Abstract
Supercritical CO2 (ScCO2)-enhanced shale gas recovery technology offers dual advantages: improving shale gas recovery while reducing CO2 emissions. The permeability of shale reservoirs during CO2 displacement of CH4 is a crucial issue in evaluating the efficacy of shale gas production and CO2 sequestration. In this study, ScCO2 fracturing and displacement experiments were carried out for shale samples, and the fracturing and permeability characteristics of shale were analyzed. The findings indicate that ScCO2 significantly enhances fracturing and permeability, with an overall increase in permeability by three orders of magnitude. Higher injection pressures and lower stress lead to an earlier breakthrough of CO2. The CH4 production rate after CO2 displacement is higher than that under conventional recovery conditions. The cumulative flow of CH4 initially rises with increasing pressure of injection, but subsequently declines throughout the later phases of displacement, leading to a reduced CO2 storage rate and CH4 generation rate. High stress can inhibit CO2 injection and CH4 outflow, reduce CH4 production rate, and promote shale to preferentially adsorb CO2, resulting in higher CO2 storage rate.
1. Introduction
Shale gas, a vital unconventional fossil fuel resource, contributes significantly to balancing energy supply and security through its efficient exploitation [1]. However, traditional water-based fracturing techniques face critical challenges: excessive water consumption and reservoir damage caused by fracturing fluids, hindering the sustainable development of shale gas fields [2]. Consequently, the CO2-enhanced shale gas recovery (CO2-ESGR) technology has been developed. This technology utilizes the unique physical and chemical properties of ScCO2, to replace water-based fluids, simultaneously enhancing shale gas recovery and enabling CO2 storage [3,4,5,6]. Furthermore, CO2-ESGR offers substantial environmental benefits by reducing greenhouse gas emissions and minimizing ecological disruption, aligning with global climate change mitigation efforts.
Fracturing shale gas reservoir with supercritical CO2 is essential for efficient shale gas recovery [7,8,9,10]. Zhang et al. [11] comparatively evaluated the effects of ScCO2, liquid CO2, and water on shale fracturing. Results indicated that ScCO2 lowers fracture pressure by 15% and 50% relative to liquid CO2 and water, correspondingly. The uneven cracks appear in the shale after fracturing, and in contrast to hydraulic cracking, more cracks are produced in the shale after ScCO2 fracturing [12]. Verdon et al. [13] found that ScCO2 fracturing achieves efficacy comparable to hydraulic fracturing while requiring significantly lower initiation pressure. Additionally, fracture propagation morphology during ScCO2 fracturing has been analyzed [14,15,16,17]. Zhou et al. [18] found that the crack propagation process during ScCO2 fracturing of shale can be divided into four stages: the evolution stage of the damage zone, the crack initiation stage, the post-fracturing instability stage, and the crack arrest stage. Zhao et al. [19] observed fractures extending primarily along natural fractures, bedding planes, and interlayers, with ScCO2 inducing more fracture branches than hydraulic fracturing. Despite these advances in understanding fracture behavior and post-fracturing permeability changes, the fundamental mechanisms governing CO2/CH4 migration within the fractured shale matrix remain inadequately explored.
The dynamic characteristics of shale gas displacement by CO2 can dynamically characterize the flow patterns of CO2/CH4 during CO2-ESGR process [20,21,22,23,24], which is critical for evaluating gas recovery and storage capacity. Current understanding of this process relies heavily on numerical simulations, supplemented by experimental studies that primarily utilize the column kinetics method [25,26]. Du et al. [27] performed column kinetics tests with CO2, N2, and CO2/N2 mixtures to displace CH4 in shale powder. Breakthrough curves indicated that N2 injection results in the lowest injected-gas breakthrough time and CH4 recovery, whereas CO2 injection provides the highest breakthrough time and recovery. Liu et al. [28] performed CO2 displacement experiments on shale cores via nuclear magnetic resonance (NMR), monitoring real-time changes in free and adsorbed CH4. Results showed that CO2 injection improves adsorbed CH4 recovery by 25%. Iddphonce et al. [29] used the displacement kinetics method to conduct pure CH4 and CO2-CH4 mixed gas production experiments on shale samples. In the early phase of production, when pressure was elevated, gas recovery predominantly depended on pressure difference. Nonetheless, in the subsequent phase when the pressure decreased, competitive adsorption became prominent. The gas recovery driving mechanism shifted from relying primarily on reservoir pressure difference to competitive adsorption as the pressure decreases. A significant limitation of these prior experiments is their use of shale powder, which fails to account for the critical influence of confining stress. There are few experimental studies on the kinetics of shale gas displacement by CO2 under in situ conditions, and it is necessary to further study the kinetics of shale gas displacement by CO2 under multi-field coupling conditions.
In this study, ScCO2 fracturing test and displacement test were carried out on shale samples, and the kinetics of CO2 displacing shale gas and the fracturing properties of shale were analyzed. The variation in shale permeability before and after fracturing was discussed, respectively. The mechanism of ScCO2 displacing shale gas under different stress and injection pressure conditions was revealed, and its impact on CO2 storage rate and shale gas recovery during displacement was analyzed. The findings may provide a theoretical foundation for optimizing CO2-ESGR.
2. Experimental Procedure
2.1. Sample Preparation
Samples of shale were taken from the Changning region of the Sichuan Basin’s Wufeng Formation. One of China’s best areas for the discovery and production of marine shale gas is the Sichuan Basin, which is situated on the northwest edge of the Upper Yangtze Platform. Black siliceous and carbonaceous shale make up the majority of the well-preserved Wufeng Formation, which has thicknesses ranging from 100 to 500 m and reservoir depths between 1500 and 4000 m [30].
For this experiment, outcrop samples of the Wufeng Formation were selected. All shale samples were obtained from intact blocks without visible fractures. Cores were drilled parallel to bedding planes and machined into cylindrical samples (diameter: 100 mm; height: 200 mm). Post-processing dimensional tolerances were controlled within ±1 mm for height/diameter and ±0.02 mm for end-face flatness. To simulate CO2-ESGR and storage processes, a 14 mm-diameter borehole (depth: 140 mm) was drilled axially in each sample to represent a wellbore. After grinding, a high-strength fracturing pipe was inserted, and the epoxy resin and curing agent were uniformly mixed and sealed [31]. The experimental sample is shown in Figure 1.

Figure 1.
Experimental sample diagram.
2.2. Experimental Method
A self-developed supercritical CO2 fracturing and permeability experimental device for shale gas reservoir was used (Figure 2). This apparatus enables fracturing and displacement experiments under multi-field coupling. The system includes gas supply system, pressure chamber, triaxial cell, gas detection system, and control/data-acquisition system. For precise flow measurement within the gas supply and detection systems, a D07-11 model flowmeter (Beijing Huacheng Electronics Co., Ltd., Beijing, China) was selected, offering an accuracy of ±1%. To monitor and control pressure within the pressure chamber and triaxial cell, a Keller PAA-33X model pressure sensor (The company Keller Pressure, Winterthur, Switzerland) was employed, providing high accuracy of ±0.1%. For gas composition analysis within the gas detection system, a GC-2060 model gas chromatograph (Shanghai Ruimin Instrument Co., Ltd., Shanghai, China) was utilized, with an accuracy of ±1.5%.

Figure 2.
Schematic diagram of experimental equipment for shale gas displacement by CO2.
In order to simulate the CO2-ESGR and CO2 storage process and analyze the evolution mechanism of shale gas reservoir permeability characteristics during ScCO2 fracturing displacement process, the fracturing experiments were carried out on each shale sample, and the changes in shale permeability characteristics and fracture propagation before and after fracturing were analyzed. Fracturing shale sample was selected to carry out the experiment of ScCO2 displacing CH4 in shale under the stress and temperature.
2.2.1. Fracturing Experiment
ScCO2 fracturing was carried out under simulated reservoir conditions (axial pressure = 10 MPa, confining pressure = 10 MPa, temperature = 50 °C, injection rate: 30 mL/min). Shale permeability tests (gas: He, pressure: 0–5 MPa) were carried out under the same stress and temperature conditions before and after fracturing, and CT scan was carried out before and after fracturing.
2.2.2. Displacement Experiment
Fractured shale sample was used for CO2 displacement experiments under different injection pressures and stress conditions. For displacement experiments with variable injection pressures, axial and confining pressures were set to 15 MPa, initial CH4 pressure to 5 MPa, and CO2 injection pressures to 6 MPa and 9 MPa, respectively, to explore the effect of different phases of CO2 on the displacement effect of CH4. A conventional recovery experiment with 5 MPa CH4 was carried out as the comparison group. In the displacement experiment under different stress conditions, the initial pressure of CH4 is set to 5 MPa, the injection pressure of CO2 is set to 9 MPa, and the axial pressure and confining pressure are set to 10 MPa, 15 MPa and 20 MPa, respectively.
3. Results and Discussion
3.1. Permeability Characteristics of Shale Before and After Fracturing
3.1.1. Pressure Curve Analysis in Fracturing Process
Figure 3 shows the pressure-time curve during the fracturing of shale with supercritical CO2. The fracture pressure of the shale sample was 31.4 MPa. As the fracturing process progressed, the pressure curve initially increased rapidly, then gradually stabilized, followed by a rapid surge until the sample fractured and the pressure subsequently declined. Based on the changes in pressure, the fracturing process consists of four steps.: (Ⅰ) cavity filling, (Ⅱ) slow pressure accumulation, (Ⅲ) rapid pressure increase, and (Ⅳ) fracturing failure. During stage Ⅰ, the injected CO2 gas rapidly entered the non-porous cavities and large pores of the shale, leading to rapidly rise in CO2 pressure. In stage Ⅱ, due to the high compressibility of CO2, the pressure increased slowly as the gas was gradually injected. In addition, CO2 in the large pores began to diffuse into the micro-pores of the shale, causing the pre-existing cracks within the shale to gradually open and expand. In stage Ⅲ, as CO2 continued to be injected, when the CO2 pressure reached 7.38 MPa, it transitioned into a supercritical state, and CO2 pressure began to increase rapidly. This is because the phase transition of CO2 at the supercritical state causes a rapid increase in its density and a significant reduction in its compressibility. The continuous injection of CO2 led to a rapid increase in pressure and the rapid expansion of cracks until the shale fractured. In stage Ⅳ, the fracturing of the shale created penetrating cracks, increasing the internal volume of the shale and thus rapidly decline the CO2 pressure, which also resulted in an increase in shale permeability.
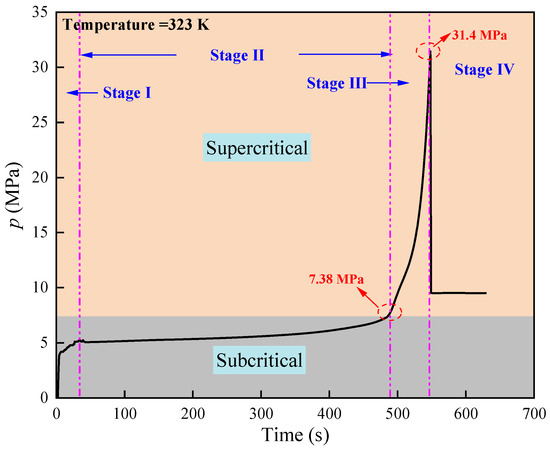
Figure 3.
Pressure-time curve of supercritical CO2 fracturing shale.
The CT scans of the shale samples before and after fracturing are displayed in Figure 4. Prior to fracturing, the shale samples’ cross-sections were uniform, have smooth surfaces, and devoid of visible scanning cracks. The shale had few pre-existing cracks, indicating good sample integrity and extremely low permeability. After fracturing with supercritical CO2, the shale samples exhibited distinct fractures on both the upper and lower surfaces, with similar crack patterns on the upper and lower surfaces, indicating that the cracks had penetrated from top to bottom. The cracks in the sample all extended perpendicular to the direction of the minimum principal stress. The crack propagation pattern showed that the cracks in the shale mainly extended along the bedding planes.
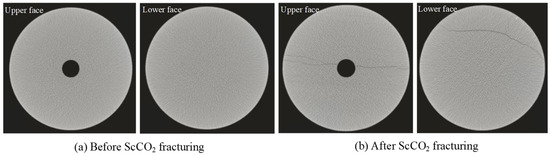
Figure 4.
CT scan of shale sample before and after fracturing.
3.1.2. Analysis of Permeability Characteristics
Figure 5 shows the permeability curves of the shale before and after fracturing under the same stress conditions. Before fracturing, the permeability of the shale samples under different He pressures ranged from 1.38 to 9.54 × 10−5 mD. After fracturing with ScCO2, the permeability of the shale samples ranged from 1.32 to 2.47 × 10−2 mD, representing an increase in three orders of magnitude, indicating a significant enhancement in permeability due to CO2 fracturing. Figure 5 shows that as the He pressure increased, the permeability of the unfractured shale declined rapidly at first and then gradually plateaued. In contrast, for the fractured shale, the permeability initially declined and then gradually rose with increasing gas pressure. This is primarily because the unfractured shale is tight rock with few natural fractures, and its stress sensitivity is low under external stress. The flow of gas within the shale is mainly dominated by Knudsen flow, transitional flow, and slip flow. As the pressure increases, the mean free path of gas molecules diminishes, the probability of interactions between molecules and pore walls decreases, and the probability of intermolecular collisions increases, leading to a decrease in Knudsen diffusion and slip effects. Although the effective stress gradually decreases as increasing pressure, its low stress sensitivity results in a relatively minor impact on permeability, causing the permeability to decrease with increasing pressure. For the fractured shale, the macroscopic fractures after fracturing leads to the dominance of continuous flow and slip flow in the gas flow within the shale. The macroscopic fractures in the shale close and open, exhibiting high stress sensitivity under the effect of effective stress. As the gas pressure increases, the slip effect gradually weakens, causing the permeability to decrease by approximately 51.0% [7]. However, with a further rise in gas pressure, the effective stress exerted on the shale under constant stress conditions gradually decreases, and this effect plays a dominant role in the permeability, which leads to a partial recovery of permeability by about 18.4% from its lowest value.
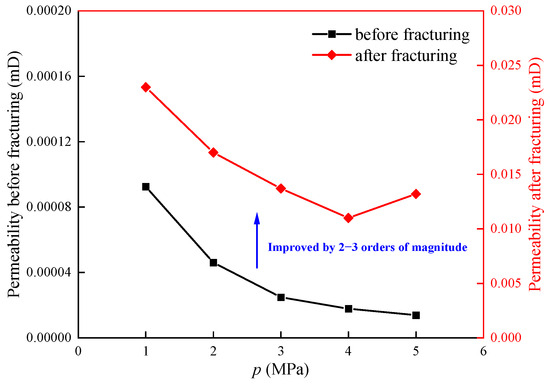
Figure 5.
Shale permeability curve before and after fracturing under the same stress condition.
3.2. Breakthrough Curves of CO2 Displacement of CH4
Figure 6 shows the breakthrough curves of CO2 displacement of CH4 under varying injection pressures (a) and stress conditions (b). The changes in the molar fractions of CH4 and CO2 over time can be categorized into three primary stages: In the first stage, CO2 mixes with CH4 within the shale fractures, leading to dispersion. The presence of CH4 hinders the movement of CO2, which migrates towards the outlet under pressure differences, displacing CH4. In the second stage, CO2 breakthrough occurs within the shale, and CO2 is detected at the outlet. A significant amount of free CH4 is rapidly discharged from the fractures, causing a rapid increase in the molar fraction of CO2. Simultaneously, CO2 begins to diffuse into the shale matrix and competes with CH4 for adsorption. Due to the shale’s preferred CO2 adsorption, the adsorbed CH4 gradually desorbed. In the third stage, the growth rate of CO2 mole fraction and the decline rate of CH4 gradually decrease. Since CH4 in the shale fracture is discharged in large quantities in the second stage, the monitored CH4 is mainly CH4 desorbed in the matrix, so the change in gas mole fraction of CO2 and CH4 gradually slows down.
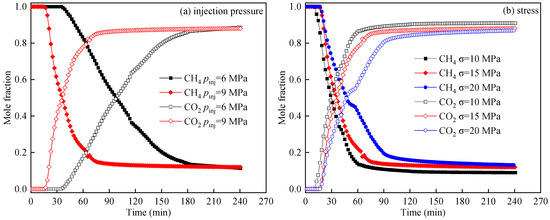
Figure 6.
Breakthrough curves of CO2 displacement CH4 under different injection pressure (a) and stress (b).
From Figure 6a, it can be observed that as the CO2 injection pressure increases, the breakthrough curves become steeper. At an injection pressure of 6 MPa, the breakthrough time of CO2 is 34.9 min, which decreases to 14.9 min at 9 MPa. Under higher injection pressures in the second stage, the increase rate in the molar fraction of CO2 is higher, and the time to reach the third stage is 171.7 min at 6 MPa and 71.7 min at 9 MPa. This is primarily due to the higher-pressure difference between the inlet and outlet under the same stress conditions, along with reduced effective stress on the shale, which accelerates CO2 migration. Additionally, the diffusion rate and dispersion coefficient of CO2 increase with pressure, enhancing CO2/CH4 mixing and facilitating displacement [27].
From Figure 6b, it is evident that as stress increases from 10 MPa to 20 MPa, CO2 breakthrough times are 8.6, 14.9, and 16.76 min, respectively. Under higher stress in the second stage, the increase rate in CO2 molar fraction is lower. The time to reach the third stage is 56.6, 71.7, and 92.8 min as increasing stress. This occurs because increased effective stress compresses internal fractures, reducing CO2 flow rate under identical injection pressure. Reduced fracture width also decreases CO2/CH4 diffusion rates, slowing displacement.
3.3. Flow Curve of CO2 Displacing CH4
Figure 7 shows the flow rate curves under varying injection pressures (a) and stress conditions (b). The CH4 flow rate declined sharply initially before decelerating gradually, while the CO2 flow rate rose rapidly upon breakthrough and then began to level off. At 240 min, the CO2 flow rate increased by 38.71% with rising injection pressure, while it decreased by up to 33.33% under increasing stress. In the initial displacement stage, CH4 in the shale fracture is largely displaced by high-pressure CO2, and the CH4 flow rate is high. With the mixing of CO2 and CH4, CH4 in the fracture gradually decreases. Through continuously injecting CO2 into CH4, the CH4 flow rate decline precipitously, while the CO2 flow rate rises correspondingly. As the CH4 in the fracture is discharged, the flow curves of CH4 and CO2 gradually slow down. At this time, the flow of CH4 is mainly contributed by the desorbed CH4. By comparing Figure 6 and Figure 7, it can be found that the time from the second stage to the third stage also has a good consistency. In the third stage, with the continuous adsorption of CO2 by shale, the permeability gradually decreases, so the flow of CH4 and CO2 also gradually diminish.
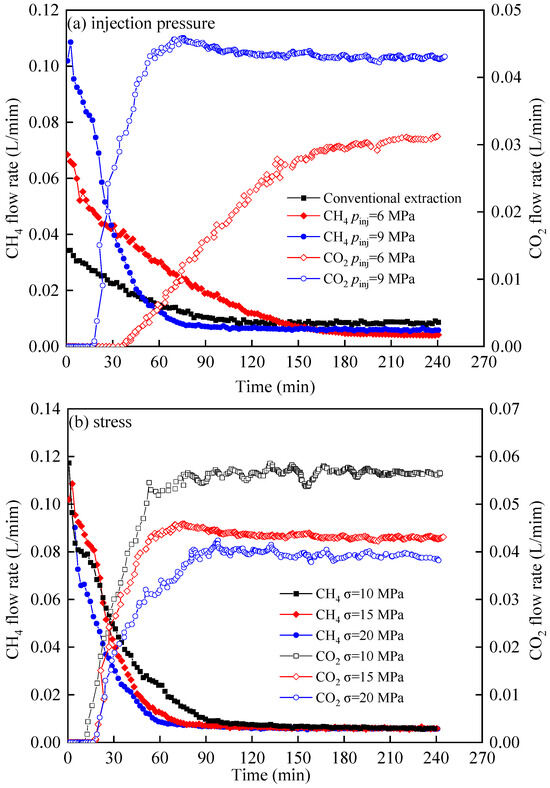
Figure 7.
Flow rate curves of CO2 displacement CH4 under different injection pressure (a) and stress (b).
Figure 7a shows that the CH4 flow curves under the three conditions. During the initial stage of conventional recovery and CO2 displacement, the CH4 flow rate gradually rises in response to higher CO2 injection pressure, while in the later stage of displacement, the flow of CH4 is higher under the conditions of conventional recovery and lower displacement pressure. This is because the CH4 flow in the initial stage of displacement is mainly dominated by free CH4 in the fracture, the flow of CH4 and CO2 at the outlet of the shale is higher under higher displacement pressure, and the decrease rate of free CH4 in the shale fracture is also higher. Therefore, in the middle stage of displacement, the CH4 under conventional recovery and lower displacement pressure conditions is higher. In the later stage, compared with the conventional recovery conditions, the flow of CH4 under CO2 displacement conditions is lower, which may also be caused by the further swelling of shale adsorption of CO2 [27]. From Figure 7b, it can be found that the CH4 and CO2 flow rates decline with increasing stress, it is induced by the alteration of effective stress response resulting from the variation in external stress. The closure of the fracture will hinder the injection of CO2 and the displacement of CH4.
3.4. Parameters of CO2 Displacement of CH4
Table 1 shows the parameters of CO2 displacement of CH4 under varying injection pressures and stress conditions. Compared to the CH4 production rate under conventional recovery conditions, the CH4 production rate after CO2 displacement is higher, indicating that CO2 displacement of CH4 within the shale effectively increases CH4 production. Additionally, it can be observed from Table 1 that as the injection pressure increases, the CH4 production rate and CO2 storage rate decrease, even with a higher total injection of CO2. As stress increases, the recovery rate of CH4 decreases, whilst the storage rate of CO2 increases. Figure 8 shows the cumulative flow rate curves of CH4 under different injection pressures and stress conditions during CO2 displacement. It is evident that in the initial stage of displacement, the cumulative flow rate of CH4 is higher as increasing injection pressure, while in the later stages of displacement, the cumulative flow rate of CH4 is lower. This is primarily because, in the initial stage of displacement, the higher injection pressure leads to a large amount of CH4 being discharged from the fractures, resulting in a higher cumulative flow rate of CH4 at this time. As the CH4 within the fractures continues to be discharged, in the later stages of displacement, the higher injection pressure leads to a decrease in the mass transfer coefficient between the shale matrix and fractures, i.e., the diffusion rate of gas from the matrix to the fractures decreases. Therefore, the role of CO2 in displacing and desorbing CH4 decreases [25], leading to a slower rate of increase in the cumulative flow rate of CH4 in the later stages, which is also a reason for the decrease in the CH4 production rate and CO2 storage rate. The fractures within the shale are further compressed under high stress conditions, leading to a decrease in the fluidity of CH4 within the fractures and a decrease in the production rate. Additionally, high stress promotes the selective adsorption of CO2 by the shale, which may also be a reason for the increase in the CO2 storage rate. Therefore, it is of great significance to select appropriate injection pressure according to reservoir conditions to ensure CH4 recovery and CO2 storage rate. Although excessive injection pressure can greatly increase CH4 production in the short term, it is not conducive to the recovery of CH4 in shale matrix in the later stage.

Table 1.
Parameters of CO2 displacement CH4 under different injection pressure and stress.
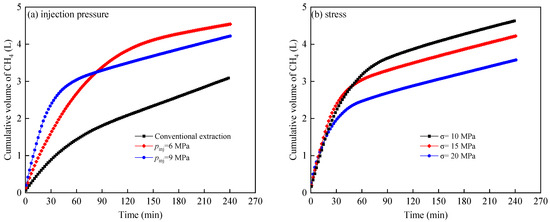
Figure 8.
Cumulative flow curve of CH4 under different injection pressure (a) and stress (b).
4. Conclusions
In this study, ScCO2 fracturing tests and displacement tests under multi-field coupling conditions were carried out innovatively, and the kinetics of CO2 displacing shale gas and the fracturing properties of shale under different stress and pressure conditions were analyzed, which filled the relevant research gaps. The specific conclusions are as follows:
(1) The pressure curve of the ScCO2 fracturing process showed a trend of rapid increase first and then gradually stabilized, followed by a rapid increase until the sample was fractured and then decreased. According to the pressure change, the fracturing process consists of four steps: (I) cavity filling, (II) slow pressure accumulation, (III) rapid pressure increase, and (IV) fracturing failure.
(2) The permeability of unfractured shale is extremely low. The permeability of shale samples before fracturing is 1.38–9.54 × 10−5 mD, while the overall permeability increases by three orders of magnitude after fracturing, indicating that ScCO2 has a good fracturing effect. As the gas pressure increases, the permeability of unfractured shale decreases, while that of fractured shale decreases first and then increases gradually. This primarily results from the enlargement of pore size in shale during fracturing, which leads to the dominance of internal continuous flow and slippage flow.
(3) Higher injection pressures and lower stresses cause CO2 to break through earlier. Compared to the CH4 production rate under conventional recovery conditions, the CH4 production rate has increased after CO2 displacement. In the initial stage of displacement, as the injection pressure increases, the cumulative flow rate of CH4 is higher, but in the later stage of displacement, the cumulative flow rate of CH4 is lower, leading to a decrease in the CH4 production rate and CO2 storage rate. High stress can inhibit CO2 injection and CH4 outflow, reducing the CH4 production rate. However, high stress also promotes the preferential adsorption of CO2 by shale, concluding in a higher CO2 storage rate.
Author Contributions
Conceptualization, K.Y.; methodology, H.Y.; software, Y.S.; validation, Y.L.; investigation, Y.S.; data curation, Y.S.; writing—original draft preparation, Y.S.; writing—review and editing, K.Y.; supervision, Q.C.; project administration, Q.C.; funding acquisition, K.Y. and Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Geological Disaster Patterns and Mitigation Strategies Under River–Reservoir Hydrodynamics in the Three Gorges Reservoir Fluctuation Zone (5000002024CC20004), the National Natural Science Foundation of China (52404145, 52404115), the China Postdoctoral Science Foundation (2024M753494), Chongqing’s Special Funding for Postdoctoral Research Projects (2024CQBSHTB3059), the Chongqing Natural Science Foundation General Project (CSTB2025NSCQ-GPX0557).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to commercial collaboration and ongoing research.
Conflicts of Interest
Yongchang Liang was employed by the company Sichuan Anxin Kechuang Technology Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement and Funding statement. This change does not affect the scientific content of the article.
References
- Yi, J.; Qi, Z.; Li, X.; Liu, H.; Zhou, W. Spatial correlation-based machine learning framework for evaluating shale gas production potential: A case study in southern Sichuan Basin, China. Appl. Energy 2024, 357, 122483. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, J.; Xian, X.; Dong, Z.; Kuang, N.; Peng, Y.; Jiang, H. Feasibility of the CO2-ESGR technique for providing carbon-negative shale gas: A life cycle assessment. J. Clean. Prod. 2024, 484, 144353. [Google Scholar] [CrossRef]
- Chang, X.; Lin, S.; Yang, C.; Wang, K.; Liu, S.; Guo, Y. A critical review of ScCO2-enhanced gas recovery and geologic storage in shale reservoirs. Gas Sci. Eng. 2024, 123, 205317. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, W.; Chen, Z.; Wei, J. Numerical simulation of CO2 sequestration in shale gas reservoirs at reservoir scale coupled with enhanced gas recovery. Energy 2023, 277, 127657. [Google Scholar] [CrossRef]
- Kasala, E.; Wang, J.; Majid, A.; Nadege, M. Enhancing CO2 storage capacity and methane (CH4) production in the Yanchang shale gas reservoir: A simulation study on influencing factors and optimization strategies. Fuel 2025, 388, 134535. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, J.; Xian, X.; Zhou, L.; Zhang, C.; Lu, Z.; Yin, H. Changes of wettability of shale exposed to supercritical CO2-water and its alteration mechanism: Implication for CO2 geo-sequestration. Fuel 2024, 357, 129942. [Google Scholar] [CrossRef]
- Hu, N.; Xian, X.; Zhou, L.; Tang, J.; Kang, Y.; Wang, H. Supercritical CO2 fracking for enhanced shale gas recovery and CO2 sequestration: Results, status and future challenges. Adv. Geo-Energy Res. 2019, 3, 207–224. [Google Scholar] [CrossRef]
- Memon, S.; Feng, R.; Ali, M.; Bhatti, M.A.; Giwelli, A.; Keshavarz, A.; Sarmadivaleh, M. Supercritical CO2-Shale interaction induced natural fracture closure: Implications for ScCO2 hydraulic fracturing in shales. Fuel 2022, 313, 122682. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, W.; Xu, Z.; Liu, S.; Wei, C. A review of experimental apparatus for supercritical CO2 fracturing of shale. J. Petrol. Sci. Eng. 2022, 208, 109515. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, A. Supercritical carbon dioxide utilization for hydraulic fracturing of shale reservoir, and geo-storage: A review. Energy Fuels 2023, 37, 14604–14621. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Tang, J.; Zhou, Z.; Liao, Y. Experimental study on fracture initiation and propagation in shale using supercritical carbon dioxide fracturing. Fuel 2017, 190, 370–378. [Google Scholar] [CrossRef]
- Shen, W.; Ma, T.; Zuo, L.; Yang, X.; Cai, J. Advances and prospects of supercritical CO2 for shale gas extraction and geological sequestration in gas shale reservoirs. Energy Fuels 2024, 38, 789–805. [Google Scholar] [CrossRef]
- Verdon, J.P.; Kendall, J.M.; Maxwell, S.C. A comparison of passive seismic monitoring of fracture stimulation from water and CO2 injection. Geophysics 2010, 75, MA1–MA7. [Google Scholar] [CrossRef]
- Lyu, Q.; Tan, J.; Li, L.; Ju, Y.; Busch, A.; Wood, D.A.; Hu, R. The role of supercritical carbon dioxide for recovery of shale gas and sequestration in gas shale reservoirs. Energy Environ. Sci. 2021, 14, 4203–4227. [Google Scholar] [CrossRef]
- Jia, Y.; Lu, Y.; Elsworth, D.; Fang, Y.; Tang, J. Surface characteristics and permeability enhancement of shale fractures due to water and supercritical carbon dioxide fracturing. J. Petrol. Sci. Eng. 2018, 165, 284–297. [Google Scholar] [CrossRef]
- Hazra, B.; Vishal, V.; Sethi, C.; Chandra, D. Impact of supercritical CO2 on shale reservoirs and its implication for CO2 sequestration. Energy Fuels 2022, 36, 9882–9903. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Li, X.; Lin, C. Experimental study on the supercritical CO2 fracturing of shale considering anisotropic effects. J. Petrol. Sci. Eng. 2019, 173, 932–940. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.; Wang, Y. Experimental investigation on fracture propagation modes in supercritical carbon dioxide fracturing using acoustic emission monitoring. Int. J. Rock. Mech. Min. 2018, 110, 111–119. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; He, J. A laboratory investigation of fracture propagation induced by supercritical carbon dioxide fracturing in continental shale with interbeds. J. Petrol. Sci. Eng. 2018, 166, 739–746. [Google Scholar] [CrossRef]
- Wang, M.; Huang, K.; Xie, W.; Dai, X. Current research into the use of supercritical CO2 technology in shale gas exploitation. Int. J. Min. Sci. Technol. 2019, 29, 739–744. [Google Scholar] [CrossRef]
- Xie, W.; Chen, S.; Wang, M.; Yu, Z.; Wang, H. Progress and prospects of supercritical CO2 application in the exploitation of shale gas reservoirs. Energy Fuels 2021, 35, 18370–18384. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Zhang, J.; Xiao, S.; Zhang, Z.; Shen, Y.; He, J. Displacement mechanism of oil in shale inorganic nanopores by supercritical carbon dioxide from molecular dynamics simulations. Energy Fuels 2017, 31, 738–746. [Google Scholar] [CrossRef]
- Du, X.; Gu, M.; Duan, S.; Xian, X. The influences of CO2 injection pressure on CO2 dispersion and the mechanism of CO2–CH4 displacement in shale. J. Energy Res. Tech. 2018, 140, 012907. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, G.; Chen, K.; Zhang, Y.; Jiang, L.; Song, Y. Molecular dynamic simulation on the dynamic process of CH4 displacement by CO2 in shale pores. Energy Fuels 2024, 38, 7057–7067. [Google Scholar] [CrossRef]
- Du, X.D.; Gu, M.; Duan, S.; Xian, X.F. Investigation of CO2–CH4 displacement and transport in shale for enhanced shale gas recovery and CO2 sequestration. J. Energy Res. Tech. 2017, 139, 012909. [Google Scholar] [CrossRef]
- Sun, J.; Chen, Z.; Wang, X.; Zhang, Y.; Qin, Y.; Chen, C.; Zhou, W. Displacement characteristics of CO2 to CH4 in heterogeneous surface slit pores. Energy Fuels 2023, 37, 2926–2944. [Google Scholar] [CrossRef]
- Du, X.; Gu, M.; Liu, Z.; Zhao, Y.; Sun, F.; Wu, T. Enhanced shale gas recovery by the injections of CO2, N2, and CO2/N2 mixture gases. Energy Fuels 2019, 33, 5091–5101. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Liu, D. Experimental evaluation of CO2 enhanced recovery of adsorbed-gas from shale. Int. J. Coal Geol. 2017, 179, 211–218. [Google Scholar] [CrossRef]
- Iddphonce, R.; Wang, J. Investigation of CO2 and CH4 competitive adsorption during enhanced shale gas production. J. Petrol. Sci. Eng. 2021, 205, 108802. [Google Scholar] [CrossRef]
- Ou, C.; Li, C.; Rui, Z.; Ma, Q. Lithofacies distribution and gas-controlling characteristics of the Wufeng-Longmaxi black shales in the southeastern region of the Sichuan Basin, China. J. Pet. Sci. Eng. 2018, 165, 269–283. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Jiang, Y.; Xian, X.; Liu, Q.; Zhang, D.; Tan, J. Supercritical carbon dioxide fracturing in shale and the coupled effects on the permeability of fractured shale: An experimental study. Nat. Gas. Sci. Eng. 2016, 36, 369–377. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).