Integrating Advanced Radiological Imaging to Enhance Sex Estimation Beyond Classical Anthropological Methods: Population-Specific Models Based on Paranasal Sinuses Volume and Craniometric Data
Abstract
1. Introduction
2. Materials and Methods
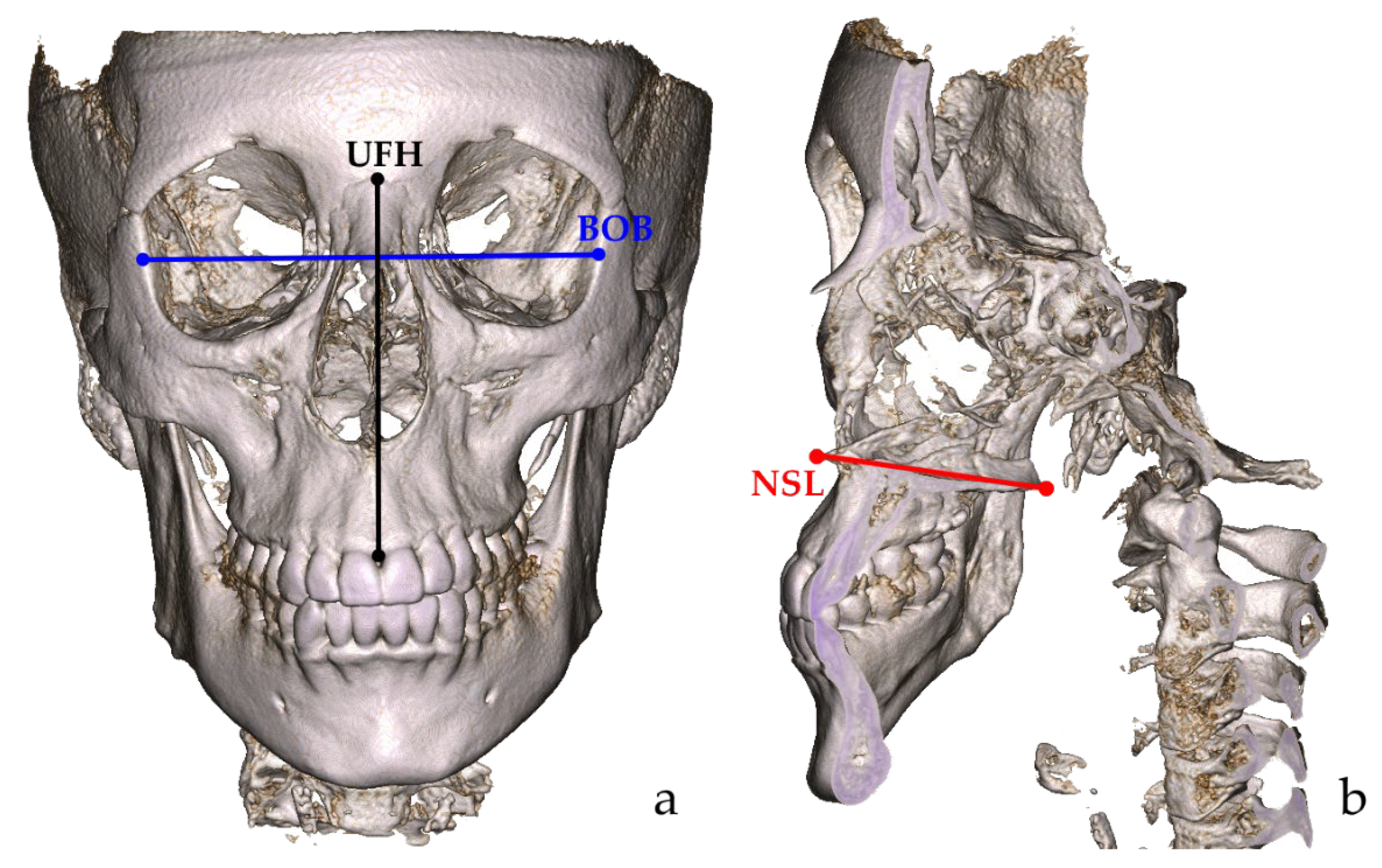
2.1. Volumetric and Linear Measurements
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Offiah, C.E. The Utility of Forensic Radiology in Evaluation of Soft Tissue Injury. In Current Practice in Forensic Medicine; Wiley: Hoboken, NJ, USA, 2022; pp. 143–165. [Google Scholar]
- Cappella, A.; Amadasi, A.; Gaudio, D.; Gibelli, D.; Borgonovo, S.; Di Giancamillo, M.; Cattaneo, C. The Application of Cone-Beam CT in the Aging of Bone Calluses: A New Perspective? Int. J. Leg. Med. 2013, 127, 1139–1144. [Google Scholar] [CrossRef]
- Petaros, A.; Lindblom, M.; Cunha, E. Combining Anthropology and Imaging to Reconstruct Antemortem Trauma for Identification Purposes. Forensic Sci. Res. 2024, 9, owae048. [Google Scholar] [CrossRef]
- Lemos, Y.V.; Furtado, A.N.; Lima, A.Z.; Dionísio, A.S.; Araújo, R.M.; Cunha, E. Human Identification by Medical Findings in a Forensic Anthropology Context. Forensic Sci. Res. 2024, 9, owae041. [Google Scholar] [CrossRef] [PubMed]
- Dedouit, F.; Ducloyer, M.; Elifritz, J.; Adolphi, N.L.; Yi-Li, G.W.; Decker, S.; Ford, J.; Kolev, Y.; Thali, M. The Current State of Forensic Imaging–Clinical Forensic Imaging. Int. J. Leg. Med. 2025, 139, 1639–1646. [Google Scholar] [CrossRef]
- De Boer, H.H.; Blau, S.; Delabarde, T.; Hackman, L. The Role of Forensic Anthropology in Disaster Victim Identification (DVI): Recent Developments and Future Prospects. Forensic Sci. Res. 2018, 4, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Vallis, J. The Role of Radiography in Disaster Victim Identification. In Human Remains: Another Dimension; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–69. [Google Scholar]
- Khmara, N.; Baumeister, R.; Schweitzer, W.; Thali, M.; Ampanozi, G. Virtopsy Concept Around the World: Institute-Based Survey of Worldwide Forensic Postmortem Imaging. Forensic Imaging 2024, 37, 200595. [Google Scholar] [CrossRef]
- Loth, S.R.; İşcan, M.Y. ANTHROPOLOGY | Sex Determination. In Encyclopedia of Forensic Sciences; Siegel, J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; pp. 252–260. [Google Scholar]
- Robinson, M.S.; Bidmos, M.A. The Skull and Humerus in the Determination of Sex: Reliability of Discriminant Function Equations. Forensic Sci. Int. 2009, 186, e1–e86. [Google Scholar] [CrossRef]
- Messer, D.L.; Getz, S.M. Effect of Sex Misclassification on the Skeletal Biological Profile. In Sex Estimation of the Human Skeleton; Elsevier: Amsterdam, The Netherlands, 2020; pp. 53–72. [Google Scholar]
- Thomas, R.M.; Parks, C.L.; Richard, A.H. Accuracy Rates of Sex Estimation by Forensic Anthropologists Through Comparison with DNA Typing Results in Forensic Casework. J. Forensic Sci. 2016, 61, 1307–1310. [Google Scholar] [CrossRef]
- Krishan, K.; Chatterjee, P.M.; Kanchan, T.; Kaur, S.; Baryah, N.; Singh, R.K. A Review of Sex Estimation Techniques During Examination of Skeletal Remains in Forensic Anthropology Casework. Forensic Sci. Int. 2016, 261, e1–e165. [Google Scholar] [CrossRef]
- Franklin, D. Estimation of Skeletal Sex. In Encyclopedia of Forensic Sciences, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 292–303. [Google Scholar]
- Stock, M.K. Analyses of the Postcranial Skeleton for Sex Estimation. In Sex Estimation of the Human Skeleton; Elsevier: Amsterdam, The Netherlands, 2020; pp. 113–130. [Google Scholar]
- Klales, A.R. Sex Estimation Using Pelvis Morphology. In Sex Estimation of the Human Skeleton; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–93. [Google Scholar]
- Garvin, H.M. Adult Sex Estimation from Cranial Morphological Traits. In Sex Estimation of the Human Skeleton; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–112. [Google Scholar]
- Spradley, M.K.; Jantz, R.L. Sex Estimation in Forensic Anthropology: Skull Versus Postcranial Elements. J. Forensic Sci. 2011, 56, 289–296. [Google Scholar] [CrossRef]
- Chapman, T.; Lefevre, P.; Semal, P.; Moiseev, F.; Sholukha, V.; Louryan, S.; Rooze, M.; Van Sint Jan, S. Sex Determination Using the Probabilistic Sex Diagnosis (DSP: Diagnose Sexuelle Probabiliste) Tool in a Virtual Environment. Forensic Sci. Int. 2014, 234, e1–e189. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.L. Sexing Skulls Using Discriminant Function Analysis of Visually Assessed Traits. Am. J. Phys. Anthropol. 2008, 136, 39–50. [Google Scholar] [CrossRef]
- Cappella, A.; Bertoglio, B.; Di Maso, M.; Mazzarelli, D.; Affatato, L.; Stacchiotti, A.; Sforza, C.; Cattaneo, C. Sexual Dimorphism of Cranial Morphological Traits in an Italian Sample: A Population-Specific Logistic Regression Model for Predicting Sex. Biology 2022, 11, 1202. [Google Scholar] [CrossRef]
- Ousley, S.D.; Jantz, R.L. Fordisc 3 and Statistical Methods for Estimating Sex and Ancestry. In A Companion to Forensic Anthropology; Dirkmaat, D.C., Ed.; Wiley-Blackwell Publishing: Oxford, UK, 2012; pp. 400–412. [Google Scholar]
- Manthey, L.; Jantz, R.L.; Bohnert, M.; Jellinghaus, K. Secular Change of Sexually Dimorphic Cranial Variables in Euro-Americans and Germans. Int. J. Leg. Med. 2017, 131, 1113–1118. [Google Scholar] [CrossRef]
- Kimmerle, E.H.; Ross, A.; Slice, D. Sexual Dimorphism in America: Geometric Morphometric Analysis of the Craniofacial Region*. J. Forensic Sci. 2008, 53, 54–57. [Google Scholar] [CrossRef]
- Gillet, C.; Costa-Mendes, L.; Rérolle, C.; Telmon, N.; Maret, D.; Savall, F. Sex Estimation in the Cranium and Mandible: A Multislice Computed Tomography (MSCT) Study Using Anthropometric and Geometric Morphometry Methods. Int. J. Leg. Med. 2020, 134, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Acharya, A.B.; Sattur, A.P.; Naikmasur, V.G. Are Frontal Sinuses Useful Indicators of Sex? J. Forensic Leg. Med. 2013, 20, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Anees, W.; Moreira, D.; Arakelyan, M.; Vieira, W.; Paranhos, L.R.; Franco, A. Umbrella Review: CT of Frontal, Maxillary and Sphenoidal Sinuses for Sexual Dimorphism. J. Forensic Leg. Med. 2025, 111, 102838. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Bavandi, R.; Esmaili, M. Frontal Sinus Agenesis Using Computed Tomography. J. Craniofacial Surg. 2011, 22, e48–e51. [Google Scholar] [CrossRef]
- Ozgursoy, O.B.; Comert, A.; Yorulmaz, I.; Tekdemir, I.; Elhan, A.; Kucuk, B. Hidden Unilateral Agenesis of the Frontal Sinus: Human Cadaver Study of a Potential Surgical Pitfall. Am. J. Otolaryngol. 2010, 31, 231–234. [Google Scholar] [CrossRef]
- Pereira, J.G.D.; Santos, J.B.S.; de Sousa, S.P.; Franco, A.; Silva, R.H.A. Frontal Sinuses as Tools for Human Identification: A Systematic Review of Imaging Methods. Dentomaxillofacial Radiol. 2021, 50, 20200599. [Google Scholar] [CrossRef]
- Güldner, C.; Pistorius, S.M.; Diogo, I.; Bien, S.; Sesterhenn, A.; Werner, J.A. Analysis of Pneumatization and Neurovascular Structures of the Sphenoid Sinus Using Cone-Beam Tomography (CBT). Acta Radiol. 2012, 53, 214–219. [Google Scholar] [CrossRef]
- Teke, H.Y.; Duran, S.; Canturk, N.; Canturk, G. Determination of Gender by Measuring the Size of the Maxillary Sinuses in Computerized Tomography Scans. Surg. Radiol. Anat. 2007, 29, 9–13. [Google Scholar] [CrossRef]
- Khaitan, T.; Kabiraj, A.; Ginjupally, U.; Jain, R. Cephalometric Analysis for Gender Determination Using Maxillary Sinus Index: A Novel Dimension in Personal Identification. Int. J. Dent. 2017, 2017, 7026796. [Google Scholar] [CrossRef] [PubMed]
- Quatrehomme, G.; Fronty, P.; Sapanet, M.; Grévin, G.; Bailet, P.; Ollier, A. Identification by Frontal Sinus Pattern in Forensic Anthropology. Forensic Sci. Int. 1996, 83, 147–153. [Google Scholar] [CrossRef]
- Christensen, A.M. Assessing the Variation in Individual Frontal Sinus Outlines. Am. J. Phys. Anthropol. 2005, 127, 291–295. [Google Scholar] [CrossRef]
- Smith, V.A.; Christensen, A.M.; Myers, S.W. The Reliability of Visually Comparing Small Frontal Sinuses*. J. Forensic Sci. 2010, 55, 1413–1415. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.M.; Hatch, G.M. Advances in the Use of Frontal Sinuses for Human Identification. In New Perspectives in Forensic Human Skeletal Identification; Elsevier: Amsterdam, The Netherlands, 2018; pp. 227–240. [Google Scholar]
- Li, Y.; Xu, C.; Yu, D.; Xiong, T.; Zhao, H.; Xue, H.; Liang, W.B.; Deng, Z.H.; Zhang, L. Computer-Aided Superimposition of the Frontal Sinus via 3D Reconstruction for Comparative Forensic Identification. Int. J. Leg. Med. 2021, 135, 1993–2001. [Google Scholar] [CrossRef]
- Beaini, T.L.; Duailibi-Neto, E.F.; Chilvarquer, I.; Melani, R.F.H. Human Identification Through Frontal Sinus 3D Superimposition: Pilot Study with Cone Beam Computer Tomography. J. Forensic Leg. Med. 2015, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, M.; Miyasaka, S.; Sato, H.; Seta, S. Classification System of Frontal Sinus Patterns by Radiography. Its Application to Identification of Unknown Skeletal Remains. Forensic Sci. Int. 1987, 34, 289–299. [Google Scholar] [CrossRef]
- Soares, C.B.R.B.; Almeida, M.S.C.; Lopes, P.D.M.L.; Beltrão, R.V.; dos Anjos Pontual, A.; Ramos-Perez, F.M.D.M.; Figueroa, J.N.; Pontual, M.L.D.A. Human Identification Study by Means of Frontal Sinus Imaginological Aspects. Forensic Sci. Int. 2016, 262, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Gibelli, D.; Cappella, A.; Toluian, T.; Pittino, C.V.; Carlo, M.; Oliva, G. Segmentation Procedures for the Assessment of Paranasal Sinuses Volumes. Neuroradiol. J. 2021, 34, 13–20. [Google Scholar] [CrossRef]
- Sridhar, M.; Bagewadi, A.; Lagali-Jirge, V.; Lokesh Kumar, S.; Panwar, A.; Keluskar, V. Reliability of Gender Determination from Paranasal Sinuses and Its Application in Forensic Identification—A Systematic Review and Meta-Analysis. Forensic Sci. Med. Pathol. 2022, 19, 409–439. [Google Scholar] [CrossRef]
- Christoloukas, N.; Mitsea, A.; Rontogianni, A.; Angelopoulos, C. Gender Determination Based on CBCT Maxillary Sinus Analysis: A Systematic Review. Diagnostics 2023, 13, 3536. [Google Scholar] [CrossRef]
- Cohen, O.; Warman, M.; Fried, M.; Shoffel-Havakuk, H.; Adi, M.; Halperin, D.; Lahav, Y. Volumetric Analysis of the Maxillary, Sphenoid and Frontal Sinuses: A Comparative Computerized Tomography Based Study. Auris Nasus Larynx 2018, 45, 96–102. [Google Scholar] [CrossRef]
- de Mendonça, D.S.; Ribeiro, E.C.; de Barros Silva, P.G.; Rodrigues, A.A.; Kurita, L.M.; de Aguiar, A.S.W.; Tuji, F.M.; Neves, F.S.; Carvalho, F.S.R.; Costa, F.W.G. Diagnostic Accuracy of Paranasal Sinus Measurements on Multislice Computed Tomography for Sex Estimation: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Forensic Sci. 2022, 67, 2151–2164. [Google Scholar] [CrossRef]
- Robles, M.; Nakhaeizadeh, S.; Rando, C.; Morgan, R.M. Human Identification: An Investigation of 3D Models of Paranasal Sinuses to Establish a Biological Profile on a Modern UK Population. Int. J. Leg. Med. 2024, 138, 1411–1424. [Google Scholar] [CrossRef]
- Uthman, A.T.; Al-Rawi, N.H.; Al-Naaimi, A.S.; Al-Timimi, J.F. Evaluation of Maxillary Sinus Dimensions in Gender Determination Using Helical CT Scanning. J. Forensic Sci. 2011, 56, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kannampurath, A.; Leela Srikantannair, S.; Mathew, P.; SivaPrasad, T. Maxillary Sinus in Gender Determination: A Morphometric Analysis Using Cone Beam Computed Tomography. Forensic Sci. Med. Pathol. 2023, 20, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.C.L.; Walewski, L.Â.; de Souza Tolentino, E.; Iwaki, L.C.V.; Silva, M.C. Three-Dimensional Analysis of the Maxillary Sinus for Determining Sex and Age in Human Identification. Forensic Imaging 2020, 22, 200395. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Bakhtavar, K.; Moarefdoost, J.; Kamali, A.; Rafeifar, S. Frontal Sinus Parameters in Computed Tomography and Sex Determination. Leg. Med. 2016, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Peckmann, T.R.; Orr, K.; Meek, S.; Manolis, S.K. Sex Determination from the Talus in a Contemporary Greek Population Using Discriminant Function Analysis. J. Forensic Leg. Med. 2015, 33, 14–19. [Google Scholar] [CrossRef]
- Ruiz Mediavilla, E.; Perea Pérez, B.; Labajo González, E.; Sánchez Sánchez, J.A.; Santiago Sáez, A.; Dorado Fernández, E. Determining Sex by Bone Volume from 3D Images: Discriminating Analysis of the Tali and Radii in a Contemporary Spanish Reference Collection. Int. J. Leg. Med. 2012, 126, 623–631. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hekimoglu, Y.; Sasani, H.; Etli, Y.; Keskin, S.; Tastekin, B.; Asirdizer, M. Sex Estimation from the Paranasal Sinus Volumes Using Semiautomatic Segmentation, Discriminant Analyses, and Machine Learning Algorithms. Am. J. Forensic Med. Pathol. 2023, 44, 311–320. [Google Scholar] [CrossRef]
- Michel, J.; Paganelli, A.; Varoquaux, A.; Piercecchi-Marti, M.; Adalian, P.; Leonetti, G.; Dessi, P. Determination of Sex: Interest of Frontal Sinus 3D Reconstructions. J. Forensic Sci. 2015, 60, 269–273. [Google Scholar] [CrossRef]
- Radulesco, T.; Michel, J.; Mancini, J.; Dessi, P.; Adalian, P. Sex Estimation from Human Cranium: Forensic and Anthropological Interest of Maxillary Sinus Volumes. J. Forensic Sci. 2018, 63, 805–808. [Google Scholar] [CrossRef]
- Thottungal, R.R.; Obertova, Z.; Flavel, A.; Franklin, D. Sex Estimation of Frontal Sinus Volume from Computed Tomography Scans in a Western Australian Adult Population. Anthropol. Anz. 2024, 81, 161–167. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Abdel-Karim, R.I.; Ibrahim, M.S.; Dar, U.F. Comparative Study of the Reliability of Frontal and Maxillary Sinuses in Sex Identification Using Multidetector Computed Tomography Among Egyptians. Forensic Imaging 2020, 22, 200390. [Google Scholar] [CrossRef]
- Wanzeler, A.M.V.; Alves-Júnior, S.M.; Ayres, L.; da Costa Prestes, M.C.; Gomes, J.T.; Tuji, F.M. Sex Estimation Using Paranasal Sinus Discriminant Analysis: A New Approach via Cone Beam Computerized Tomography Volume Analysis. Int. J. Leg. Med. 2019, 133, 1977–1984. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-Guided 3D Active Contour Segmentation of Anatomical Structures: Significantly Improved Efficiency and Reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Simmons-Ehrhardt, T.L.; Ehrhardt, C.J.; Monson, K.L. Evaluation of the Suitability of Cranial Measurements Obtained from Surface-Rendered CT Scans of Living People for Estimating Sex and Ancestry. J. Forensic Radiol. Imaging 2019, 19, 100338. [Google Scholar] [CrossRef]
- Costa, H.N.; Slavicek, R.; Sato, S. A Computerized Tomography Study of the Morphological Interrelationship Between the Temporal Bones and the Craniofacial Complex. J. Anat. 2012, 220, 544–554. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Kawarai, Y.; Fukushima, K.; Ogawa, T.; Nishizaki, K.; Gunduz, M.; Fujimoto, M.; Masuda, Y. Volume Quantification of Healthy Paranasal Cavity by Three-Dimensional CT Imaging. Acta Otolaryngol. Suppl. 1999, 540, 45–49. [Google Scholar] [PubMed]
- Čechová, M.; Dupej, J.; Brůžek, J.; Bejdová, Š.; Horák, M.; Velemínská, J. Sex Estimation Using External Morphology of the Frontal Bone and Frontal Sinuses in a Contemporary Czech Population. Int. J. Leg. Med. 2019, 133, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.G.G.; Duailibi-Neto, E.F.; Beaini, T.L.; da Silva, R.L.B.; Chilvarquer, I. The Frontal Sinus Cavity Exhibits Sexual Dimorphism in 3D Cone-Beam CT Images and Can Be Used for Sex Determination. J. Forensic Sci. 2018, 63, 692–698. [Google Scholar] [CrossRef]
- Emirzeoglu, M.; Sahin, B.; Bilgic, S.; Celebi, M.; Uzun, A. Volumetric Evaluation of the Paranasal Sinuses in Normal Subjects Using Computer Tomography Images: A Stereological Study. Auris Nasus Larynx 2007, 34, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Heussen, N.; Peters, F.; Steiner, T.; Hölzle, F.; Modabber, A. Is the Maxillary Sinus Really Suitable in Sex Determination? A Three-Dimensional Analysis of Maxillary Sinus Volume and Surface Depending on Sex and Dentition. J. Craniofacial Surg. 2015, 26, e723–e726. [Google Scholar] [CrossRef]
- Farias Gomes, A.; de Oliveira Gamba, T.; Yamasaki, M.C.; Groppo, F.C.; Haiter Neto, F.; Possobon, R.D.F. Development and Validation of a Formula Based on Maxillary Sinus Measurements as a Tool for Sex Estimation: A Cone Beam Computed Tomography Study. Int. J. Leg. Med. 2019, 133, 1241–1249. [Google Scholar] [CrossRef]
- Urooge, A.; Patil, B.A. Sexual Dimorphism of Maxillary Sinus: A Morphometric Analysis Using Cone Beam Computed Tomography. J. Clin. Diagn. Res. 2017, 11, ZC67–ZC70. [Google Scholar] [CrossRef]
- Saccucci, M.; Cipriani, F.; Carderi, S.; Di Carlo, G.; D’Attilio, M.; Rodolfino, D.; Festa, F.; Polimeni, A. Gender Assessment Through Three-Dimensional Analysis of Maxillary Sinuses by Means of Cone Beam Computed Tomography. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 185–193. [Google Scholar]
- Bangi, B.B.; Ginjupally, U.; Nadendla, L.K.; Vadla, B. 3D Evaluation of Maxillary Sinus Using Computed Tomography: A Sexual Dimorphic Study. Int. J. Dent. 2017, 2017, 9017078. [Google Scholar] [CrossRef]
- Gibelli, D.; Cellina, M.; Gibelli, S.; Oliva, A.G.; Codari, M.; Termine, G.; Sforza, C. Volumetric Assessment of Sphenoid Sinuses through Segmentation on CT Scan. Surg. Radiol. Anat. 2018, 40, 193–198. [Google Scholar] [CrossRef]
- Ramos, B.C.; Manzi, F.R.; Vespasiano, A.I. Volumetric and Linear Evaluation of the Sphenoidal Sinus of a Brazilian Population, in Cone Beam Computed Tomography. J. Forensic Leg. Med. 2021, 77, 102097. [Google Scholar] [CrossRef] [PubMed]
- Yonetsu, K.; Watanabe, M.; Nakamura, T. Age-Related Expansion and Reduction in Aeration of the Sphenoid Sinus: Volume Assessment by Helical CT Scanning. Am. J. Neuroradiol. 2000, 21, 179–182. [Google Scholar] [PubMed]
- Nejaim, Y.; Farias Gomes, A.; Valadares, C.V.; Costa, E.D.; Peroni, L.V.; Groppo, F.C.; Haiter-Neto, F. Evaluation of Volume of the Sphenoid Sinus According to Sex, Facial Type, Skeletal Class, and Presence of a Septum: A Cone-Beam Computed Tomographic Study. Br. J. Oral Maxillofac. Surg. 2019, 57, 336–340. [Google Scholar] [CrossRef]
- Oliveira, J.M.M.; Alonso, M.B.C.C.; de Sousa e Tucunduva, M.J.A.P.; Fuziy, A.; Scocate, A.C.R.N.; Costa, A.L.F. Volumetric Study of Sphenoid Sinuses: Anatomical Analysis in Helical Computed Tomography. Surg. Radiol. Anat. 2017, 39, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, B.; Durmaz, S.; Kanat, A.; Yemis, T.; Ozdemir, C.; Celiker, F.B. The Gender-Related Volumetric Side Asymmetries in Sphenoid Sinuses and Their Clinical Significance. Eur. Arch. Oto-Rhino-Laryngol. 2025, 282, 2567–2570. [Google Scholar] [CrossRef]
- Yan, Y.; Guo, F.; Liu, J.; Yu, M.; Huang, Y. Anatomical Variants, Pneumatization Classification, and Volumetric Studies of the Sphenoid Sinus with High-Resolution Computed Tomography. J. Craniofacial Surg. 2021, 32, 2542–2545. [Google Scholar] [CrossRef]
- Wu, Z.-X.; Bu, W.-Q.; Tang, Y.; Guo, Y.-X.; Guo, Y.-C.; Wang, F.; Meng, H.-T. Sex Estimation Using Maxillary Sinus Volume for Chinese Subjects Based on Cone-Beam Computed Tomography. BMC Oral Health 2024, 24, 253. [Google Scholar] [CrossRef]
- Salim, H.; Yanarates, G.; Golpinar, M.; Komut, E.; Aydoğdu, G. Sex Estimation with Three-Dimensional Analysis of the Maxillary Sinus from Computed Tomography Images. J. Craniofacial Surg. 2024, 35, 2288–2290. [Google Scholar] [CrossRef]
- Cappella, A.; Gibelli, D.; Vitale, A.; Zago, M.; Dolci, C.; Sforza, C.; Cattaneo, C. Preliminary Study on Sexual Dimorphism of Metric Traits of Cranium and Mandible in a Modern Italian Skeletal Population and Review of Population Literature. Leg. Med. 2020, 44, 101695. [Google Scholar] [CrossRef] [PubMed]
- Bartholdy, B.P.; Sandoval, E.; Hoogland, M.L.P.; Schrader, S.A. Getting Rid of Dichotomous Sex Estimations: Why Logistic Regression Should Be Preferred over Discriminant Function Analysis. J. Forensic Sci. 2020, 65, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Konigsberg, L.W.; Frankenberg, S.R. Multivariate Ordinal Probit Analysis in the Skeletal Assessment of Sex. Am. J. Phys. Anthropol. 2019, 169, 385–387. [Google Scholar] [CrossRef]
- Klales, A.R.; Ousley, S.D.; Vollner, J.M. A Revised Method of Sexing the Human Innominate Using Phenice’s Nonmetric Traits and Statistical Methods. Am. J. Phys. Anthropol. 2012, 149, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lesciotto, K.M.; Klales, A.R. Sex Estimation Using Metrics of the Innominate: A Test of the DSP2 Method. J. Forensic Sci. 2025, 70, 249–257. [Google Scholar] [CrossRef]
- Rojas González, N.; Obertová, Z.; Franklin, D. Validation and Recalibration of Sex Estimation Methods Using Pubic Nonmetric Traits for the Chilean Population. Int. J. Leg. Med. 2024, 138, 2071–2080. [Google Scholar] [CrossRef]
- Gupta, A.; Billings, B.K.; Hummel, S.; Grosskopf, B. Evaluating Morphological Methods for Sex Estimation on Isolated Human Skeletal Materials: Comparisons of Accuracies Between German and South African Skeletal Collections. Forensic Sci. 2022, 2, 574–584. [Google Scholar] [CrossRef]
- Indra, L.; Lösch, S. Forensic Anthropology Casework from Switzerland (Bern): Taphonomic Implications for the Future. Forensic Sci. Int. Rep. 2021, 4, 100222. [Google Scholar] [CrossRef]
- Klales, A.R. Practitioner Preferences for Sex Estimation from Human Skeletal Remains. In Sex Estimation of the Human Skeleton; Elsevier: Amsterdam, The Netherlands, 2020; pp. 11–23. [Google Scholar]
- Colman, K.L.; de Boer, H.H.; Dobbe, J.G.G.; Liberton, N.P.T.J.; Stull, K.E.; van Eijnatten, M.; Streekstra, G.J.; Oostra, R.-J.; van Rijn, R.R.; van der Merwe, A.E. Virtual Forensic Anthropology: The Accuracy of Osteometric Analysis of 3D Bone Models Derived from Clinical Computed Tomography (CT) Scans. Forensic Sci. Int. 2019, 304, 109963. [Google Scholar] [CrossRef] [PubMed]
- Colman, K.L.; Dobbe, J.G.G.; Stull, K.E.; Ruijter, J.M.; Oostra, R.-J.; van Rijn, R.R.; van der Merwe, A.E.; de Boer, H.H.; Streekstra, G.J. The Geometrical Precision of Virtual Bone Models Derived from Clinical Computed Tomography Data for Forensic Anthropology. Int. J. Leg. Med. 2017, 131, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazak, N.; Butaric, L.N.; Garvin, H.M. Age-Related Changes to Frontal Sinus Traits and Implications for Forensic Identification. Forensic Anthropol. 2023, 6, 192. [Google Scholar] [CrossRef]
- Aktuna Belgin, C.; Colak, M.; Adiguzel, O.; Akkus, Z.; Orhan, K. Three-Dimensional Evaluation of Maxillary Sinus Volume in Different Age and Sex Groups Using CBCT. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 1493–1499. [Google Scholar] [CrossRef]
- Iturralde-Garrote, A.; Sanz, J.L.; Forner, L.; Melo, M.; Puig-Herreros, C. Volumetric Changes of the Paranasal Sinuses with Age: A Systematic Review. J. Clin. Med. 2023, 12, 3355. [Google Scholar] [CrossRef]
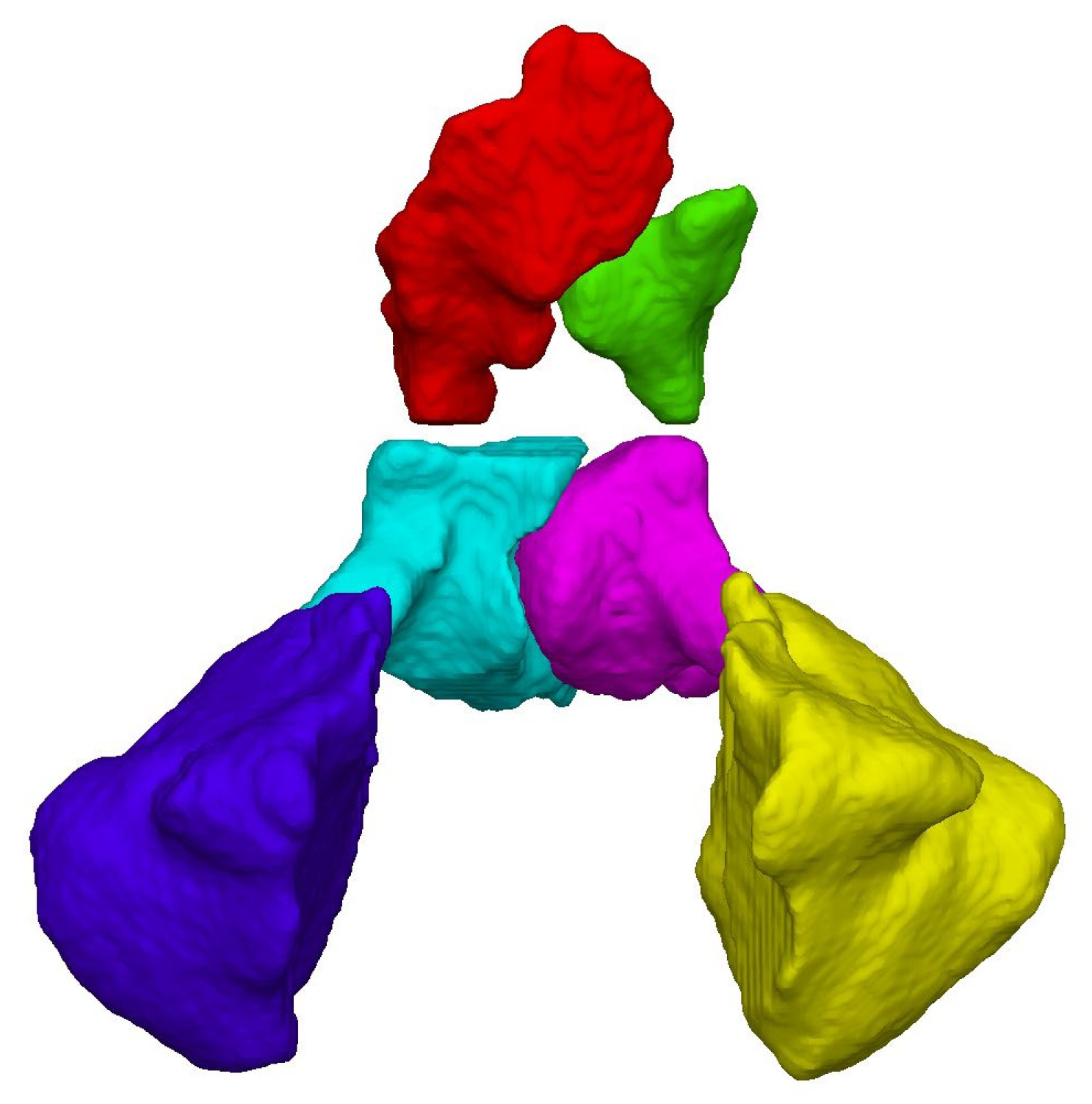
| Female | Male | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | p-Value | |
| Age (years) | 58.4 | 21.1 | 20 | 96 | 44.9 | 21.0 | 18 | 96 | <0.001 § |
| Paranasal sinuses volume (cm3) | |||||||||
| RFS | 2.52 | 1.61 | 0.15 | 9.54 | 4.56 | 2.42 | 0.43 | 13.69 | <0.001 * |
| LFS | 3.34 | 2.28 | 0.35 | 12.65 | 5.01 | 2.97 | 0.46 | 19.34 | <0.001 * |
| TFS | 5.86 | 3.24 | 0.66 | 17.19 | 9.57 | 4.41 | 2.35 | 27.44 | <0.001 * |
| RMS | 13.87 | 3.66 | 4.94 | 23.85 | 17.32 | 5.12 | 4.92 | 30.86 | <0.001 * |
| LMS | 13.92 | 3.64 | 1.98 | 21.83 | 16.89 | 5.35 | 5.57 | 30.37 | <0.001 * |
| TMS | 27.79 | 6.48 | 13.15 | 44.45 | 34.21 | 9.83 | 10.49 | 60.53 | <0.001 * |
| RSS | 4.18 | 2.58 | 0.08 | 11.90 | 5.20 | 3.29 | 0.36 | 16.18 | 0.012 |
| LSS | 4.05 | 2.20 | 0.20 | 10.93 | 5.44 | 3.07 | 0.39 | 14.65 | <0.001 * |
| TSS | 8.23 | 3.06 | 2.67 | 15.87 | 10.64 | 4.61 | 3.09 | 29.80 | <0.001 * |
| TV | 41.88 | 9.39 | 18.78 | 68.01 | 54.42 | 15.00 | 26.89 | 100.53 | <0.001 * |
| Cranial linear measurement (mm) | |||||||||
| BOB | 94.73 | 3.42 | 84.23 | 103.8 | 97.71 | 4.99 | 69.98 | 107.8 | <0.001 * |
| UFH | 66.30 | 4.89 | 50.19 | 78.64 | 71.25 | 5.23 | 53.19 | 90.64 | <0.001 * |
| NSL | 52.21 | 3.17 | 44.02 | 61.65 | 55.40 | 4.09 | 47.79 | 66.87 | <0.001 * |
| Predictor | Estimate | p-Value | Odds Ratios (95% CI) | VIF |
|---|---|---|---|---|
| Intercept | −4.170 | <0.001 * | 0.015 (0.003–0.078) | |
| RFS | 0.422 | <0.001 * | 1.526 (1.236–1.883) | 1.308 |
| LFS | 0.126 | 0.115 | 1.134 (0.970–1.327) | 1.401 |
| RMS | 0.154 | 0.007 * | 1.166 (1.043–1.305) | 2.217 |
| LMS | −0.034 | 0.543 | 0.967 (0.868–1.078) | 2.417 |
| RSS | 0.008 | 0.913 | 1.008 (0.877–1.158) | 1.255 |
| LSS | 0.070 | 0.382 | 1.072 (0.917–1.253) | 1.366 |
| Training sample | |||||
| Predicted | Accuracy rates | ||||
| Female | Male | Total | |||
| True | Female | 68 | 23 | 91 | Sensitivity: 68/91 × 100 = 74.7% |
| Male | 26 | 61 | 87 | Specificity: 67/87 × 100 = 77.0% | |
| Total | 94 | 84 | 178 | Accuracy: 129/178 × 100 = 72.5% | |
| Validation sample | |||||
| Predicted | Accuracy rates | ||||
| Female | Male | Total | |||
| True | Female | 17 | 6 | 23 | Sensitivity: 17/23 × 100 = 73.9% |
| Male | 3 | 18 | 21 | Specificity: 18/21 × 100 = 85.7% | |
| Total | 20 | 24 | 44 | Accuracy: 35/44 × 100 = 79.5% | |
| Predictor | Estimate | p-Value | Odds Ratio (95% CI) | VIF |
|---|---|---|---|---|
| Intercept | −29.969 | <0.001 * | –) | |
| RFS | 0.313 | 0.009 * | 1.367 (1.079–1.733) | 1.430 |
| LFS | 0.165 | 0.094 | 1.180 (0.972–1.431) | 1.410 |
| RMS | 0.112 | 0.081 | 1.119 (0.986–1.268) | 2.279 |
| LMS | −0.034 | 0.584 | 0.967 (0.858–1.09) | 2.437 |
| RSS | 0.075 | 0.381 | 1.077 (0.912–1.273) | 1.272 |
| LSS | 0.102 | 0.270 | 1.108 (0.923–1.329) | 1.395 |
| BOB | 0.040 | 0.396 | 1.041 (0.949–1.142) | 1.332 |
| UFH | 0.168 | <0.001 * | 1.183 (1.084–1.292) | 1.325 |
| NSL | 0.202 | 0.002 * | 1.224 (1.079–1.389) | 1.263 |
| Training sample | |||||
| Predicted | Accuracy rates | ||||
| Female | Male | Total | |||
| True | Female | 73 | 18 | 91 | Sensitivity: 73/91 × 100 = 80.2% |
| Male | 20 | 67 | 87 | Specificity: 67/87 × 100 = 77.0% | |
| Total | 93 | 85 | 178 | Accuracy: 140/178 × 100 = 78.7% | |
| Validation sample | |||||
| Predicted | Accuracy rates | ||||
| Female | Male | Total | |||
| True | Female | 17 | 6 | 23 | Sensitivity: 17/23 × 100 = 73.9% |
| Male | 4 | 17 | 21 | Specificity: 17/21 × 100 = 81.0% | |
| Total | 21 | 23 | 44 | Accuracy: 34/44 × 100 = 77.3% | |
| First Author, Year | Population | Sample Size | Sinuses | Equation | Accuracy | Sensitivity; Specificity |
|---|---|---|---|---|---|---|
| Studies using the volume of one or two pairs of sinuses | ||||||
| Wu et al., 2024 [82] | Chinese | Training: 179 (140 M; 139 F) Test: 70 (35 M; 35 F) | LMS | p = | Training: 78.57% Test: 76.67% | Training: 91.43%; 65.71% Test: 86.67%; 66.67% |
| RMS | p = | Training: 74.29% Test: 73.33% | Training: 97.14%; 51.43% Test: 93.33%; 53.33% | |||
| Thottungal et al., 2024 [57] | Western Australian | 99 (47 M; 52 F) | TFS | p = | 57.20% | 60.8%; 44.4% |
| LFS | p = | 57.30% | 64.7%; 53.3% | |||
| RFS | p = | 62.50% | 56.9%: 46.7% | |||
| Michel et al., 2015 [55] | French | 69 (34 M; 35 F) | TFS | p(Male) = | 72.5% | Not provided |
| p(Female) = | ||||||
| Radulesco et al., 2018 [56] | French | 103 (50 M; 53 F) | LMS, RMS | / | 68.00% | 66.0%; 70.0% |
| Studies using the volume of one or two pairs of sinuses coupled with linear measurements | ||||||
| Choi et al., 2018 [68] | Brazilian | 130 (65 M; 65 F) | TFS | p = | 80.00% | 87.69%; 72.31% |
| Ibrahim et al., 2020 [58] | Egyptian | 100 (50 M; 50 F) | RFS, LFS | df = 0.664 × RAP + 0.542 × LAP + 0.337 × RCC + 0.428 × LCC + 0.662 × RT + 0.526 × LT + 0.448 × RFS + 0.334 × LFS | 94.00% | 92.0%; 96.0% |
| RMS, LMS | df = 0.578 × RCC + 0.485 × LCC + 0.689 × LT + 0.368 × LFS | 92.00% | 92.0%; 92.0% | |||
| Salim et al., 2024 [83] | Turkish | 232 (116 M; 116 F) | LMS, RMS | / | 68.20% | 77.6%; 58.6% |
| LMS | / | 61.20% | 93.1%; 29.3% | |||
| RMS | / | 60.30% | 58.6%; 62.0% | |||
| Studies using the volume of all sinuses | ||||||
| Hekimoglu et al., 2023 [54] | Turkish | 100 (50 M; 50 F) | RFS, RLS, RMS, LMS, RSS, LSS | df(Male) = −8.139 + 0.411 × RFS + 0.161 × LFS + 0.114 × RMS + 0.359 × LMS + 0.632 × RSS + 0.265 × LSS | 68.00% | 74.00%; 62.00% |
| df(Female) = −5.421 + 0.094 × RFS + 0.073 × LFS + 0.045 × RMS + 0.435 × LMS + 0.534 × RSS + 0.143 × LSS | ||||||
| Studies using the volume of all sinuses, and all the volumes coupled with cranial linear measurements | ||||||
| Wanzeler et al., 2019 [59] | Brazilian | 163 (80 M; 83 F) | TFS, RMS, LMS, RSS, LSS | Not provided | 94.48% | 92.77%; 96.25% |
| TFS, RMS, LMS, RSS, LSS, and foramen magnum measurements | Not Provided | 100% | 100%; 100% | |||
| Present study | Italian | 222 (108 M; 114 F) | RFS, LFS, RMS, LMS, RSS, LSS | Training: 72.5% Validation: 73.9% | Training: 74.7%; 77.0% Validation: 85.7%; 79.5% | |
| RFS, LFS, RMS, LMS, RSS, LSS, and cranial measurements | Training: 78.7% Validation: 73.9% | Training: 80.2%; 77.0% Validation: 81.0%; 77.3% | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solazzo, R.; Palamenghi, A.; Mazzarelli, D.; Cellina, M.; Sforza, C.; Cattaneo, C.; Gibelli, D.; Cappella, A. Integrating Advanced Radiological Imaging to Enhance Sex Estimation Beyond Classical Anthropological Methods: Population-Specific Models Based on Paranasal Sinuses Volume and Craniometric Data. Appl. Sci. 2025, 15, 10232. https://doi.org/10.3390/app151810232
Solazzo R, Palamenghi A, Mazzarelli D, Cellina M, Sforza C, Cattaneo C, Gibelli D, Cappella A. Integrating Advanced Radiological Imaging to Enhance Sex Estimation Beyond Classical Anthropological Methods: Population-Specific Models Based on Paranasal Sinuses Volume and Craniometric Data. Applied Sciences. 2025; 15(18):10232. https://doi.org/10.3390/app151810232
Chicago/Turabian StyleSolazzo, Riccardo, Andrea Palamenghi, Debora Mazzarelli, Michaela Cellina, Chiarella Sforza, Cristina Cattaneo, Daniele Gibelli, and Annalisa Cappella. 2025. "Integrating Advanced Radiological Imaging to Enhance Sex Estimation Beyond Classical Anthropological Methods: Population-Specific Models Based on Paranasal Sinuses Volume and Craniometric Data" Applied Sciences 15, no. 18: 10232. https://doi.org/10.3390/app151810232
APA StyleSolazzo, R., Palamenghi, A., Mazzarelli, D., Cellina, M., Sforza, C., Cattaneo, C., Gibelli, D., & Cappella, A. (2025). Integrating Advanced Radiological Imaging to Enhance Sex Estimation Beyond Classical Anthropological Methods: Population-Specific Models Based on Paranasal Sinuses Volume and Craniometric Data. Applied Sciences, 15(18), 10232. https://doi.org/10.3390/app151810232









