Development and Performance Evaluation of Translucent Concrete Incorporating Activated Copper Tailings as Cementitious Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Sample Preparation
2.2.1. Preparation of Cementitious Specimens and Experimental Methods
- Raw material measurement
- 2.
- Mixing process
- 3.
- Molding and compaction
- 4.
- Curing
- 5.
- Testing
2.2.2. Preparation and Experimental Methods for Light-Transmitting Concrete Specimens
- Fiber embedding
- 2.
- Mixing and casting
- 3.
- Curing and testing
| Scheme | Total Mass/g | Activator | Activator Content in Total Binder/wt% | Water-to-Solid Ratio | Cement Content in Light-Transmitting Concrete/wt% | Polypropylene Fiber Content/wt% |
|---|---|---|---|---|---|---|
| Cementitious paste A | 100 | NaOH | 10 | 3:10 | – | – |
| 100 | NaOH | 20 | 7:20 | – | – | |
| 100 | NaOH | 30 | 2:5 | – | – | |
| 100 | NaOH | 40 | 9:20 | – | – | |
| 100 | NaOH | 50 | 1:2 | – | – | |
| Cementitious paste B | 100 | CaO | 10 | 3:10 | – | – |
| 100 | CaO | 20 | 7:20 | – | – | |
| 100 | CaO | 30 | 2:5 | – | – | |
| 100 | CaO | 40 | 9:20 | – | – | |
| 100 | CaO | 50 | 1:2 | – | – | |
| Cementitious paste C | 100 | Na2SiO3 | 10 | 3:10 | – | – |
| 100 | Na2SiO3 | 20 | 7:20 | – | – | |
| 100 | Na2SiO3 | 30 | 2:5 | – | – | |
| 100 | Na2SiO3 | 40 | 9:20 | – | – | |
| 100 | Na2SiO3 | 50 | 1:2 | – | – | |
| Cementitious paste D | 100 | Na2CO3 | 10 | 3:10 | – | – |
| 100 | Na2CO3 | 20 | 7:20 | – | – | |
| 100 | Na2CO3 | 30 | 2:5 | – | – | |
| 100 | Na2CO3 | 40 | 9:20 | – | – | |
| 100 | Na2CO3 | 50 | 1:2 | – | – | |
| Concrete specimen | 100 | CaO | 10 | 1:2 | 9:1 | – |
| 100 | CaO | 10 | 1:2 | 8:2 | – | |
| 100 | CaO | 10 | 1:2 | 7:3 | – | |
| 100 | CaO | 10 | 1:2 | 6:4 | – | |
| 100 | CaO | 10 | 1:2 | 5:5 | – | |
| Light-transmitting concrete specimen | 100 | CaO | 10 | 1:2 | 6:4 | 1 |
| 100 | CaO | 10 | 1:2 | 6:4 | 2 | |
| 100 | CaO | 10 | 1:2 | 6:4 | 3 | |
| 100 | CaO | 10 | 1:2 | 6:4 | 4 | |
| 100 | CaO | 10 | 1:2 | 6:4 | 5 |
2.3. Characterization of Material Methods
3. Results and Discussion
3.1. Optimization of the Performance of Copper-Tailing-Based Cementitious Materials
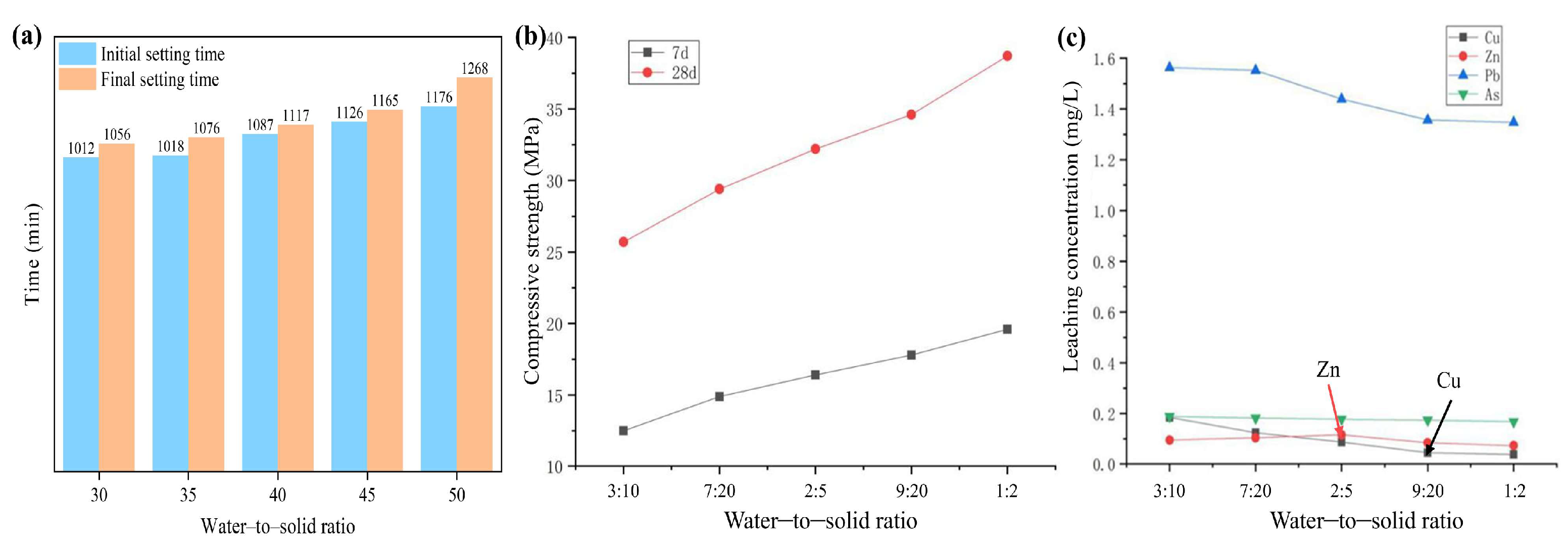
3.1.1. Study on the Effect of Water-to-Solid Ratio on the Performance of Cementitious Materials
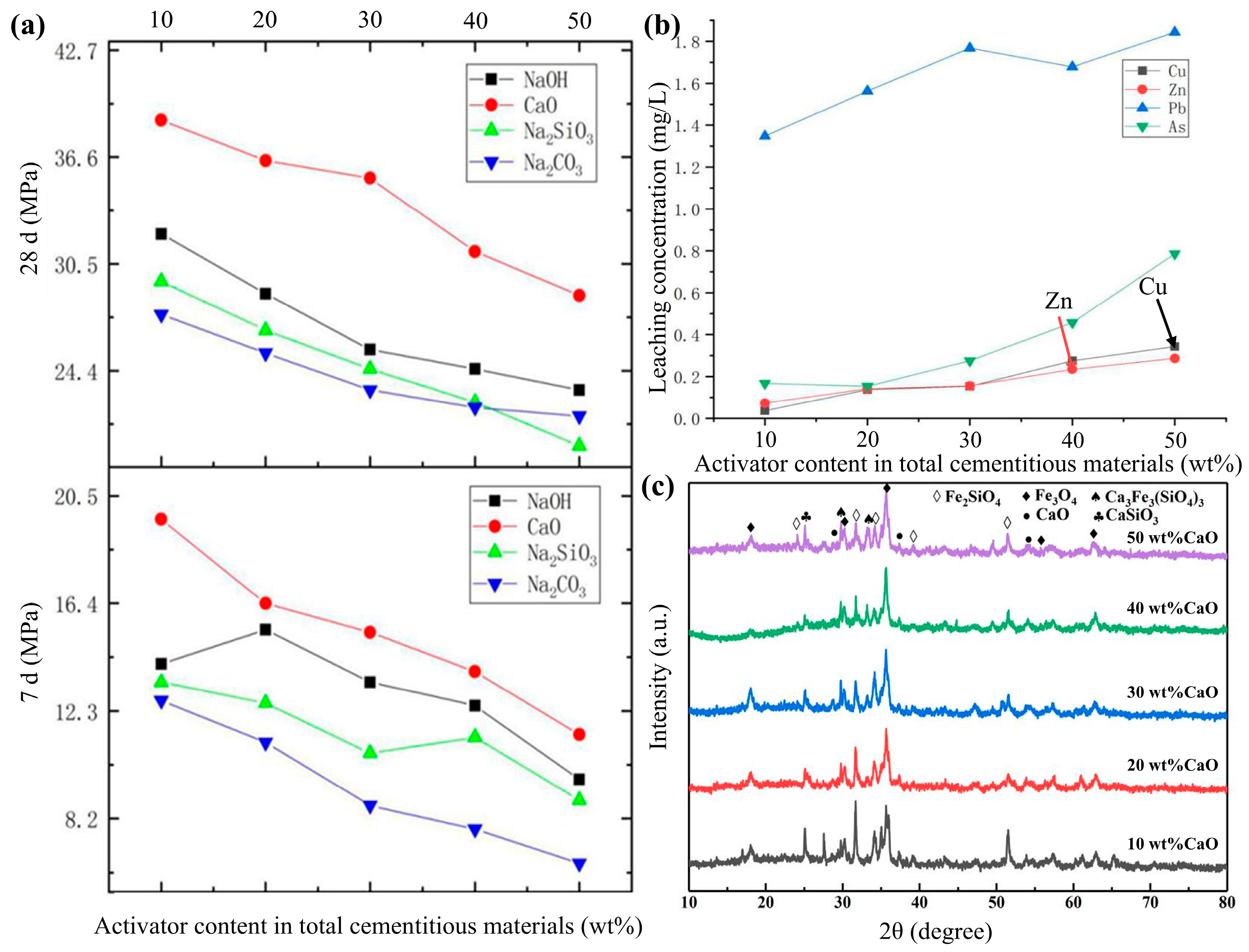
3.1.2. Investigation of the Effects of Different Activators and Their Dosages on the Performance of Cementitious Materials
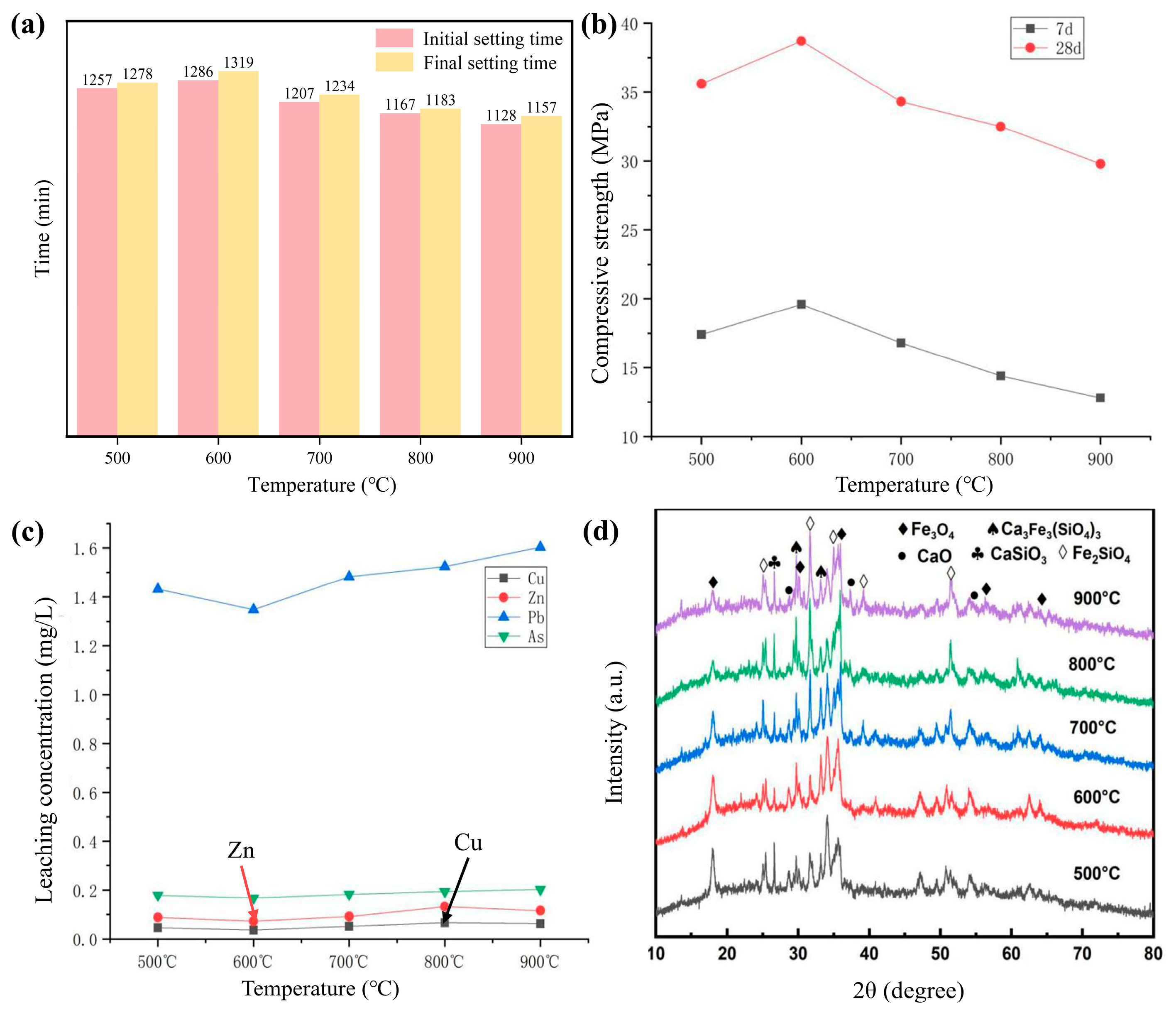
3.1.3. Effect of Calcination Temperature on the Performance of Cementitious Materials
3.2. Investigation of the Performance of Copper-Tailing-Based Light-Transmitting Concrete
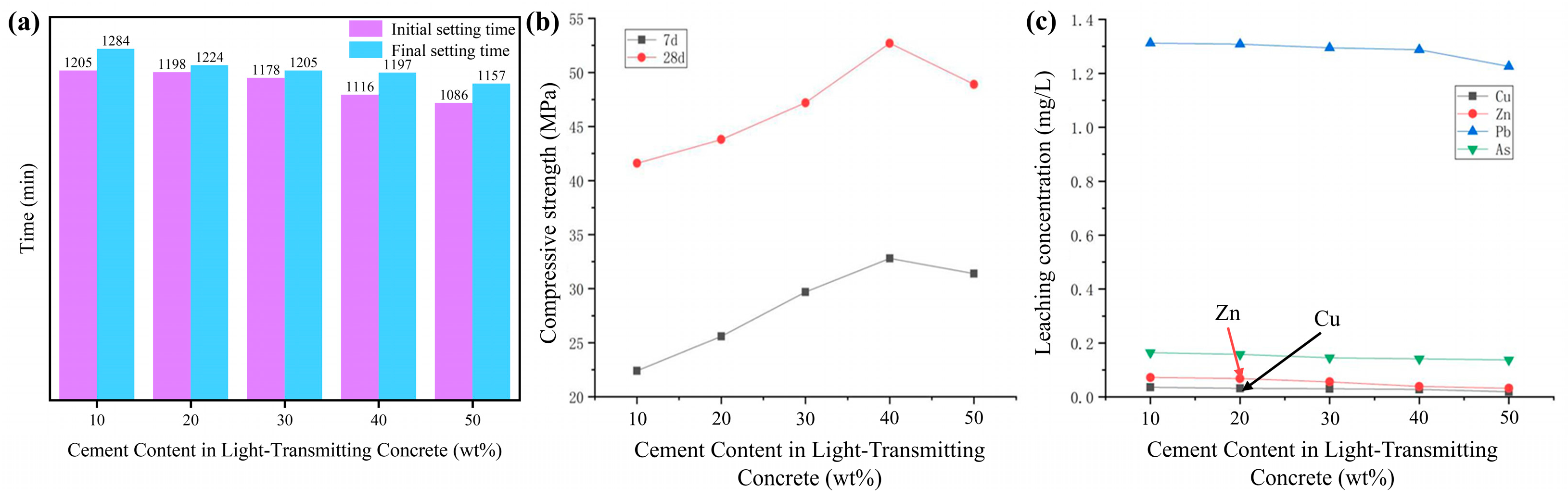
3.2.1. Effect of Different Cement Contents on the Setting Time of Light-Transmitting Concrete
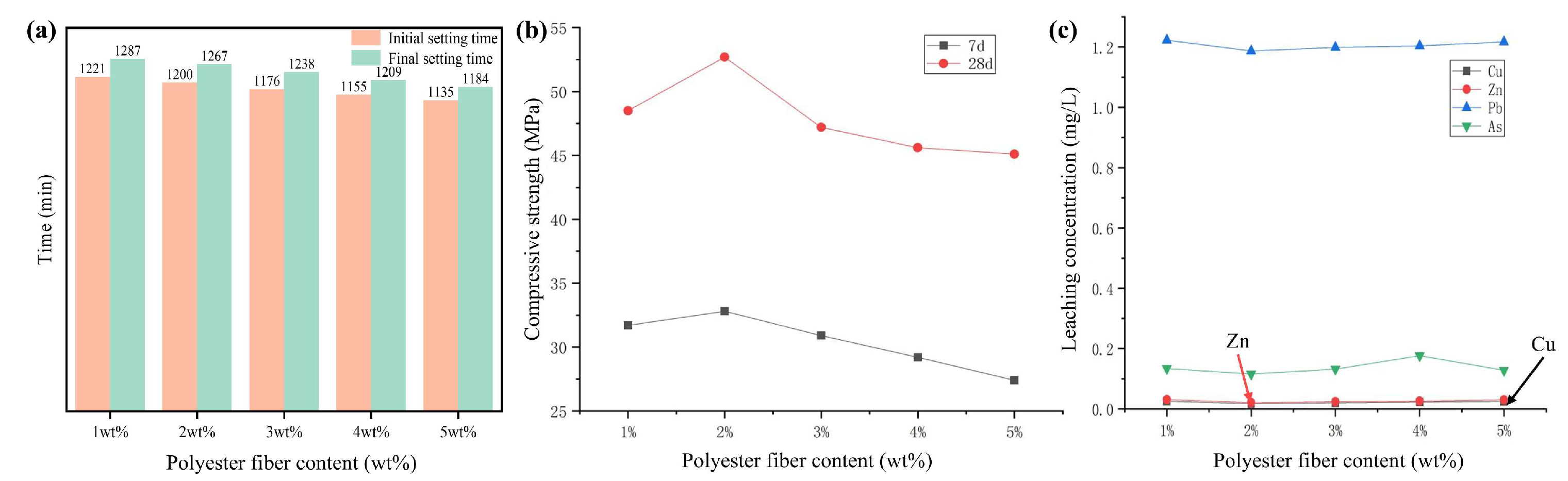
3.2.2. Effect of Light-Transmitting Specimen Mix Proportions on the Setting Time of Light-Transmitting Concrete
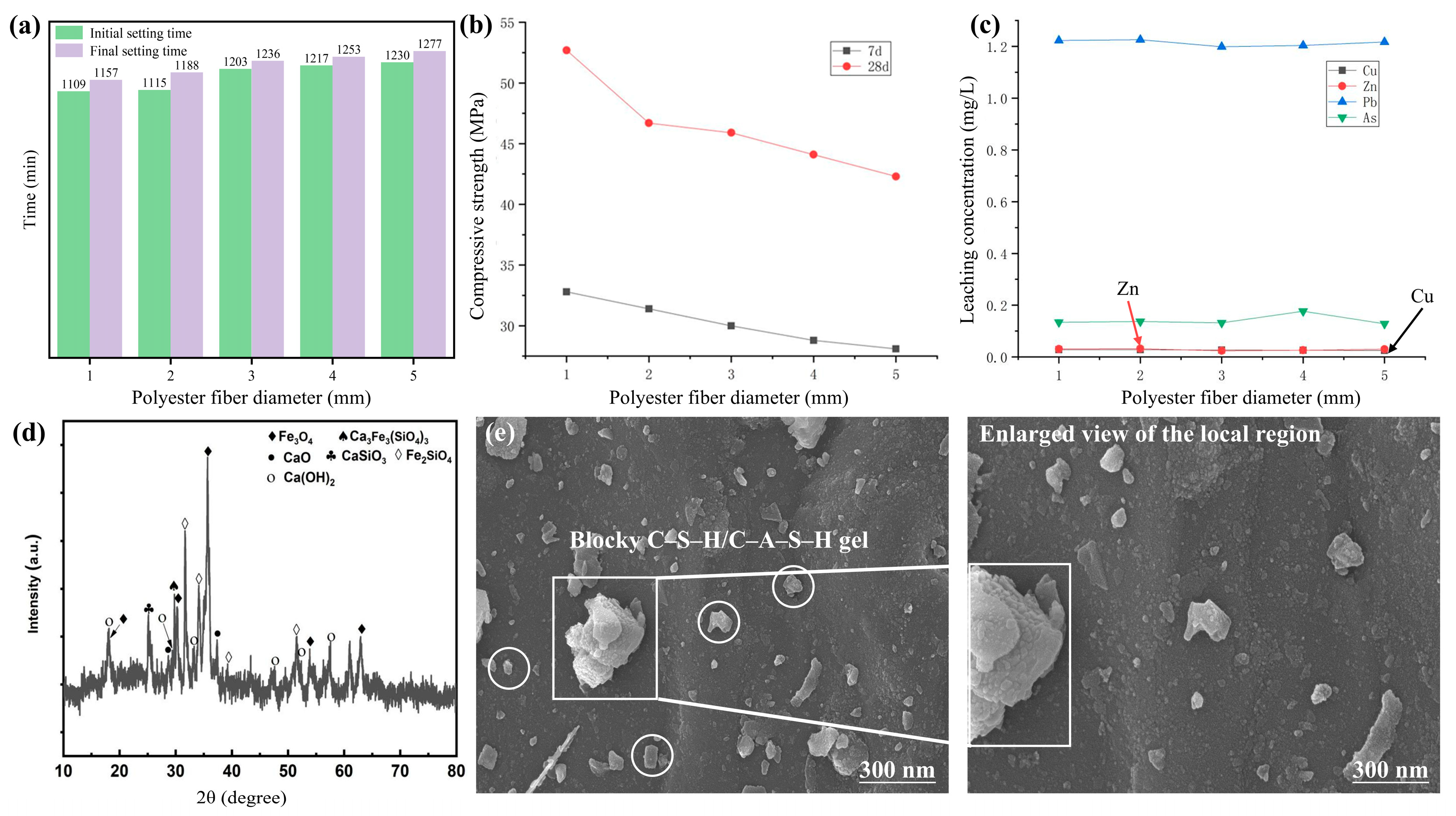
3.2.3. Effect of Light-Transmitting Material Diameter on the Performance of Light-Transmitting Concrete
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akintayo, B.D.; Babatunde, O.M.; Olanrewaju, O.A. Comparative analysis of cement production methods using a life cycle assessment and a multicriteria decision-making approach. Sustainability 2024, 16, 484. [Google Scholar] [CrossRef]
- Bărbulescu, A.; Hosen, K. Cement Industry Pollution and Its Impact on the Environment and Population Health: A Review. Toxics 2025, 13, 587. [Google Scholar] [CrossRef]
- Dunant, C.F.; Joseph, S.; Prajapati, R.; Allwood, J.M. Electric recycling of Portland cement at scale. Nature 2024, 629, 1055–1061. [Google Scholar] [CrossRef]
- Das, A.P.; van Hullebusch, E.D.; Akçil, A. Sustainable Management of Mining Waste and Tailings: A Circular Economy Approach; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Caratti, A.; Trapani, F.; Fina, A.; Bicchi, C.; Liberto, E.; Stephen, E.R.; Tao, Q.; Geschwender, D.; Cordero, C. Boosting Comprehensive Two-Dimensional Gas Chromatography with Artificial Intelligence: Computer Vision Helps to See What We Smell. In Abstract Book; KU Leuven: Leuven, Belgium, 2024; pp. 7–8. [Google Scholar]
- Barrera-Rojas, J.; Muro-Medina, C.V.; Palacios-Hinestroza, H.; Flores-Payán, V.; Osuna-Laveaga, D.R.; Sulbarán-Rangel, B. Transforming Waste into Value: The Role of Physicochemical Treatments in Circular Water Management. Limnol. Rev. 2025, 25, 42. [Google Scholar] [CrossRef]
- Jwaida, Z.; Dulaimi, A.; Mashaan, N.; Othuman Mydin, M.A. Geopolymers: The green alternative to traditional materials for engineering applications. Infrastructures 2023, 8, 98. [Google Scholar] [CrossRef]
- Kumar, S.G.K.M.; Kinuthia, J.M.; Oti, J.; Adeleke, B.O. Geopolymer Chemistry and Composition: A Comprehensive Review of Synthesis, Reaction Mechanisms, and Material Properties—Oriented with Sustainable Construction. Materials 2025, 18, 3823. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, W.; Tian, J.; Wu, X.; Zheng, M. Development of Optical Fiber Light-Transmitting Concrete (LTC)—A Review. Buildings 2024, 15, 104. [Google Scholar] [CrossRef]
- Bharti, G.; Hurukadli, P.; Bharath, B.; Nagavi, J.C.; Sharma, P.K.; Shukla, B.K. Innovative Light-Emitting Concrete: Enhancing Energy Efficiency and Aesthetic Appeal in Sustainable Infrastructure. In Intelligent Infrastructure and Smart Materials: Sustainable Technologies for a Greener Future; Springer: Berlin/Heidelberg, Germany, 2025; pp. 55–74. [Google Scholar]
- Luhar, I.; Luhar, S.; Savva, P.; Theodosiou, A.; Petrou, M.F.; Nicolaides, D. Light transmitting concrete: A review. Buildings 2021, 11, 480. [Google Scholar] [CrossRef]
- Yunshan, L.; Chengli, X.; Peiming, Z.; Haocheng, Q.; Xudong, L.; Liming, L. Integrative research on the mechanisms of acupuncture mechanics and interdisciplinary innovation. Biomed. Eng. OnLine 2025, 24, 30. [Google Scholar] [CrossRef] [PubMed]
- Knabikaite, I.; Eisinas, A.; Baltakys, K. Al3+ influence on the formation of calcium silicates by using two step synthesis. In Proceedings of the NBCM 2020: International Conference on Nanostructured Bioceramic Materials, Vilnius, Lithuania, 1–3 December 2020; Conference Book. Vilnius University Press: Vilnius, Lithuania, 2020. [Google Scholar]
- Peys, A.; White, C.E.; Rahier, H.; Blanpain, B.; Pontikes, Y. Alkali-activation of CaO-FeOx-SiO2 slag: Formation mechanism from in-situ X-ray total scattering. Cem. Concr. Res. 2019, 122, 179–188. [Google Scholar] [CrossRef]
- Sogutoglu, L.-C.; Steiger, M.; Houben, J.; Biemans, D.; Fischer, H.R.; Donkers, P.; Huinink, H.; Adan, O.C. Understanding the hydration process of salts: The impact of a nucleation barrier. Cryst. Growth Des. 2019, 19, 2279–2288. [Google Scholar] [CrossRef]
- Xu, S.; Cao, D.; Liu, Y.; Wang, Y. Role of additives in crystal nucleation from solutions: A review. Cryst. Growth Des. 2021, 22, 2001–2022. [Google Scholar] [CrossRef]
- Marvila, M.T.; Azevedo, A.R.G.d.; Vieira, C.M.F. Reaction mechanisms of alkali-activated materials. Rev. IBRACON Estrut. Mater. 2021, 14, e14309. [Google Scholar] [CrossRef]
- Wan, X.; Ren, L.; Lv, T.; Wang, D.; Wang, B. Research on alkali-activated systems based on solid waste-derived activators: A review. Sustainability 2025, 17, 254. [Google Scholar] [CrossRef]
- Yu, X.; Shi, J.; He, Z.; Yalçınkaya, Ç.; Revilla-Cuesta, V.; Gencel, O. Review of the materials composition and performance evolution of green alkali-activated cementitious materials. Clean Technol. Environ. Policy 2023, 25, 1439–1459. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Wang, Z.; Liu, W.; Ma, Y.; Quan, Z.; Wang, X. Effects of particle packing structure on molten salts migration behavior and thermal properties in composite phase change materials. Sol. Energy 2025, 301, 113922. [Google Scholar] [CrossRef]
- Wang, B.; Pan, K.; Zhao, C.; Luo, X.; Sun, M. Particle size optimization for enhancing densification and mechanical properties of powder metallurgy titanium. Mater. Today Commun. 2025, 48, 113446. [Google Scholar] [CrossRef]
- Wei, L.; Zuo, W.; Pan, H.; Lyu, K.; Zhang, W.; She, W. Rational design of lightweight cementitious composites with reinforced mechanical property and thermal insulation: Particle packing, hot pressing method, and microstructural mechanisms. Compos. Part B Eng. 2021, 226, 109333. [Google Scholar] [CrossRef]
- Alsarhan, H.; Al-Fakih, A. Performance and sustainability of industrial by-products-based alkali-activated concrete: A review. Multiscale Multidiscip. Model. Exp. Des. 2025, 8, 215. [Google Scholar] [CrossRef]
- Liao, Z.; Xue, W.; Liao, L.; Hao, R.; Shen, L.; Cui, D. A Study on the Effect of Different Cementitious Materials on the Mechanical Properties and Microscopic Characteristics of Alkali-Activated Green Ultra-High Performance Concrete (GUHPC). Materials 2025, 18, 2163. [Google Scholar] [CrossRef]
- Chousidis, N. Mechanical Properties and Performance of CNT–Reinforced Mortars (CEM II/B–L and CEM I) for Crack Bridging and Protective Coating Applications. Buildings 2025, 15, 2296. [Google Scholar] [CrossRef]
- Sathyan, D. Unveiling the combined influence of higher molecular weight polyethylene glycol and superplasticizer chemistry on fresh, mechanical, and microstructural performance of internally cured mortar. Mater. Res. Express 2024, 11, 075502. [Google Scholar] [CrossRef]
- Fei, X.-P.; Guo, L.-P.; Du, H.-J.; Li, J.-Y.; Shen, H.-R.; Chen, H.-T. Performance evolution of high ductility cementitious composites under different curing regimes: Hydration kinetics, microstructure development, and mechanical behavior. Constr. Build. Mater. 2025, 492, 142728. [Google Scholar] [CrossRef]
- GB/T 5086.3-2007; Standard for Pollution Control on Hazardous Waste Storage. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2007.
- Genua, F.; Lancellotti, I.; Leonelli, C. Geopolymer-based stabilization of heavy metals, the role of chemical agents in encapsulation and adsorption. Polymers 2025, 17, 670. [Google Scholar] [CrossRef]
- Liu, J.; Wu, D.; Tan, X.; Yu, P.; Xu, L. Review of the interactions between conventional cementitious materials and heavy metal ions in stabilization/solidification processing. Materials 2023, 16, 3444. [Google Scholar] [CrossRef]
- Burgos-Cara, A.; Putnis, C.V.; Rodriguez-Navarro, C.; Ruiz-Agudo, E. Hydration effects on the stability of calcium carbonate pre-nucleation species. Minerals 2017, 7, 126. [Google Scholar] [CrossRef]
- John, E.; Matschei, T.; Stephan, D. Nucleation seeding with calcium silicate hydrate—A review. Cem. Concr. Res. 2018, 113, 74–85. [Google Scholar] [CrossRef]
- Hendry, G.A.; Houghton, J.D.; Brown, S.B. Tansley Review No. 11. The degradation of chlorophyll-A biological enigma. New Phytol. 1987, 107, 255–302. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, K.; Xu, Z. High-Temperature Mechanical and Microstructural Properties of Well Cement Modified with Ethylene-Vinyl Acetate Polymer and Polypropylene Fibers for Geothermal Well Applications. Polymers 2025, 17, 1587. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Ye, G. The effect of activating solution on the mechanical strength, reaction rate, mineralogy, and microstructure of alkali-activated fly ash. J. Mater. Sci. 2012, 47, 4568–4578. [Google Scholar] [CrossRef]
- Qian, X.; Qin, Y.; Liu, Y.; Hu, C.; Wang, F.; Hu, S. New insight into energy-saving calcination of limestone: Preparation, characterization, and application of partially calcined limestone. ACS Sustain. Chem. Eng. 2023, 11, 16227–16239. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, T.; Cheng, Z.; Liu, L.; Li, C.; Luo, M.; Li, H.; Lv, R.; Cheng, Z. Synergistic Effects of Composite Modifiers on Mechanical Properties and Freeze-Thaw Durability of Multi-Solid Waste Cement Mortar. J. Build. Eng. 2025, 111, 113299. [Google Scholar] [CrossRef]
- Mandal, A.; Rajput, S.P. Advances in alkali-activation of ceramic waste-based pozzolana in concrete and mortar: A comprehensive review. Waste Biomass Valorization 2025, 16, 3309–3330. [Google Scholar] [CrossRef]
- Li, Z.; Jing, Y.; Zhu, R.; Yu, Q.; Qiu, X. Sustainable soil rehabilitation with multiple network structures of layered double hydroxide beads: Immobilization of heavy metals, fertilizer release, and water retention. J. Hazard. Mater. 2024, 478, 135385. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.; Marques, A.P.; Guerreiro, A.; Magan, N.; Castro, P.M. Removal of heavy metals using different polymer matrixes as support for bacterial immobilisation. J. Hazard. Mater. 2011, 191, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Huang, B.; Saafi, M.; Ye, J.; Lambert, C. Carrot-based covalently bonded saccharides as a new 2D material for healing defective calcium-silicate-hydrate in cement: Integrating atomistic computational simulation with experimental studies. Compos. Part B Eng. 2020, 199, 108235. [Google Scholar] [CrossRef]
- Huang, S.; Zujovic, Z.; Huang, Z.; Gao, W.; Cao, P. Crystallization of a high-strength lithium disilicate glass-ceramic: An XRD and solid-state NMR investigation. J. Non-Cryst. Solids 2017, 457, 65–72. [Google Scholar] [CrossRef]
- Rößler, C.; Steiniger, F.; Ludwig, H.M. Characterization of C–S–H and C–A–S–H phases by electron microscopy imaging, diffraction, and energy dispersive X-ray spectroscopy. J. Am. Ceram. Soc. 2017, 100, 1733–1742. [Google Scholar]
- Castillo, H.; Collado, H.; Droguett, T.; Vesely, M.; Garrido, P.; Palma, S. State of the art of geopolymers: A review. e-Polymers 2022, 22, 108–124. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, P.; Shao, L.; Chen, C.; Liu, X.; Ma, Y.; Zhang, L.; Geng, G. Quantifying the immobilization mechanisms of heavy metals by Calcium Silicate Hydrate (CSH): The case of Cu2+. Cem. Concr. Res. 2024, 186, 107695. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N. Mechanisms of heavy metal immobilisation using geopolymerisation techniques—A review. J. Adv. Concr. Technol. 2018, 16, 124–135. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, K.; Zhang, L.; Li, A.; Chen, C.; Huang, J.; Zhang, Y. Mechanical property and heavy metal leaching behavior enhancement of municipal solid waste incineration fly ash during the pressure-assisted sintering treatment. J. Environ. Manag. 2022, 301, 113856. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, W.; Zhang, S.; Li, J.; Suraneni, P. Optimal mixture designs for heavy metal encapsulation in municipal solid waste incineration fly ash. Appl. Sci. 2020, 10, 6948. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, M.; Wang, F.; Jacques, D.; Shen, X.; Zhang, J.; Gao, C.; Zeng, H.; Liu, J.; Liu, J. Heating-induced transformations in calcium silicate hydrate (CSH): In-situ investigations of composition, structure, and morphology. Cem. Concr. Res. 2025, 190, 107819. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Xu, L.; Zhu, Z.; Zhou, Y.; Pan, F.; Wu, K. Insight into the local CSH structure and its evolution mechanism controlled by curing regime and Ca/Si ratio. Constr. Build. Mater. 2022, 333, 127388. [Google Scholar] [CrossRef]
- Gou, J.; Li, S.; Jiang, C.; Li, Z.; You, G. A Study on the Influence of Gypsum and Ca (OH)2 on the Mechanical Properties and Hydration Behavior of Multi-Component Solid Waste-Based Cementitious Materials. Materials 2025, 18, 1964. [Google Scholar] [CrossRef]
- Zheng, Q.; Jiang, J.; Li, X.; Bustillo, K.C.; Zheng, H. In situ TEM observation of calcium silicate hydrate nanostructure at high temperatures. Cem. Concr. Res. 2021, 149, 106579. [Google Scholar] [CrossRef]
- Henderson, C.M.B. Composition, thermal expansion and phase transitions in framework silicates: Revisitation and review of natural and synthetic analogues of nepheline-, feldspar-and leucite-mineral groups. Solids 2021, 2, 1–49. [Google Scholar] [CrossRef]
- Kiss, E.E.; Panić, S.N. Accelerated physical and chemical transformations in ceramics processing. J. Serbian Chem. Soc. 2019, 84, 1055–1071. [Google Scholar] [CrossRef]
- Liu, Y.; Su, Y.; Xu, G.; Chen, Y.; You, G. Research progress on controlled low-strength materials: Metallurgical waste slag as cementitious materials. Materials 2022, 15, 727. [Google Scholar] [CrossRef]
- Peng, J.; Wu, Z.; Pan, Y.; Wang, J.; Wang, J.; Ren, S.; Xu, H. Enhancing the hydration properties of copper tailings cement through improving the interfacial transition zone by calcium-based activators. Miner. Eng. 2025, 232, 109542. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, A.; Deng, Y.; Cui, Y.; Yu, Z.; Yu, C. Strength performance of cement/slag-based stabilized soft clays. Constr. Build. Mater. 2019, 211, 909–918. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of heavy metals from industrial wastewater by chemical precipitation: Mechanisms and sludge characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Wang, B.; Ding, W.; Fan, C.; Liu, F.; Lu, W.; Yang, H. Solidification performance and mechanism of CSH gel for Pb (II), Zn (II), and Cd (II). J. Build. Eng. 2025, 99, 111464. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, L.; Liao, L.; Bai, Y.; Hu, J. Study on synthesis of CSH gel and its immobilization of heavy metals. Crystals 2024, 14, 864. [Google Scholar] [CrossRef]
- Amabilis-Sosa, L.E.; Valenzuela, E.I.; Quezada-Renteria, J.A.; Pat-Espadas, A.M. Biochar-assisted bioengineered strategies for metal removal: Mechanisms, key considerations, and perspectives for the treatment of solid and liquid matrixes. Sustainability 2022, 14, 17049. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Y.; Geng, Z.; Xu, X.; Yu, W.; Hubao, A.; Chen, J. Structure, fractality, mechanics and durability of calcium silicate hydrates. Fractal Fract. 2021, 5, 47. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Chang, J. Adsorption behavior and solidification mechanism of Pb (II) on synthetic CSH gel with low and high Ca/Si ratios in highly alkaline environments. J. Environ. Chem. Eng. 2024, 12, 113871. [Google Scholar] [CrossRef]
- Bažant, Z.P.; Rahimi-Aghdam, S. Century-long durability of concrete structures: Expansiveness of hydration and chemo-mechanics of autogenous shrinkage and swelling. In Computational Modelling of Concrete Structures; CRC Press: Boca Raton, FL, USA, 2018; pp. 15–23. [Google Scholar]
- Bentz, D.P.; Jensen, O.M. Mitigation strategies for autogenous shrinkage cracking. Cem. Concr. Compos. 2004, 26, 677–685. [Google Scholar] [CrossRef]
- Marsiske, M.R.; Debus, C.; Di Lorenzo, F.; Bernard, E.; Churakov, S.V.; Ruiz-Agudo, C. Immobilization of (aqueous) cations in low pH MSH cement. Appl. Sci. 2021, 11, 2968. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Zhang, Q.; Zhao, Y.; Feng, Y.; Yuan, H. Synergistic effect of OH-rich fibers and mineral capsules on the self-healing properties of cement mortar. Cem. Concr. Compos. 2023, 137, 104913. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, Z.; Zhang, J.; Zhu, Y.; Zhang, L.; Fan, Y.; Zhou, X.; Tang, S.; Lu, Y.; Li, W. Research on the aging mechanism of polypropylene nonwoven geotextiles under simulated heavy metal aging scenarios. Geotext. Geomembr. 2024, 52, 1240–1250. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Z.; Lu, S.; Shi, X. An amidoxime-functionalized polypropylene fiber: Competitive removal of Cu (II), Pb (II) and Zn (II) from wastewater and subsequent sequestration in cement mortar. J. Clean. Prod. 2020, 274, 123049. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Z.; Wang, M.; Lu, S.; Chi, L. Functionalized PP fiber to improve compressive strength and solidification/stabilization performance of cement with heavy metals. Constr. Build. Mater. 2021, 278, 122412. [Google Scholar] [CrossRef]
- Rohollah, R.; Almeida, F.C. Effect of superabsorbent polymers on microstructure and strength of blended cements mortars reinforced by polymeric fibre. Cement 2022, 9, 100041. [Google Scholar] [CrossRef]

| Fe2O3 | SiO2 | CaO | Na2O | MgO | MnO | ZnO | K2O | PbO | |
|---|---|---|---|---|---|---|---|---|---|
| Content | 43.14 | 36.36 | 3.41 | 2.26 | 1.79 | 1.70 | 1.36 | 1.09 | 0.57 |
| Activator Content in Cementitious Materials/wt% | NaOH | CaO | Na2SiO3 | Na2CO3 | ||||
|---|---|---|---|---|---|---|---|---|
| Initial Setting Time/min | Final Setting Time/min | Initial Setting Time/min | Final Setting Time/min | Initial Setting Time/min | Final Setting Time/min | Initial Setting Time/min | Final Setting Time/min | |
| 10 | 1287 | 1308 | 1412 | 1478 | 1324 | 1365 | 1223 | 1274 |
| 20 | 1256 | 1289 | 1389 | 1423 | 1294 | 1315 | 1197 | 1213 |
| 30 | 1207 | 1254 | 1307 | 1386 | 1245 | 1286 | 1167 | 1204 |
| 40 | 1176 | 1203 | 1276 | 1324 | 1189 | 1243 | 1134 | 1178 |
| 50 | 1121 | 1176 | 1235 | 1285 | 1145 | 1189 | 1113 | 1165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, G.; Li, S.; Li, Z.; He, Z.; Li, K.; Ning, P.; Wang, X.; Sun, X. Development and Performance Evaluation of Translucent Concrete Incorporating Activated Copper Tailings as Cementitious Material. Appl. Sci. 2025, 15, 10228. https://doi.org/10.3390/app151810228
An G, Li S, Li Z, He Z, Li K, Ning P, Wang X, Sun X. Development and Performance Evaluation of Translucent Concrete Incorporating Activated Copper Tailings as Cementitious Material. Applied Sciences. 2025; 15(18):10228. https://doi.org/10.3390/app151810228
Chicago/Turabian StyleAn, Guangdong, Siyang Li, Zhaorui Li, Zhaohui He, Kai Li, Ping Ning, Xiangyu Wang, and Xin Sun. 2025. "Development and Performance Evaluation of Translucent Concrete Incorporating Activated Copper Tailings as Cementitious Material" Applied Sciences 15, no. 18: 10228. https://doi.org/10.3390/app151810228
APA StyleAn, G., Li, S., Li, Z., He, Z., Li, K., Ning, P., Wang, X., & Sun, X. (2025). Development and Performance Evaluation of Translucent Concrete Incorporating Activated Copper Tailings as Cementitious Material. Applied Sciences, 15(18), 10228. https://doi.org/10.3390/app151810228







