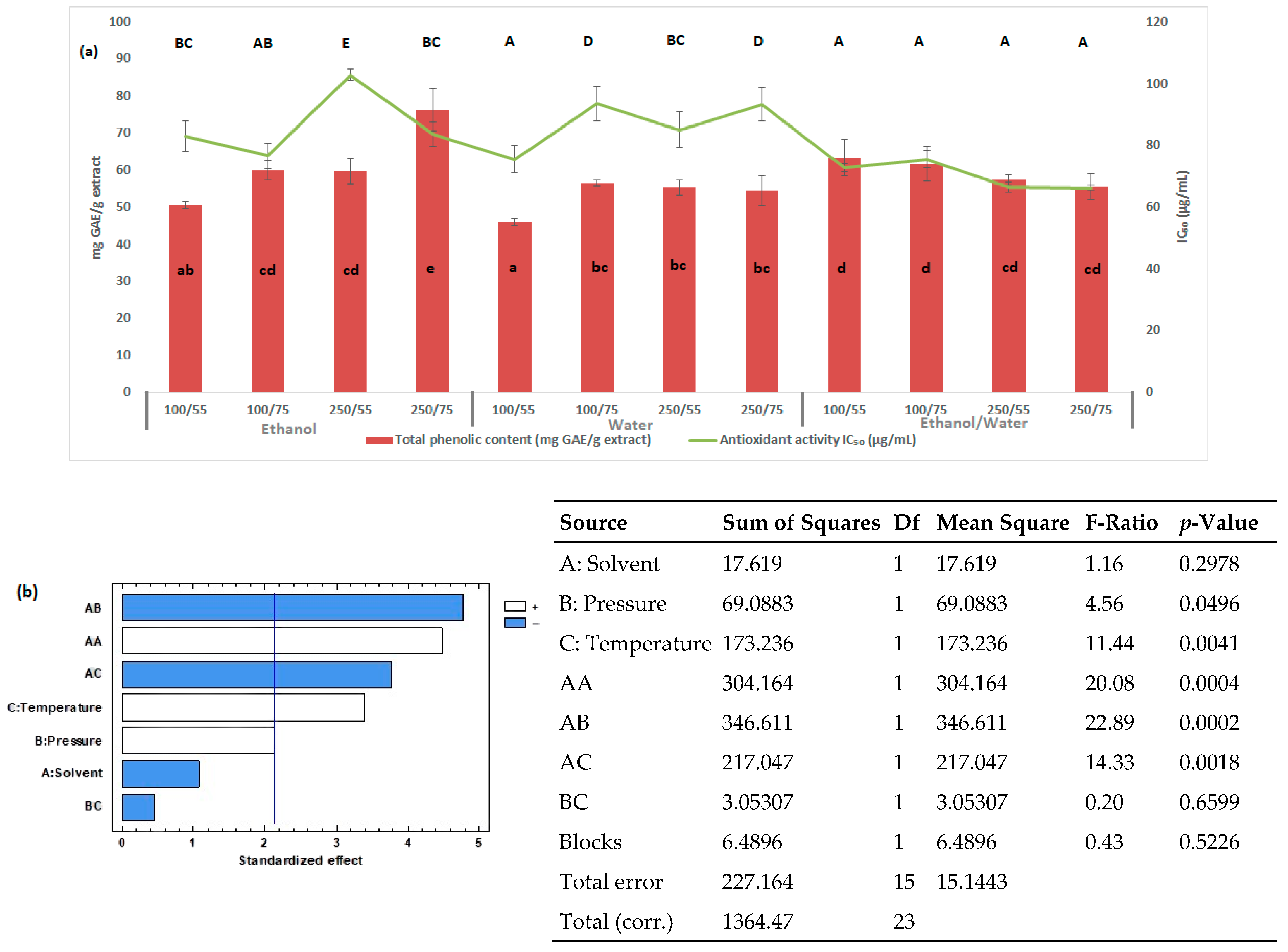
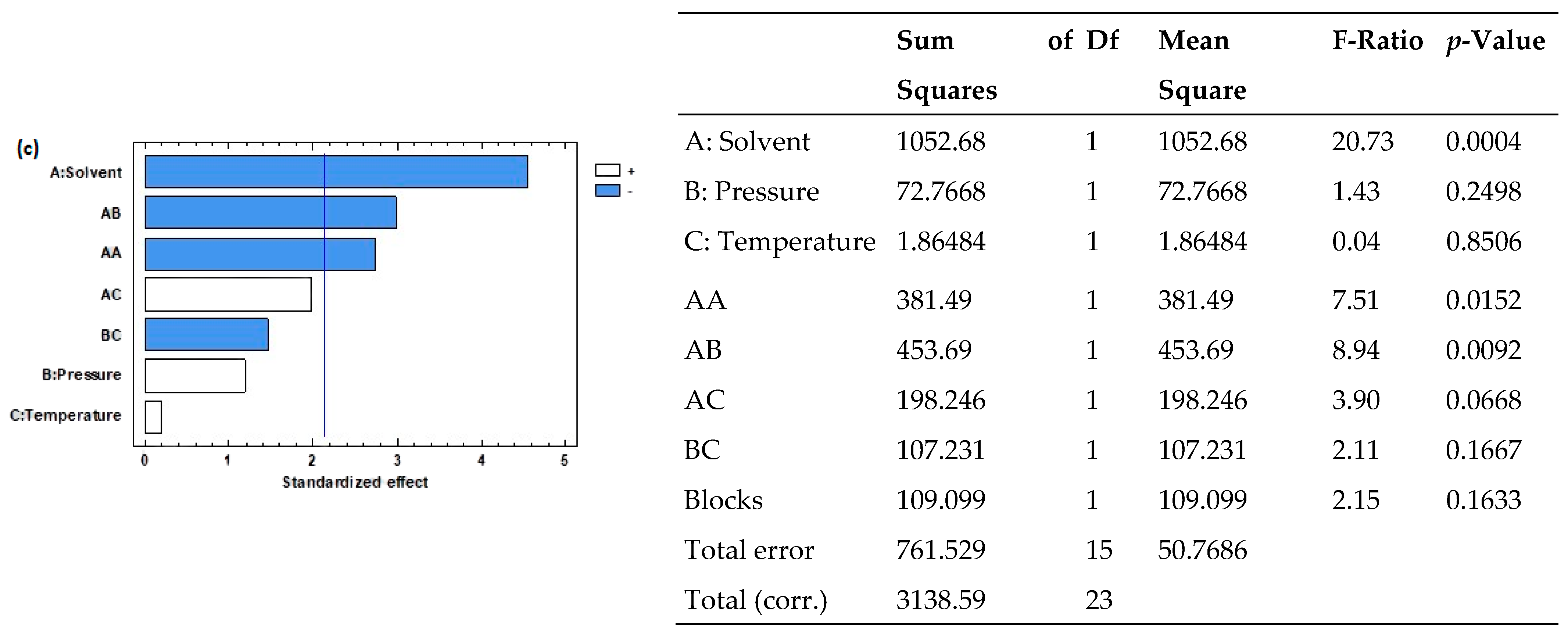
3.2. Pressurized Liquid Extraction
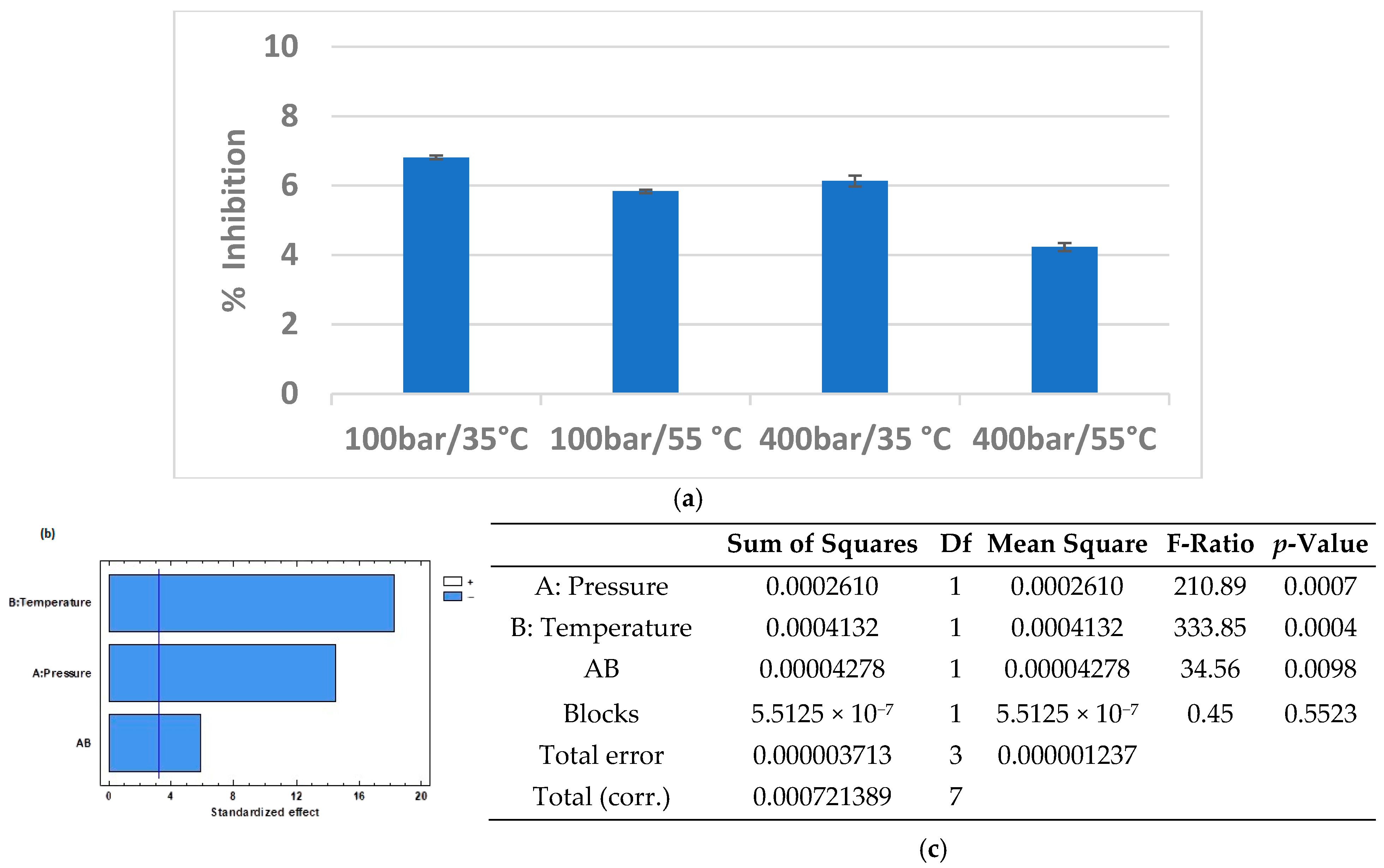
3.3. Microbial Capacity
3.4. Impregnation into PLA Filaments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical fluid extraction of seed oils—A short review of current trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Dias, A.L.B.; Rodrigues, K.P.; Hatami, T.; Mei, L.H.I.; Martínez, J. Supercritical fluid adsorption of natural extracts: Technical, practical, and theoretical aspects. J. CO2 Util. 2022, 56, 101865. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Ahmed Khalil, A.; Dina, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-Related Matrices. Food Rev. Int. 2022, 38, 1166–1196. [Google Scholar] [CrossRef]
- Zhang, J.; Gunal-Koroglu, D.; Echeverria-Jaramillo, E.; Subasi, B.G. Recent advances in valorization of wastes and byproducts of berry processing and food applications. In Berry Fruits; Elsevier: Amsterdam, The Netherlands, 2025; pp. 343–383. [Google Scholar] [CrossRef]
- Dashtian, K.; Kamalabadi, M.; Ghoorchian, A.; Ganjali, M.R.; Rahimi-Nasrabadi, M. Integrated supercritical fluid extraction of essential oils. J. Chromatogr. A 2024, 1733, 465240. [Google Scholar] [CrossRef] [PubMed]
- FLAVEX. Organic Products by FLAVEX. 2024. Available online: https://share.google/bXakuLr8R8KWaO8v3 (accessed on 21 June 2025).
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The Application of Supercritical Fluid Extraction in Phenolic Compounds Isolation from Natural Plant Materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Wu, H.; Sakai, K.; Zhang, J.; McClements, D.J. Plant-based meat analogs: Color challenges and coloring agents. Food Nutr. Health 2024, 1, 4. [Google Scholar] [CrossRef]
- Phasex. Natural Products Extraction [Internet]. 2024. Available online: https://www.phasex4scf.com/natural-product-extraction (accessed on 21 June 2025).
- Zhang, J.; Li, Y. Berry pomace as a potential ingredient for plant-based meat analogs. Food Biomacromol. 2024, 1, 127–139. [Google Scholar] [CrossRef]
- Eden Labs. CO2 Extraction Process [Internet]. 2024. Available online: https://www.edenlabs.com/co2-extraction/co2-extraction-process/ (accessed on 21 June 2025).
- Hayrapetyan, G.; Trchounian, K.; Buon, L.; Noret, L.; Pinel, B.; Lagrue, J.; Assifaoui, A. Sequential extraction of high-value added molecules from grape pomaces using supercritical fluids with water as a co-solvent. RSC Sustain. 2023, 1, 2014–2023. [Google Scholar] [CrossRef]
- de Souza Correa, M.; Boschen, N.L.; Rodrigues, P.R.P.; Corazza, M.L.; de Paula Scheer, A.; Ribani, R.H. Supercritical CO2 with co-solvent extraction of blackberry (Rubus spp. Xavante cultivar) seeds. J. Supercrit. Fluids 2022, 189, 105702. [Google Scholar] [CrossRef]
- Sánchez-Gomar, I.; Benítez-Camacho, J.; Cejudo-Bastante, C.; Casas, L.; Moreno-Luna, R.; Mantell, C.; Durán-Ruíz, M. Pro-Angiogenic Effects of Natural Antioxidants Extracted from Mango Leaf, Olive Leaf and Red Grape Pomace over Endothelial Colony-Forming Cells. Antioxidants 2022, 11, 851. [Google Scholar] [CrossRef]
- Oukebdane, K. Pressurized liquid extraction and gas chromatography/mass spectrometry for determination of nitrated polycyclic aromatic hydrocarbons adsorbed on refractory diesel particles. Curr. Chem. Lett. 2024, 13, 255–264. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. [Google Scholar]
- Silva de Oliveira, C.R.; de Oliveira, P.V.; Pellenz, L.; Lange de Aguiar, C.R.; da Silva Júnior, A.H. Supercritical fluid technology as a sustainable alternative method for textile dyeing: An approach on waste, energy, and CO2 emission reduction. J. Environ. Sci. 2024, 140, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Chen, B.; Dai, J.; Zhang, K. Using supercritical carbon dioxide as solvent to replace water in polyethylene terephthalate (PET) fabric dyeing procedures. J. Clean. Prod. 2010, 18, 1009–1014. [Google Scholar] [CrossRef]
- Wilson, A. Dyecoo Technology at the Tipping Point [Internet]. 2021. Available online: https://www.innovationintextiles.com/dyecoo-technology-at-the-tipping-point/?utm_source=chatgpt.com (accessed on 21 June 2025).
- Eren, H.A.; Avinc, O.; Eren, S. Supercritical carbon dioxide for textile applications and recent developments. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 082011. [Google Scholar] [CrossRef]
- Weidner, E. Impregnation via supercritical CO2—What we know and what we need to know. J. Supercrit. Fluids 2018, 134, 220–227. [Google Scholar] [CrossRef]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-assisted impregnation/deposition of polymeric materials with pharmaceutical, nutraceutical, and biomedical applications: A review (2015–2021). J. Supercrit. Fluids 2022, 191, 105763. [Google Scholar] [CrossRef]
- Dias, A.M.A.; Braga, M.E.M.; Seabra, I.J.; Ferreira, P.; Gil, M.H.; de Sousa, H.C. Development of natural-based wound dressings impregnated with bioactive compounds and using supercritical carbon dioxide. Int. J. Pharm. 2011, 408, 9–19. [Google Scholar] [CrossRef]
- Castañeda, R.; Cáceres, A.; Velásquez, D.; Rodríguez, C.; Morales, D.; Castillo, A. Medicinal plants used in traditional Mayan medicine for the treatment of central nervous system disorders: An overview. J. Ethnopharmacol. 2022, 283, 114746. [Google Scholar] [CrossRef]
- Zavala-Ocampo, L.M.; López-Camacho, P.Y.; Aguirre-Hernández, E.; Cárdenas-Vázquez, R.; Bonilla-Jaime, H.; Basurto-Islas, G. Neuroprotective effects of Petiveria alliacea on scopolamine-induced learning and memory impairment mouse model. J. Ethnopharmacol. 2024, 318, 116881. [Google Scholar] [CrossRef]
- Murillo, N.; Lasso, P.; Urueña, C.; Pardo-Rodriguez, D.; Ballesteros-Ramírez, R.; Betancourt, G.; Rojas, L.; Cala, M.P.; Fiorentino, S. Petiveria alliacea Reduces Tumor Burden and Metastasis and Regulates the Peripheral Immune Response in a Murine Myeloid Leukemia Model. Int. J. Mol. Sci. 2023, 24, 12972. [Google Scholar] [CrossRef] [PubMed]
- Lasso, P.; Rojas, L.; Arévalo, C.; Urueña, C.; Murillo, N.; Fiorentino, S. Natural Products Induce Different Anti-Tumor Immune Responses in Murine Models of 4T1 Mammary Carcinoma and B16-F10 Melanoma. Int. J. Mol. Sci. 2023, 24, 16698. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Abolade, O.; Ogunlakin, A.D.; Akintayo, C.O.; Olukiran, O.S.; Odesanmi, O.E.; Ajayi-Odoko, O.A. Petiveria alliacea L. extract protects against streptozotocin-induced type-2 diabetes by modulating the cAMP/PKA/CREB/cFOS pathway. Phytomedicine Plus 2024, 4, 100596. [Google Scholar] [CrossRef]
- Olomieja, A.O.; Olanrewaju, I.O.; Ayo-Ajayi, J.I.; Jolayemi, G.E.; Daniel, U.O.; Mordi, R.C. Antimicrobial and Antioxidant Properties of Petiveria alliacea. IOP Conf. Ser. Earth. Environ. Sci. 2021, 655, 012015. [Google Scholar] [CrossRef]
- Lopes-Martins, R.A.B.; Pegoraro, D.H.; Woisky, R.; Penna, S.C.; Sertié, J.A.A. The anti-Inflammatory and analgesic effects of a crude extract of Petiveria alliacea L. (Phytolaccaceae). Phytomedicine 2002, 9, 245–248. [Google Scholar] [CrossRef]
- Gunawan, V.A.; Soetjipto, H.; Mustika, A. Hypoglicemic and Antioxidant Activity of Petiveria alliacea in Diabetic Rat Models. Biomol. Health Sci. J. 2020, 3, 19. [Google Scholar] [CrossRef]
- Montes-Lobato, J.R.; Machado, N.D.; Cejudo-Bastante, C.; Mantell-Serrano, C.; Casas-Cardoso, L. Supercritical Impregnation of Olive Leaf Extract in Poly(L-Lactic Acid-co-Caprolactone) Filaments: An Environmentally Friendly Approach to Obtaining Active Biomedical Materials. Polymers 2025, 17, 1464. [Google Scholar] [CrossRef]
- Chávez Pisco, J.; Burgos Briones, G.; Alcívar Cedeño, U.; Sántos Dávila, J. Cómo reducir la-vulnerabilidad ante eventos meteorológicos extremos en una finca integral. Rev. Colón Cienc. Tecnol. Neg. 2021, 8, 1–12. Available online: https://revistas.up.ac.pa/index.php/revista_colon_ctn/article/view/2236 (accessed on 1 June 2025).
- Burgos-Briones, G.A.; Verano-Naranjo, L.; Cejudo-Bastante, C.; Dueñas-Rivadeneira, A.A.; Mantell-Serrano, C.; Casas-Cardoso, L. Extraction of Bioactive Compounds from Prestonia mollis Leaves and Their Impregnation into Polylactic Acid Using High-Pressure Technologies: Potential for Biomedical Application. Antioxidants 2023, 12, 1864. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison Between Folin-Ciocalteu and Prussian Blue Assays to Estimate the Total Phenolic Content of Juices and Teas Using 96-Well Microplates. J. Food. Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food. Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Manual del Ingeniero Químico; McGraw-Hill: Nueva York, NY, USA, 1984. [Google Scholar]
- Moreno, E.; Reza, J.; Trejo, A. Extraction of polycyclic aromatic hydrocarbons from soil using water under subcritical conditions. Polycycl. Aromat. Compd. 2007, 27, 239–260. [Google Scholar] [CrossRef]
- Fernández-González, V.; Concha-Graña, E.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Pressurized hot water extraction coupled to solid-phase microextraction–gas chromatography–mass spectrometry for the analysis of polycyclic aromatic hydrocarbons in sediments. J. Chromatogr. A 2008, 1196–1197, 65–72. [Google Scholar] [CrossRef]
- Björklund, E.; Nilsson, T.; Bøwadt, S. Pressurised liquid extraction of persistent organic pollutants in environmental analysis. TrAC Trends Anal. Chem. 2000, 19, 434–445. [Google Scholar] [CrossRef]
- Cheong, M.W.; Tan, A.A.A.; Liu, S.Q.; Curran, P.; Yu, B. Pressurised liquid extraction of volatile compounds in coffee bean. Talanta 2013, 115, 300–307. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Md Saleh, N. Parameter Effects and Optimisation in Supercritical Fluid Extraction of Phenolic Compounds from Labisia pumila. Separations 2022, 9, 385. [Google Scholar] [CrossRef]
- Atwi-Ghaddar, S.; Zerwette, L.; Destandau, E.; Lesellier, E. Supercritical Fluid Extraction (SFE) of Polar Compounds from Camellia sinensis Leaves: Use of Ethanol/Water as a Green Polarity Modifier. Molecules 2023, 28, 5485. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Allawzi, M.; Al-Otoom, A.; Allaboun, H.; Al-Zoubi, A. Supercritical fluid extraction of useful compounds from sage. Nat. Sci. 2012, 4, 544–551. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.; Idris, A.M.; Simal-Gándara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Zavala-Ocampo, L.M.; Aguirre-Hernández, E.; López-Camacho, P.Y.; Cárdenas-Vázquez, R.; Dorazco-González, A.; Basurto-Islas, G. Acetylcholinesterase inhibition and antioxidant activity properties of Petiveria alliacea L. J. Ethnopharmacol. 2022, 292, 115239. [Google Scholar] [CrossRef]
- Lawal, I.O.; Sowunmi, L.I.; Olaniyi, M.B.; Jegede, A.E.; Akanni, F.O.; Akinwumi, I.A.; Babalola, O.Y. Ethnobotany, chemistry and toxicity of Petivera alliacea: A review. Funct. Food Sci. 2024, 4, 69–82. [Google Scholar] [CrossRef]
- Fatimah, N.; Mustika, A.; Sudjarwo, S.A.; Noor, N.S. Metabolite profiling based on UPLC-QTOF-MS/MS and evaluation of Petiveria alliacea Leaves extract as an in silico anti-inflammatory. J. Med. Pharm. Chem. Res. 2024, 6, 344–361. [Google Scholar] [CrossRef]
- Urueña, C.; Cifuentes, C.; Castañeda, D.; Arango, A.; Kaur, P.; Asea, A.; Fiorentino, S. Petiveria alliacea extracts uses multiple mechanisms to inhibit growth of human and mouse tumoral cells. BMC Complement. Altern. Med. 2008, 8, 60. [Google Scholar] [CrossRef]
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiro, M.C.; Prediger, R.D.; Ferraz Maia, C.S.; Fontes, E.A. Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Alvarado, D.; Monagas, M.J. Proanthocyanidin Characterization, Antioxidant and Cytotoxic Activities of Three Plants Commonly Used in Traditional Medicine in Costa Rica: Petiveria alliacea L., Phyllanthus niruri L. and Senna reticulata Willd. Plants 2017, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Camel, V. Recent extraction techniques for solid matrices—Supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: Their potential and pitfalls. Analyst 2001, 126, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, J.; Veriansyah, B.; Kim, J.D.; Lee, Y.W.; Oh, S.G.; Tjandrawinata, R.R. Extraction of bioactive components from Centella asiatica using subcritical water. J. Supercrit. Fluids 2009, 48, 211–216. [Google Scholar] [CrossRef]
- Hassasroudsari, M.; Chang, P.; Pegg, R.; Tyler, R. Antioxidant Capacity of Bioactives Extracted from Canola Meal by Subcritical Water, Ethanolic and Hot Water Extraction. Food Chem. 2009, 114, 717–726. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Evaluation of the Extraction Efficiency of Thermally Labile Bioactive Compounds in Gastrodia elata Blume by Pressurized Hot Water Extraction and Microwave-Assisted Extraction. J. Chromatogr. A 2008, 1182, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, M.; Panja, P. Recovery of Phytochemicals from Kokum (Garcinia indica Choisy) Using Pressurized Hot Water. Int. J. Food Eng. 2008, 4, 13. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Sjöberg, P.J.R.; Turner, C. Characterisation of anthocyanins in red cabbage using high resolution liquid chromatography coupled with photodiode array detection and electrospray ionization-linear ion trap mass spectrometry. Food Chem. 2008, 109, 219–226. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of Experimental Conditions on the Extraction of Phenolic Compounds from Parsley (Petroselinum crispum) Flakes Using a Pressurized Liquid Extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef]
- Scarano, P.; Guida, R.; Zuzolo, D.; Tartaglia, M.; Prigioniero, A.; Postiglione, A.; Pinto, G.; Illiano, A.; Amoresano, A.; Schicchi, R.; et al. An Endemic Plant of the Mediterranean Area: Phytochemical Characterization of Strawberry Tree (Arbutus unedo L.) Fruit Extracts at Different Ripening Stages. Front. Nutr. 2022, 9, 915994. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Laguerre, M.; Fabiano-Tixier, A.; Tenon, M.; Feuillère, N.; Bily, A.; Chemat, F. What is the best ethanol-water ratio for the extraction of antioxidants from rosemary? Impact of the solvent on yield, composition, and activity of the extracts. Electrophoresis 2018, 39, 1946–1956. [Google Scholar] [CrossRef]
- JideFaleye, F.; Olatunya, A. Assessment of the antioxidant and antibacterial activities of Petiveria alliacea and Viscum album (mistletoe). Int. J. Adv. Res. 2016, 4, 1915–1919. [Google Scholar] [CrossRef]
- Adesanya, E.O.; Oyesiku, O.O.; Adesanya, O.O.; Ogunlakin, A.D.; Odugbemi, A.I.; Egieyeh, S.A. 12 Phytochemical components and GC–MS analysis of Petiveria alliaceae L. fractions and volatile oils. In Sustainable Chemistry Research; De Gruyter: Berlin, Germany, 2023; pp. 273–286. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Aldana, E.; Herrera, M.V.; Urueña, C.; Rojas, L.Y.; Echeverri, L.F.; Costa, G.M.; Quijano, S.; Fiorentino, S. Preferential Activity of Petiveria alliacea Extract on Primary Myeloid Leukemic Blast. Evid. Based Complement. Alternat. Med. 2020, 2020, 4736206. [Google Scholar] [CrossRef]
- Zare-Zardini, H.; Alizadeh, A.; Saberian, E.; Jenča, A.; Jenca, A.; Petrášová, A.; Jenčová, J.; Hasani, S.T.; Heidari, M.A.; Sahraei, N. Enhanced Antimicrobial Efficacy and Biocompatibility of Albumin Nanoparticles Loaded with Mentha Extract Against Methicillin-Resistant Staphylococcus aureus. Sci. Rep. 2025, 15, 6548. [Google Scholar] [CrossRef] [PubMed]
- Piper, K.R.; Souza, S.S.R.; Ikhimiukor, O.O.; Workman, A.A.; Martin, I.W.; Andam, C.P. Lineage-Specific Variation in Frequency and Hotspots of Recombination in Invasive Escherichia coli. BMC Genom. 2025, 26, 190. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.B.; do Nascimento, S.C.M.; Okabe, D.H.; Pinto, A.C.G.; de Oliveira, F.R.; da Paixão, T.P.; Souza Siqueira, M.L.; Baetas, A.C.; Ataíde de Andrade, M. Antimicrobial and Anticancer Potential of Petiveria alliacea L. (Herb to “Tame the Master”): A Review. Pharmacogn. Rev. 2018, 12, 85. [Google Scholar] [CrossRef]
- Kim, S.; Kubec, R.; Musah, R.A. Antibacterial and Antifungal Activity of Sulfur-Containing Compounds from Petiveria alliacea L. J. Ethnopharmacol. 2006, 104, 188–192. [Google Scholar] [CrossRef]
- Mulyani, Y.; Sukmawati, I.K.; Sodik, J.J. Antimicrobial Activities and Mechanism of Action of Petiveria alliacea Stem Extract. Indones. J. Pharm. Clin. Res. 2018, 1, 45–55. [Google Scholar] [CrossRef]
- Kubec, R.; Kim, S.; Musah, R.A. S-Substituted Cysteine Derivatives and Thiosulfinate Formation in Petiveria alliacea—Part II. Phytochemistry 2002, 61, 675–680. [Google Scholar] [CrossRef]
- de Araujo, E.J.S.; Martínez, J. Scenarios, prospects, and challenges related to supercritical fluid impregnation in the food industry: A scoping review (2018–2023). Biofuels Bioprod. Bioref. 2024, 18, 2091–2115. [Google Scholar] [CrossRef]
- Sutil, G.A.; Andrade, K.S.; Rebelatto, E.A.; Lanza, M. Effect of Supercritical CO2 Impregnation of Piperine and Black Pepper Extract on Properties of Poly(L-Lactic Acid) Films. J. Supercrit. Fluids 2025, 216, 106441. [Google Scholar] [CrossRef]
- Machado, N.D.; Cejudo-Bastante, C.; Goñi, M.L.; Gañán, N.A.; Casas-Cardoso, L.; Mantell-Serrano, C. Screening of the Supercritical Impregnation of Olea europaea Leaves Extract into Filaments of Thermoplastic Polyurethane (TPU) and Polylactic Acid (PLA) Intended for Biomedical Applications. Antioxidants 2022, 11, 1170. [Google Scholar] [CrossRef]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Supercritical Impregnation of PLA Filaments with Mango Leaf Extract to Manufacture Functionalized Biomedical Devices by 3D Printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef]


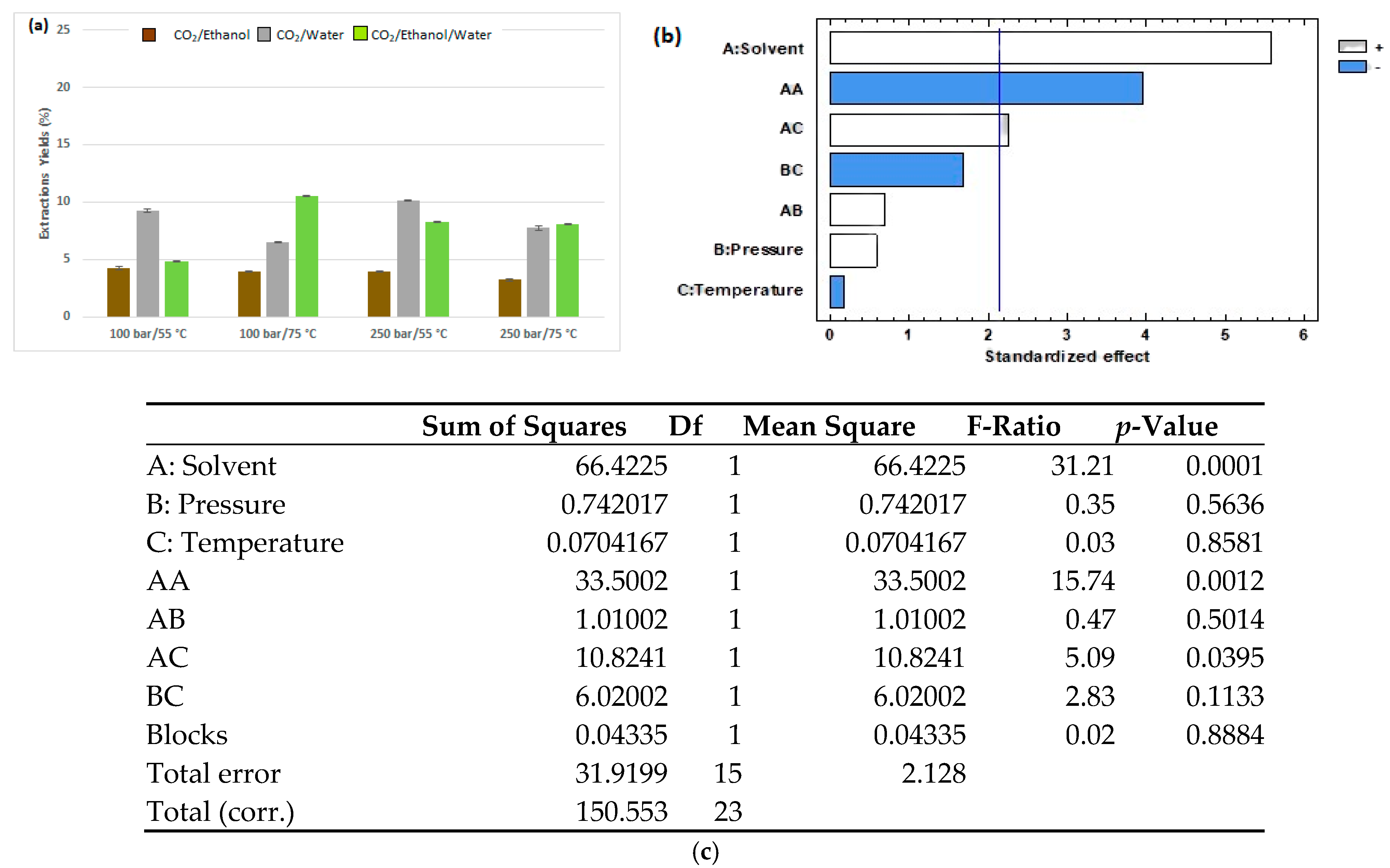


| Extraction Method | Factor | Variable | Levels |
|---|---|---|---|
| ESE | A | Solvent | CO2/ethanol (1:1 v/v, CO2/water (1:1 v/v), CO2/ethanol/water (1:0.5:0.5 v/v/v) |
| B | Pressure (bar) | 100, 250 | |
| C | Temperature (°C) | 55, 75 | |
| PLE | A | Solvent | Ethanol, water, ethanol/water (50:50 v/v) |
| B | Pressure (bar) | 100, 250 | |
| C | Temperature (°C) | 55, 75 |
| Factor | Variable | Levels |
|---|---|---|
| A | Pressure (bar) | 100, 400 |
| B | Temperature (°C) | 35, 55 |
| Concentration (µg/mL) | Inhibition of Growth (%) ± SD |
|---|---|
| 0.38 | 6.87 ± 0.061 |
| 0.95 | 31.02 ± 0.076 |
| 1.90 | 46.70 ± 0.001 |
| 4.74 | 56.04 ± 0.069 |
| 9.48 | 100.71 ± 0.002 |
| 14.21 | 100.41 ± 0.000 |
| 18.95 | 99.78 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos-Briones, G.A.; Cejudo-Bastante, C.; Dueñas-Rivadeneira, A.A.; Mantell-Serrano, C.; Casas-Cardoso, L. High-Pressure Green Technologies for the Recovery and Functionalization of Bioactive Compounds from Petiveria alliacea. Appl. Sci. 2025, 15, 9875. https://doi.org/10.3390/app15189875
Burgos-Briones GA, Cejudo-Bastante C, Dueñas-Rivadeneira AA, Mantell-Serrano C, Casas-Cardoso L. High-Pressure Green Technologies for the Recovery and Functionalization of Bioactive Compounds from Petiveria alliacea. Applied Sciences. 2025; 15(18):9875. https://doi.org/10.3390/app15189875
Chicago/Turabian StyleBurgos-Briones, Gabriel Alfonso, Cristina Cejudo-Bastante, Alex Alberto Dueñas-Rivadeneira, Casimiro Mantell-Serrano, and Lourdes Casas-Cardoso. 2025. "High-Pressure Green Technologies for the Recovery and Functionalization of Bioactive Compounds from Petiveria alliacea" Applied Sciences 15, no. 18: 9875. https://doi.org/10.3390/app15189875
APA StyleBurgos-Briones, G. A., Cejudo-Bastante, C., Dueñas-Rivadeneira, A. A., Mantell-Serrano, C., & Casas-Cardoso, L. (2025). High-Pressure Green Technologies for the Recovery and Functionalization of Bioactive Compounds from Petiveria alliacea. Applied Sciences, 15(18), 9875. https://doi.org/10.3390/app15189875











