Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases
Abstract
1. Introduction
2. Methods
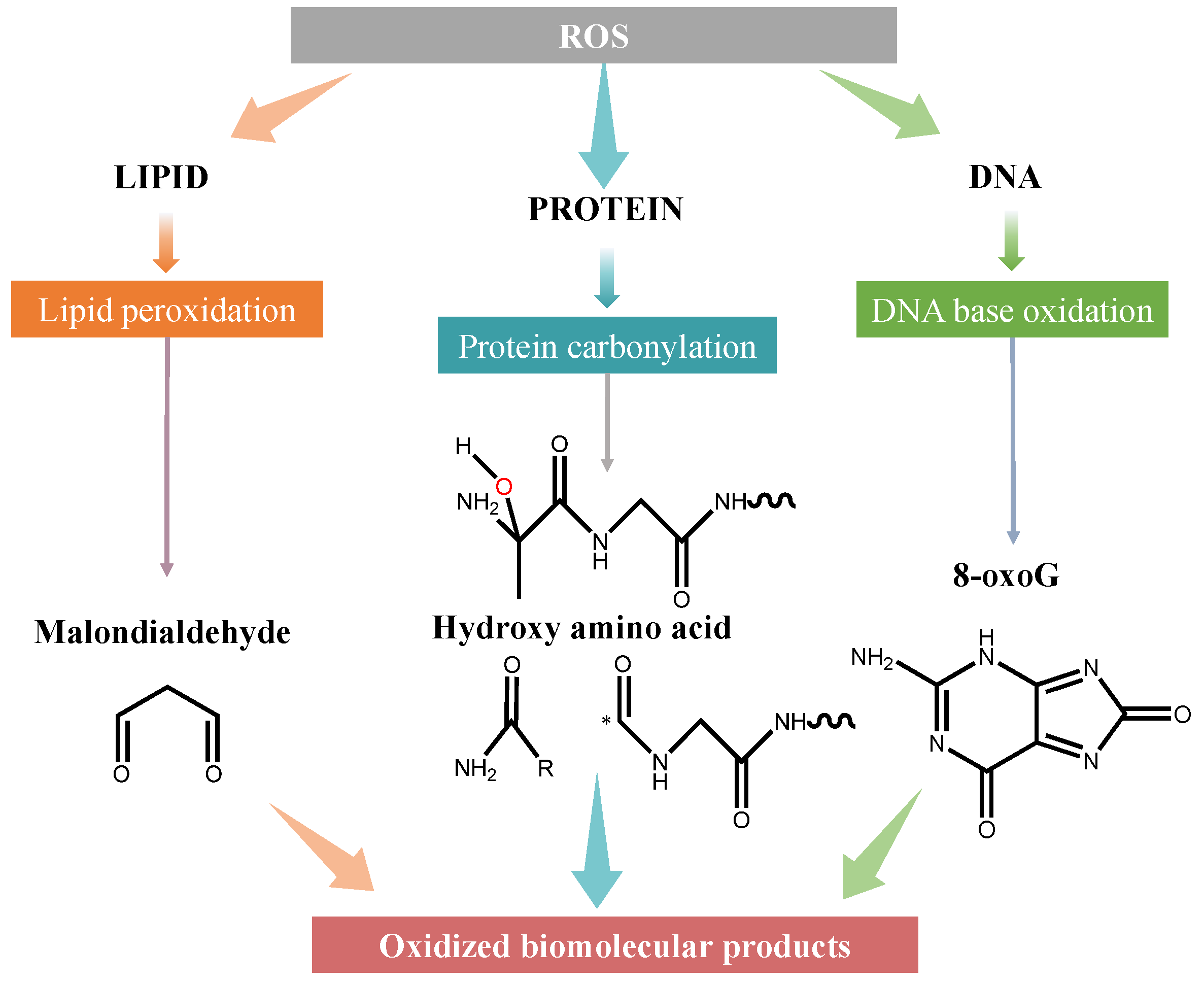
3. Mechanisms of Biomolecular Damage by ROS
3.1. Lipid Peroxidation and Cholesterol Damage
3.2. Protein Carbonylation
3.3. DNA Damage
4. Defense System Against Oxidative Stress
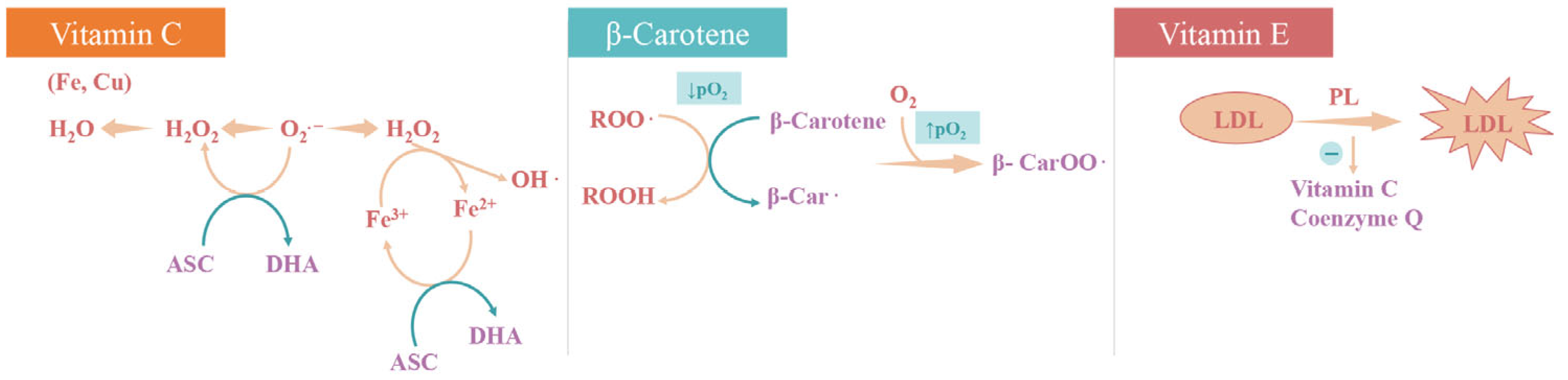
4.1. Enzymatic and Non-Enzymatic Antioxidant System
4.2. Nutritional Antioxidants
5. Oxidative Stress Repair System
6. Detection of Oxidative Stress and Antioxidant Biomarkers
6.1. Assessment of Lipid Peroxidation, Protein Oxidation, and DNA Damage
6.2. Antioxidant Activity
7. Oxidative Stress in Disease Pathogenesis
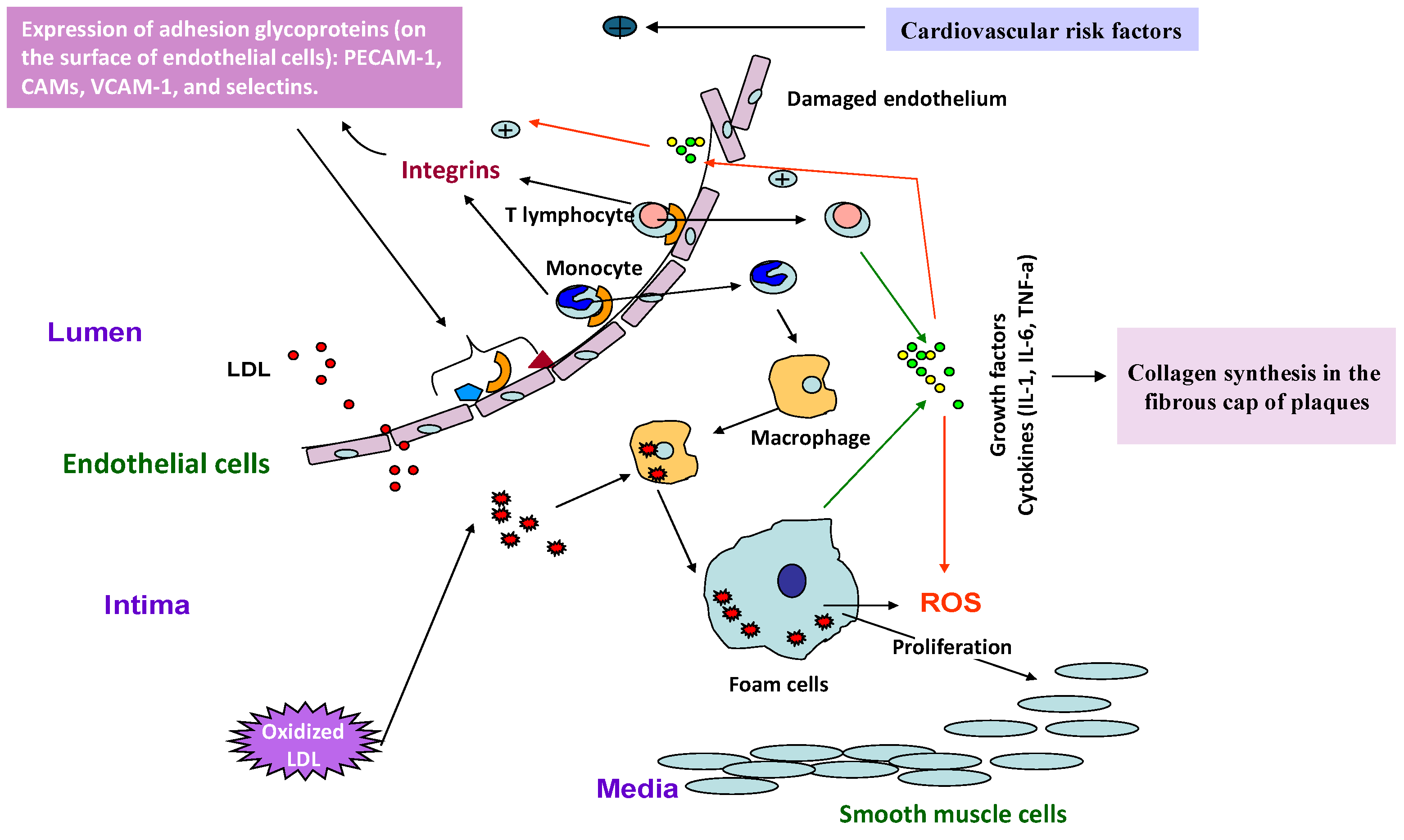
7.1. Cardiovascular Diseases
7.2. Inflammatory and Autoimmune Diseases
| Immune Molecule | Oxidative Stress Interaction | Associated Diseases |
|---|---|---|
| NF-κB 1 | Activated by ROS. Promotes pro-inflammatory cytokine production (e.g., TNF-α and IL-6). | Rheumatoid arthritis, IBD 5, and lupus |
| Nrf2 2 | Suppressed by chronic ROS. Impaired Nrf2 function increases oxidative stress and inflammation. | Multiple sclerosis, lupus, and type 1 diabetes |
| TNF-α 3 | Induces ROS generation via NADPH oxidase. Sustains inflammation and tissue damage. | Rheumatoid arthritis and psoriasis |
| T cells (Th17) | ROS modulate differentiation. Skewed Th17/Treg balance promotes autoimmunity and chronic inflammation. | Multiple sclerosis, psoriasis, and type 1 diabetes |
| Macrophages (M1/M2) | Excess ROS generated by M1 macrophages sustain inflammatory environment. | Atherosclerosis and IBD |
| B cells | ROS affect B-cell receptor signaling and survival. ROS-mediated dysregulation promotes autoantibody production. | Lupus erythematous and autoimmune thyroiditis |
| Dendritic cells (DCs) | ROS influence antigen processing and presentation. Enhanced ROS in DCs can break tolerance and activate autoreactive T cells. | Type 1 diabetes and lupus |
| Tregs 4 | High ROS impairs Treg suppressive function. Reduced immunosuppression allows unchecked autoimmune responses. | Type 1 diabetes and multiple sclerosis |
7.3. Neurodegenerative Diseases
8. Targeting Redox Imbalance in Precision Therapy
8.1. Challenges in Translating Antioxidant Research into Clinical Practice
8.2. Therapeutic Applications of Vitamins and Supplements: Evidence from Recent Clinical Studies
8.3. Targeting Redox Imbalance in Precision Therapy
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 8-oxoG | 8-Oxoguanine |
| ABTS | 2,2′-Azino-Bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) |
| AGEs | Advanced Glycation End Products |
| ALS | Amyotrophic Lateral Sclerosis |
| APOP | Advanced Protein Oxidation Products |
| ATM | Ataxia-Telangiectasia Mutated |
| ATO | Arsenic Trioxide |
| ATR | ATM and Rad3-Related |
| CAT | Catalase |
| CSCs | Cancer Stem Cells |
| DPPH | 2,2-Diphenyl-1-Picrylhydrazyl |
| EGCG | Epigallocatechin Gallate |
| FRAP | Ferric-Reducing Antioxidant Power |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GPx | Glutathione Peroxidase |
| GSH | Glutathione (Reduced Form) |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| NCDs | Non-Communicable Diseases |
| SLE | Systemic Lupus Erythematosus |
| SOD | Superoxide Dismutase |
References
- Davies, K.J. Oxidative Stress: The Paradox of Aerobic Life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, R.A. (Ed.) Handbook of Methods for Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-351-08137-5. [Google Scholar]
- Pryor, W.A. Organic Free Radicals. Chem. Eng. News Arch. 1968, 46, 70. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A. Reactive Oxygen Species and Protein Oxidation in Aging: A Look Back, a Look Ahead. Arch. Biochem. Biophys. 2002, 397, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Hollunger, G. The Interaction of Energy and Electron Transfer Reactions in Mitochondria. I. General Properties and Nature of the Products of Succinate-Linked Reduction of Pyridine Nucleotide. J. Biol. Chem. 1961, 236, 1534–1543. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding Mechanisms of Antioxidant Action in Health and Disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Therond, P. Dommages créés aux biomolécules (lipides, protéines, ADN) par le stress oxydant. Ann. Pharm. Fr. 2006, 64, 383–389. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Choi, S.-H.; Sviridov, D.; Miller, Y.I. Oxidized Cholesteryl Esters and Inflammation. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2017, 1862, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H. Front line of oxidized lipoproteins: Role of oxidized lipoproteins in atherogenesis and cardiovascular disease risk. Rinsho Byori 2010, 58, 622–630. [Google Scholar] [PubMed]
- Arsenault, B.J.; Bourgeois, R.; Mathieu, P. Do Oxidized Lipoproteins Cause Atherosclerotic Cardiovascular Diseases? Can. J. Cardiol. 2017, 33, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. GC–MS and GC–MS/MS Measurement of Malondialdehyde (MDA) in Clinical Studies: Pre-Analytical and Clinical Considerations. J. Mass Spectrom. Adv. Clin. Lab 2023, 30, 10–24. [Google Scholar] [CrossRef]
- Fanti, F.; Sergi, M.; Compagnone, D. LC-MS/MS Based Analytical Strategies for the Detection of Lipid Peroxidation Products in Biological Matrices. J. Pharm. Biomed. Anal. 2025, 256, 116681. [Google Scholar] [CrossRef]
- Wang, J.; Wu, N.; Yao, Y.; Chen, S.; Xu, L.; Zhao, Y.; Tu, Y. Protein Oxidation and Its Effect on Functional Properties of Livestock Products during the Processing and Storage: A Review. Food Chem. X 2025, 27, 102454. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein Carbonylation, Cellular Dysfunction, and Disease Progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Nyström, T. Role of Oxidative Carbonylation in Protein Quality Control and Senescence. EMBO J. 2005, 24, 1311–1317. [Google Scholar] [CrossRef]
- Frohnert, B.I.; Bernlohr, D.A. Protein Carbonylation, Mitochondrial Dysfunction, and Insulin Resistance. Adv. Nutr. 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Frohnert, B.I.; Sinaiko, A.R.; Serrot, F.J.; Foncea, R.E.; Moran, A.; Ikramuddin, S.; Choudry, U.; Bernlohr, D.A. Increased Adipose Protein Carbonylation in Human Obesity. Obesity 2011, 19, 1735–1741. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA Base Damage by Reactive Oxygen Species, Oxidizing Agents, and UV Radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA Damage Response in Cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Qi, Q.; Zhu, J.; Anderson, K.E.; Ma, X. Protoporphyrin IX-Induced Phototoxicity: Mechanisms and Therapeutics. Pharmacol. Ther. 2023, 248, 108487. [Google Scholar] [CrossRef] [PubMed]
- Willis, Z.I.; Boyd, A.S.; Cecilia Di Pentima, M. Phototoxicity, Pseudoporphyria, and Photo-Onycholysis Due to Voriconazole in a Pediatric Patient with Leukemia and Invasive Aspergillosis. J. Pediatr. Infect. Dis. Soc. 2015, 4, e22–e24. [Google Scholar] [CrossRef] [PubMed]
- Carmody, R.J.; Cotter, T.G. Signalling Apoptosis: A Radical Approach. Redox Rep. 2001, 6, 77–90. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Schmidt, H.H.H.W.; Stocker, R.; Vollbracht, C.; Paulsen, G.; Riley, D.; Daiber, A.; Cuadrado, A. Antioxidants in Translational Medicine. Antioxid. Redox Signal. 2015, 23, 1130–1143. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Yasui, M.; Kanemaru, Y.; Kamoshita, N.; Suzuki, T.; Arakawa, T.; Honma, M. Tracing the Fates of Site-Specifically Introduced DNA Adducts in the Human Genome. DNA Repair 2014, 15, 11–20. [Google Scholar] [CrossRef]
- Arat Çelik, H.E.; Yılmaz, S.; Akşahin, İ.C.; Kök Kendirlioğlu, B.; Çörekli, E.; Dal Bekar, N.E.; Çelik, Ö.F.; Yorguner, N.; Targıtay Öztürk, B.; İşlekel, H.; et al. Oxidatively-Induced DNA Base Damage and Base Excision Repair Abnormalities in Siblings of Individuals with Bipolar Disorder DNA Damage and Repair in Bipolar Disorder. Transl. Psychiatry 2024, 14, 207. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. The Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; De Araújo Brasil, A.; Monteiro Neto, J.R.; De Holanda Paranhos, L. SOD1, More than Just an Antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Glorieux, C.; Calderon, P.B. Catalase Down-Regulation in Cancer Cells Exposed to Arsenic Trioxide Is Involved in Their Increased Sensitivity to a pro-Oxidant Treatment. Cancer Cell Int. 2018, 18, 24. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; De Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione—Linking Cell Proliferation to Oxidative Stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Ghaedamini, H.; Duanghathaipornsuk, S.; Onusko, P.; Binsheheween, A.M.; Kim, D.-S. Reduced Glutathione-Modified Electrode for the Detection of Hydroxyl Free Radicals. Biosensors 2023, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and Fat-Soluble Antioxidants in Human Seminal Plasma and Serum of Fertile Males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.J.; Marko, D.M.; Fenech, R.K.; Yang, A.J.T.; MacPherson, R.E.K. Healthy Brain, Healthy Life: A Review of Diet and Exercise Interventions to Promote Brain Health and Reduce Alzheimer’s Disease Risk. Appl. Physiol. Nutr. Metab. 2020, 45, 1055–1065. [Google Scholar] [CrossRef]
- Xiong, Z.; Liu, L.; Jian, Z.; Ma, Y.; Li, H.; Jin, X.; Liao, B.; Wang, K. Vitamin E and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses. Nutrients 2023, 15, 3301. [Google Scholar] [CrossRef]
- Carlson, D.A.; True, C.; Wilson, C.G. Oxidative Stress and Food as Medicine. Front. Nutr. 2024, 11, 1394632. [Google Scholar] [CrossRef]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef]
- Yin, L.; Yan, H.; Chen, K.; Ji, Z.; Zhang, X.; Ji, G.; Zhang, B. Diet-Derived Circulating Antioxidants and Risk of Digestive System Tumors: A Mendelian Randomization Study. Nutrients 2022, 14, 3274. [Google Scholar] [CrossRef]
- Sim, M.; Hong, S.; Jung, S.; Kim, J.-S.; Goo, Y.-T.; Chun, W.Y.; Shin, D.-M. Vitamin C Supplementation Promotes Mental Vitality in Healthy Young Adults: Results from a Cross-Sectional Analysis and a Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Nutr. 2022, 61, 447–459. [Google Scholar] [CrossRef]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of L-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef]
- Nissanka, N.; Moraes, C.T. Mitochondrial DNA Damage and Reactive Oxygen Species in Neurodegenerative Disease. FEBS Lett. 2018, 592, 728–742. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Zhang, F.; Lin, J.; Yuan, H.; Nian, Q. Circulating Micronutrients Levels and Their Association with the Risk of Endometriosis. Front. Nutr. 2024, 11, 1466126. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Valls-Bellés, V.; Codoñer-Franch, P.; González, M.L.; Muñiz, P. Biodisponibilidad de Los Flavonoides de La Cerveza, Efecto Antioxidante “In Vivo”; Cerveza y salud; Centro de Información Cerveza y Salud: Madrid, Spain, 2005. [Google Scholar]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castillejo, S.; Pedret, A.; Catalán, Ú.; Valls, R.; Farràs, M.; Rubió, L.; Castañer, O.; Macià, A.; Fitó, M.; Motilva, M.J.; et al. Virgin Olive Oil Phenolic Compounds Modulate the HDL Lipidome in Hypercholesterolaemic Subjects: A Lipidomic Analysis of the VOHF Study. Mol. Nutr. Food Res. 2021, 65, 2001192. [Google Scholar] [CrossRef]
- Mena, P.; Bresciani, L.; Tassotti, M.; Rosi, A.; Martini, D.; Antonini, M.; Cas, A.D.; Bonadonna, R.; Brighenti, F.; Del Rio, D. Effect of Different Patterns of Consumption of Coffee and a Cocoa-Based Product Containing Coffee on the Nutrikinetics and Urinary Excretion of Phenolic Compounds. Am. J. Clin. Nutr. 2021, 114, 2107–2118. [Google Scholar] [CrossRef]
- Fernández-Cardero, Á.; Sierra-Cinos, J.L.; Bravo, L.; Sarriá, B. Consumption of a Coffee Rich in Phenolic Compounds May Improve the Body Composition of People with Overweight or Obesity: Preliminary Insights from a Randomized, Controlled and Blind Crossover Study. Nutrients 2024, 16, 2848. [Google Scholar] [CrossRef]
- Nguyen, C.; Coudeyre, E.; Boutron, I.; Baron, G.; Daste, C.; Lefèvre-Colau, M.-M.; Sellam, J.; Zauderer, J.; Berenbaum, F.; Rannou, F. Oral Resveratrol in Adults with Knee Osteoarthritis: A Randomized Placebo-Controlled Trial (ARTHROL). PLOS Med. 2024, 21, e1004440. [Google Scholar] [CrossRef]
- Conforti, A.; Iorio, G.G.; Di Girolamo, R.; Rovetto, M.Y.; Picarelli, S.; Cariati, F.; Gentile, R.; D’Amato, A.; Gliozheni, O.; Fioretti, B.; et al. The Impact of Resveratrol on the Outcome of the in Vitro Fertilization: An Exploratory Randomized Placebo-Controlled Trial. J. Ovarian Res. 2024, 17, 81. [Google Scholar] [CrossRef]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green Tea Catechins Prevent Low-Density Lipoprotein Oxidation via Their Accumulation in Low-Density Lipoprotein Particles in Humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Chiu, A.; Barone, M.K.; Avino, D.; Wang, F.; Coleman, C.I.; Phung, O.J. Green Tea Catechins Decrease Total and Low-Density Lipoprotein Cholesterol: A Systematic Review and Meta-Analysis. J. Am. Diet. Assoc. 2011, 111, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Méndez-del Villar, M.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Lizárraga-Valdez, R. Effect of Resveratrol Administration on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. Metab. Syndr. Relat. Disord. 2014, 12, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Calina, D.; Docea, A.; Koirala, N.; Aryal, S.; Lombardo, D.; Pasqua, L.; Taheri, Y.; Marina Salgado Castillo, C.; Martorell, M.; et al. Curcumin’s Nanomedicine Formulations for Therapeutic Application in Neurological Diseases. J. Clin. Med. 2020, 9, 430. [Google Scholar] [CrossRef]
- Liu, X.; Morris, M.C.; Dhana, K.; Ventrelle, J.; Johnson, K.; Bishop, L.; Hollings, C.S.; Boulin, A.; Laranjo, N.; Stubbs, B.J.; et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Study: Rationale, Design and Baseline Characteristics of a Randomized Control Trial of the MIND Diet on Cognitive Decline. Contemp. Clin. Trials 2021, 102, 106270. [Google Scholar] [CrossRef]
- Roberts, D.W.; Doerge, D.R.; Churchwell, M.I.; Gamboa Da Costa, G.; Marques, M.M.; Tolleson, W.H. Inhibition of Extrahepatic Human Cytochromes P450 1A1 and 1B1 by Metabolism of Isoflavones Found in Trifolium pratense (Red Clover). J. Agric. Food Chem. 2004, 52, 6623–6632. [Google Scholar] [CrossRef]
- Chan, T.S.; Galati, G.; Pannala, A.S.; Rice-Evans, C.; O’Brien, P.J. Simultaneous Detection of the Antioxidant and Pro-Oxidant Activity of Dietary Polyphenolics in a Peroxidase System. Free Radic. Res. 2003, 37, 787–794. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Rice-Evans, C. Cellular Uptake and Metabolism of Flavonoids and Their Metabolites: Implications for Their Bioactivity. Arch. Biochem. Biophys. 2004, 423, 148–161. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, J.; Wu, H.; Yu, P.; He, Z.; Tan, Y.; Wu, Y.; Song, X.; Chen, X.; Wang, Y.; et al. Serum Levels of Vitamin B12 Combined with Folate and Plasma Total Homocysteine Predict Ischemic Stroke Disease: A Retrospective Case-Control Study. Nutr. J. 2024, 23, 76. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-Lowering by B Vitamins Slows the Rate of Accelerated Brain Atrophy in Mild Cognitive Impairment: A Randomized Controlled Trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Jakubowski, H.; Zioła-Frankowska, A.; Frankowski, M.; Perła-Kaján, J.; Refsum, H.; de Jager, C.A.; Smith, A.D. B Vitamins Prevent Iron-Associated Brain Atrophy and Domain-Specific Effects of Iron, Copper, Aluminum, and Silicon on Cognition in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 84, 1039–1055. [Google Scholar] [CrossRef]
- Sartori, A.A.; Jiricny, J. Enzymology of Base Excision Repair in the Hyperthermophilic Archaeon Pyrobaculum aerophilum. J. Biol. Chem. 2003, 278, 24563–24576. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The Proteostasis Network and Its Decline in Ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Sienes Bailo, P.; Llorente Martín, E.; Calmarza, P.; Montolio Breva, S.; Bravo Gómez, A.; Pozo Giráldez, A.; Sánchez-Pascuala Callau, J.J.; Vaquer Santamaría, J.M.; Dayaldasani Khialani, A.; Cerdá Micó, C.; et al. Implicación Del Estrés Oxidativo En Las Enfermedades Neurodegenerativas y Posibles Terapias Antioxidantes. Adv. Lab. Med. 2022, 3, 351–360. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and Thioredoxin Reductase: Current Research with Special Reference to Human Disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.S.; Murphy, D.L.; Sweasy, J.B. Base Excision Repair and Cancer. Cancer Lett. 2012, 327, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of Antioxidant Activity with the Thiobarbituric Acid Reactive Substances Assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of Malondialdehyde (MDA) by High-Performance Liquid Chromatography in Serum and Liver as a Biomarker for Oxidative stressApplication to a Rat Model for Hypercholesterolemia and Evaluation of the Effect of Diets Rich in Phenolic Antioxidants from Fruits. J. Chromatogr. B 2005, 827, 76–82. [Google Scholar] [CrossRef]
- Holley, A.E.; Walker, M.K.; Cheeseman, K.H.; Slater, T.F. Measurement of N-Alkanals and Hydroxyalkenals in Biological Samples. Free Radic. Biol. Med. 1993, 15, 281–289. [Google Scholar] [CrossRef]
- Liu, W.; Morrow, J.D.; Yin, H. Quantification of F2-Isoprostanes as a Reliable Index of Oxidative Stress in Vivo Using Gas Chromatography–Mass Spectrometry (GC-MS) Method. Free Radic. Biol. Med. 2009, 47, 1101–1107. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid Peroxidation as Measured by Chromatographic Determination of Malondialdehyde. Human Plasma Reference Values in Health and Disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Boloor, K.K.; Ramasarma, T. Methods for Estimating Lipid Peroxidation: An Analysis of Merits and Demerits. Indian J. Biochem. Biophys. 2003, 40, 300–308. [Google Scholar]
- Nabih, G.A.; Sheshtawy, N.E.; Mikkawy, D.M.E.E.; Kamel, M.A. Serum Malondialdehyde as a Marker of Oxidative Stress in Rheumatoid Arthritis. Egypt. Rheumatol. Rehabil. 2024, 51, 43. [Google Scholar] [CrossRef]
- Niki, E. Biomarkers of Lipid Peroxidation in Clinical Material. Biochim. Biophys. Acta BBA—Gen. Subj. 2014, 1840, 809–817. [Google Scholar] [CrossRef]
- Rajesh, M.; Sulochana, K.N.; Coral, K.; Punitham, R.; Biswas, J.; Babu, K.; Ramakrishnan, S. Determination of Carbonyl Group Content in Plasma Proteins as a Useful Marker to Assess Impairment in Antioxidant Defense in Patients with Eales’ Disease. Indian J. Ophthalmol. 2004, 52, 139–144. [Google Scholar]
- Tiana, L.; Caib, Q.; Wei, H. Alterations of Antioxidant Enzymes and Oxidative Damage to Macromolecules in Different Organs of Rats During Aging. Free Radic. Biol. Med. 1998, 24, 1477–1484. [Google Scholar] [CrossRef]
- Colombo, G.; Reggiani, F.; Angelini, C.; Finazzi, S.; Astori, E.; Garavaglia, M.L.; Landoni, L.; Portinaro, N.M.; Giustarini, D.; Rossi, R.; et al. Plasma Protein Carbonyls as Biomarkers of Oxidative Stress in Chronic Kidney Disease, Dialysis, and Transplantation. Oxid. Med. Cell. Longev. 2020, 2020, 2975256. [Google Scholar] [CrossRef]
- Cadenas-Garrido, P.; Schonvandt-Alarcos, A.; Herrera-Quintana, L.; Vázquez-Lorente, H.; Santamaría-Quiles, A.; Ruiz De Francisco, J.; Moya-Escudero, M.; Martín-Oliva, D.; Martín-Guerrero, S.M.; Rodríguez-Santana, C.; et al. Using Redox Proteomics to Gain New Insights into Neurodegenerative Disease and Protein Modification. Antioxidants 2024, 13, 127. [Google Scholar] [CrossRef]
- Kisty, E.A.; Falco, J.A.; Weerapana, E. Redox Proteomics Combined with Proximity Labeling Enables Monitoring of Localized Cysteine Oxidation in Cells. Cell Chem. Biol. 2023, 30, 321–336.e6. [Google Scholar] [CrossRef]
- Mu, B.; Zeng, Y.; Luo, L.; Wang, K. Oxidative Stress-Mediated Protein Sulfenylation in Human Diseases: Past, Present, and Future. Redox Biol. 2024, 76, 103332. [Google Scholar] [CrossRef]
- Burger, N.; Chouchani, E.T. A New Era of Cysteine Proteomics—Technological Advances in Thiol Biology. Curr. Opin. Chem. Biol. 2024, 79, 102435. [Google Scholar] [CrossRef] [PubMed]
- Desai, H.; Andrews, K.H.; Bergersen, K.V.; Ofori, S.; Yu, F.; Shikwana, F.; Arbing, M.A.; Boatner, L.M.; Villanueva, M.; Ung, N.; et al. Chemoproteogenomic Stratification of the Missense Variant Cysteinome. Nat. Commun. 2024, 15, 9284. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, N.L.; Kennedy, D.A.; Gagné, R.; White, T.; Long, A.S.; Yauk, C.L.; White, P.A. HPLC Measurement of the DNA Oxidation Biomarker, 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine, in Cultured Cells and Animal Tissues. J. Vis. Exp. JoVE 2015, e52697. [Google Scholar] [CrossRef]
- Kumar, K.; Fornace, A.J.; Suman, S. 8-OxodG: A Potential Biomarker for Chronic Oxidative Stress Induced by High-LET Radiation. DNA 2024, 4, 221–238. [Google Scholar] [CrossRef]
- Sliwinska, A.; Kwiatkowski, D.; Czarny, P.; Toma, M.; Wigner, P.; Drzewoski, J.; Fabianowska-Majewska, K.; Szemraj, J.; Maes, M.; Galecki, P.; et al. The Levels of 7,8-Dihydrodeoxyguanosine (8-oxoG) and 8-Oxoguanine DNA Glycosylase 1 (OGG1)—A Potential Diagnostic Biomarkers of Alzheimer’s Disease. J. Neurol. Sci. 2016, 368, 155–159. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. 8-Oxoguanine and 8-Oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC-ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A Modification of the ABTS• Decolorization Method and an Insight into Its Mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J.; Shalliker, R.A. Ferric Reducing Antioxidant Potential (FRAP) of Antioxidants Using Reaction Flow Chromatography. Anal. Chim. Acta 2017, 967, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Dominguez-López, I.; Pérez, M.; Lamuela-Raventós, R.M. Total (Poly)Phenol Analysis by the Folin-Ciocalteu Assay as an Anti-Inflammatory Biomarker in Biological Samples. Crit. Rev. Food Sci. Nutr. 2024, 64, 10048–10054. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Xu, X.; Viana, M.A.; Chen, L.; Stacewicz-Sapuntzakis, M.; Duncan, C.; Bowen, P.E.; Sharifi, R. Liquid Chromatography–Mass Spectrometry of Cis- and All-Trans-Lycopene in Human Serum and Prostate Tissue after Dietary Supplementation with Tomato Sauce. J. Agric. Food Chem. 2002, 50, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Karppi, J.; Nurmi, T.; Olmedilla-Alonso, B.; Granado-Lorencio, F.; Nyyssönen, K. Simultaneous Measurement of Retinol, α-Tocopherol and Six Carotenoids in Human Plasma by Using an Isocratic Reversed-Phase HPLC Method. J. Chromatogr. B 2008, 867, 226–232. [Google Scholar] [CrossRef]
- Robitaille, L.; Hoffer, L.J. A Simple Method for Plasma Total Vitamin C Analysis Suitable for Routine Clinical Laboratory Use. Nutr. J. 2015, 15, 40. [Google Scholar] [CrossRef]
- Tang, P.H.; Miles, M.V.; Miles, L.; Quinlan, J.; Wong, B.; Wenisch, A.; Bove, K. Measurement of Reduced and Oxidized Coenzyme Q9 and Coenzyme Q10 Levels in Mouse Tissues by HPLC with Coulometric Detection. Clin. Chim. Acta 2004, 341, 173–184. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Kumar, R.; Diessl, J.; Hanna, D.A.; Banerjee, R. Rapid HPLC Method Reveals Dynamic Shifts in Coenzyme Q Redox State. J. Biol. Chem. 2024, 300, 107301. [Google Scholar] [CrossRef]
- Alkan, Ş.B.; Artaç, M.; Rakıcıoğlu, N. Dietary Antioxidant Capacity and Serum Inflammatory Biomarkers Levels in Cancer Survivors. Nutr. Cancer 2022, 74, 1243–1251. [Google Scholar] [CrossRef]
- Kim, J.H.; Baik, H.W.; Yoon, Y.S.; Joung, H.J.; Park, J.S.; Park, S.J.; Jang, E.J.; Park, S.W.; Kim, S.J.; Kim, M.J.; et al. Measurement of Antioxidant Capacity Using the Biological Antioxidant Potential Test and Its Role as a Predictive Marker of Metabolic Syndrome. Korean J. Intern. Med. 2014, 29, 31–39. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Galina Hidalgo, M.Á. Estrés oxidativo y antioxidantes. Av. Investig. Agropecu. 2018, 22, 29–46. [Google Scholar]
- Conti, E.; Musumeci, M.B.; De Giusti, M.; Dito, E.; Mastromarino, V.; Autore, C.; Volpe, M. IGF-1 and Atherothrombosis: Relevance to Pathophysiology and Therapy. Clin. Sci. 2011, 120, 377–402. [Google Scholar] [CrossRef][Green Version]
- Naik, E.; Dixit, V.M. Mitochondrial Reactive Oxygen Species Drive Proinflammatory Cytokine Production. J. Exp. Med. 2011, 208, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M. Molecular Biology’s Impact on Our Understanding of Aging. BMJ 1997, 315, 1078–1081. [Google Scholar] [CrossRef]
- Valls, V.; Peiro, C.; Muñiz, P.; Saez, G.T. Age-Related Changes in Antioxidant Status and Oxidative Damage to Lipids and Dna in Mitochondria of Rat Liver. Process Biochem. 2005, 40, 903–908. [Google Scholar] [CrossRef]
- Ma, L.; Dou, H.-L.; Wu, Y.-Q.; Huang, Y.-M.; Huang, Y.-B.; Xu, X.-R.; Zou, Z.-Y.; Lin, X.-M. Lutein and Zeaxanthin Intake and the Risk of Age-Related Macular Degeneration: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casariego, A.; Rodríguez Lavandeira, U.; Pita Gutiérrez, F.; Lugo Rodríguez, G.; Torres Carreta, J.P. Influence of Individualized Dietary Adaptation in Patients Undergoing Hematopoietic Stem Cell Transplantation. The ADITRAS Study. Nutr. Hosp. 2024, 41, 433–438. [Google Scholar] [CrossRef]
- Dakterzada, F.; Jové, M.; Cantero, J.L.; Pamplona, R.; Piñoll-Ripoll, G. Plasma and Cerebrospinal Fluid Nonenzymatic Protein Damage Is Sustained in Alzheimer’s Disease. Redox Biol. 2023, 64, 102772. [Google Scholar] [CrossRef] [PubMed]
- Cores, Á.; Carmona-Zafra, N.; Martín-Cámara, O.; Sánchez, J.D.; Duarte, P.; Villacampa, M.; Bermejo-Bescós, P.; Martín-Aragón, S.; León, R.; Menéndez, J.C. Curcumin-Piperlongumine Hybrids with a Multitarget Profile Elicit Neuroprotection in In Vitro Models of Oxidative Stress and Hyperphosphorylation. Antioxidants 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative Cell Death in Cancer: Mechanisms and Therapeutic Opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Vetrani, C.; Costabile, G.; Di Marino, L.; Rivellese, A.A. Nutrition and Oxidative Stress: A Systematic Review of Human Studies. Int. J. Food Sci. Nutr. 2013, 64, 312–326. [Google Scholar] [CrossRef]
- Ilic, D.; Forbes, K.M.; Hassed, C. Lycopene for the Prevention of Prostate Cancer. Cochrane Database Syst. Rev. 2011, 2011, CD008007. [Google Scholar] [CrossRef]
- Fraser, G.E.; Jacobsen, B.K.; Knutsen, S.F.; Mashchak, A.; Lloren, J.I. Tomato Consumption and Intake of Lycopene as Predictors of the Incidence of Prostate Cancer: The Adventist Health Study-2. Cancer Causes Control 2020, 31, 341–351. [Google Scholar] [CrossRef]
- Kapała, A.; Szlendak, M.; Motacka, E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef]
- Valls-Bellés, V.; Abad, C.; Hernández-Aguilar, M.T.; Nacher, A.; Guerrero, C.; Baliño, P.; Romero, F.J.; Muriach, M. Human Milk Antioxidative Modifications in Mastitis: Further Beneficial Effects of Cranberry Supplementation. Antioxidants 2021, 11, 51. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Hernández-Aguilar, M.T.; Navarro-Ruiz, A.; López-Jaén, A.B.; Borja-Herrero, C.; Valls-Bellés, V. Diet Supplementation During Early Lactation with Non-Alcoholic Beer Increases the Antioxidant Properties of Breastmilk and Decreases the Oxidative Damage in Breastfeeding Mothers. Breastfeed. Med. 2013, 8, 164–169. [Google Scholar] [CrossRef]
- Castedo, E.; Segovia, J.; Escudero, C.; Olmedilla, B.; Granado, F.; Blas, C.; Guardiola, J.M.; Millán, I.; Pulpón, L.A.; Ugartea, J. Ischemia-reperfusion injury during experimental heart transplantation. Evaluation of trimetazidine’s cytoprotective effect. Rev. Esp. Cardiol. 2005, 58, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Muñiz, P.; Gasco, E.; Domingo, J.V.; Valls-Belles, V. Effect of a Diet Supplemented with Alpha-Tocopherol and Beta-Carotene on ATP and Antioxidant Levels after Hepatic Ischemia-Reperfusion. J. Clin. Biochem. Nutr. 2008, 43, 13–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.; Cirillo, P.; Paglia, A.; Sasso, L.; Palma, V.; Chiariello, M. Reactive Oxygen Species and Antioxidants in the Pathophysiology of Cardiovascular Disease: Does the Actual Knowledge Justify a Clinical Approach? Curr. Vasc. Pharmacol. 2010, 8, 259–275. [Google Scholar] [CrossRef]
- Ou, H.; Huang, Z.; Mo, Z.; Xiao, J. The Characteristics and Roles of Advanced Oxidation Protein Products in Atherosclerosis. Cardiovasc. Toxicol. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Sugamura, K.; Keaney, J.F. Reactive Oxygen Species in Cardiovascular Disease. Free Radic. Biol. Med. 2011, 51, 978–992. [Google Scholar] [CrossRef]
- Tsimikas, S.; Miller, Y.I. Oxidative Modification of Lipoproteins: Mechanisms, Role in Inflammation and Potential Clinical Applications in Cardiovascular Disease. Curr. Pharm. Des. 2011, 17, 27–37. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; López-Jaén, A.B.; Muñiz, P.; Sentandreu, E.; Bellés, V.V. Mandarin Juice Improves the Antioxidant Status of Hypercholesterolemic Children. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 349–355. [Google Scholar] [CrossRef]
- Steinberg, D. The LDL Modification Hypothesis of Atherogenesis: An Update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and Redox Signaling in Myocardial Ischemia-Reperfusion Injury and Cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Codoñer-Franch, P.; Bataller Alberola, A.; Domingo Camarasa, J.V.; Escribano Moya, M.C.; Valls Bellés, V. Influence of Dietary Lipids on the Erythrocyte Antioxidant Status of Hypercholesterolaemic Children. Eur. J. Pediatr. 2009, 168, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Martínez Alvarez, J.R.; Bellés, V.V.; López-Jaén, A.B.; Marín, A.V.; Codoñer-Franch, P. Effects of Alcohol-Free Beer on Lipid Profile and Parameters of Oxidative Stress and Inflammation in Elderly Women. Nutrition 2009, 25, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Navarro-Ruiz, A.; Fernández-Ferri, M.; Arilla-Codoñer, Á.; Ballester-Asensio, E.; Valls-Bellés, V. A Matter of Fat: Insulin Resistance and Oxidative Stress: Fat, HOMA-IR, and Oxidative Stress. Pediatr. Diabetes 2012, 13, 392–399. [Google Scholar] [CrossRef]
- Rumm-Kreuter, D. Comparison of the Eating and Cooking Habits of Northern Europe and the Mediterranean Countries in the Past, Present and Future. Int. J. Vitam. Nutr. Res. 2001, 71, 141–148. [Google Scholar] [CrossRef]
- Xu, Q.; Qian, X.; Sun, F.; Liu, H.; Dou, Z.; Zhang, J. Independent and Joint Associations of Dietary Antioxidant Intake with Risk of Post-Stroke Depression and All-Cause Mortality. J. Affect. Disord. 2023, 322, 84–90. [Google Scholar] [CrossRef]
- Khosravi, A.; Bahonar, A.; Saadatnia, M.; Khorvash, F.; Maracy, M. Carotenoids as Potential Antioxidant Agents in Stroke Prevention: A Systematic Review. Int. J. Prev. Med. 2017, 8, 70. [Google Scholar] [CrossRef]
- Cui, C.; Jiang, M.; Jain, N.; Das, S.; Lo, Y.-H.; Kermani, A.A.; Pipatpolkai, T.; Sun, J. Structural Basis of Human NOX5 Activation. Nat. Commun. 2024, 15, 3994. [Google Scholar] [CrossRef]
- Zhao, Y.; Linkermann, A.; Takahashi, M.; Li, Q.; Zhou, X. Ferroptosis in Cardiovascular Disease: Regulatory Mechanisms and Therapeutic Implications. Eur. Heart J. 2025, 46, 3247–3260. [Google Scholar] [CrossRef]
- Pang, M.; Wang, S.; Shi, T.; Chen, J. Overview of MitoQ on Prevention and Management of Cardiometabolic Diseases: A Scoping Review. Front. Cardiovasc. Med. 2025, 12, 1506460. [Google Scholar] [CrossRef]
- Zheng, R.; Song, W.; Wang, C.; Du, X.; Sun, X.; Lu, C. Association between Oxidative Balance Score and Resistant Hypertension and Arterial Stiffness among US Adults: A Population-Based Study. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1901–1911. [Google Scholar] [CrossRef]
- Tao, L.; Zhou, Y.; Wu, L.; Zhu, Y.; Li, J.; Li, C.; Pan, Y.; Liu, J. Association between Hypertension and Oxidative Balance Score: Data from National Health and Nutrition Examination Survey 2005–2018. Front. Cardiovasc. Med. 2025, 12, 1538095. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Valls-Bellés, V.; Arilla-Codoñer, A.; Alonso-Iglesias, E. Oxidant Mechanisms in Childhood Obesity: The Link between Inflammation and Oxidative Stress. Transl. Res. 2011, 158, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kim, J.H.; Kannappan, R.; Reuter, S.; Dougherty, P.M.; Aggarwal, B.B. Role of Nuclear Factor κB-Mediated Inflammatory Pathways in Cancer-Related Symptoms and Their Regulation by Nutritional Agents. Exp. Biol. Med. 2011, 236, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, S.R.; Park, S.J.; Min, K.H.; Lee, K.Y.; Jin, S.M.; Yoo, W.H.; Lee, Y.C. Antioxidant Down-Regulates Interleukin-18 Expression in Asthma. Mol. Pharmacol. 2006, 70, 1184–1193. [Google Scholar] [CrossRef]
- Barbosa, A.C.S.; Mendes, P.S.; Mattos, G.; Fuchs, R.H.B.; Marques, L.L.M.; Beneti, S.C.; Heck, S.C.; Droval, A.A.; Cardoso, F.A.R. Comparative Analysis of the Use of Natural and Synthetic Antioxidants in Chicken Meat: An Update Review. Braz. J. Biol. 2023, 83, e275539. [Google Scholar] [CrossRef]
- Martínez Álvarez, J.R.; Lopez Jaen, A.B.; Cavia-Saiz, M.; Muñiz, P.; Valls-Belles, V. Beneficial Effects of Olive Oil Enriched with Lycopene on the Plasma Antioxidant and Anti-Inflammatory Profile of Hypercholesterolemic Patients. Antioxidants 2023, 12, 1458. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox Regulation of the Immune Response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 Inflammasome: Contributions to Inflammation-Related Diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Liu, W.; Yang, R.; Zhan, Y.; Yang, X.; Zeng, H.; Chen, B.; Zeng, J.; Hu, T.; Hu, J.; Xiao, Q.; et al. Lactate and Lactylation: Emerging Roles in Autoimmune Diseases and Metabolic Reprogramming. Front. Immunol. 2025, 16, 1589853. [Google Scholar] [CrossRef]
- Liu, L.; De Leeuw, K.; Arends, S.; Doornbos-van Der Meer, B.; Bulthuis, M.L.C.; Van Goor, H.; Westra, J. Biomarkers of Oxidative Stress in Systemic Lupus Erythematosus Patients with Active Nephritis. Antioxidants 2023, 12, 1627. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Orekhov, N.A.; Churov, A.V.; Starodubtseva, I.A.; Beloyartsev, D.F.; Kovyanova, T.I.; Sukhorukov, V.N.; Orekhov, A.N. Mitochondrial Dysfunction in Systemic Lupus Erythematosus: Insights and Therapeutic Potential. Diseases 2024, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.; Stoleriu, G.; Nedelcu, A.H.; Perju, S.N.; Gavrilovici, C.; Baciu, G.; Mihai, C.M.; Chisnoiu, T.; Morariu, I.D.; Grigore, E.; et al. Overview of Oxidative Stress in Systemic Lupus Erythematosus. Antioxidants 2025, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K. Oxidative Stress in SLE T Cells, Is NRF2 Really the Target to Treat? Front. Immunol. 2021, 12, 633845. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; Salgado-Cámara, P.; García-Martín, E.; Agúndez, J.A.G. Oxidative Stress Markers in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 6289. [Google Scholar] [CrossRef] [PubMed]
- Piñar-Morales, R.; Durán, R.; Bautista-García, A.; García-Mansilla, M.J.; Aliaga-Gaspar, P.; Vives-Montero, F.; Barrero-Hernández, F.J. The Impact of Oxidative Stress on Symptoms Associated with Multiple Sclerosis. Sci. Rep. 2025, 15, 22983. [Google Scholar] [CrossRef]
- Izumi, Y.; Koyama, Y. Nrf2-Independent Anti-Inflammatory Effects of Dimethyl Fumarate: Challenges and Prospects in Developing Electrophilic Nrf2 Activators for Neurodegenerative Diseases. Antioxidants 2024, 13, 1527. [Google Scholar] [CrossRef]
- Djordjevic, K.; Milojevic Samanovic, A.; Veselinovic, M.; Zivkovic, V.; Mikhaylovsky, V.; Mikerova, M.; Reshetnikov, V.; Jakovljevic, V.; Nikolic Turnic, T. Oxidative Stress Mediated Therapy in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1938. [Google Scholar] [CrossRef]
- Giordo, R.; Posadino, A.M.; Maccioccu, P.; Capobianco, G.; Zinellu, A.; Erre, G.L.; Pintus, G. Sera from Rheumatoid Arthritis Patients Induce Oxidative Stress and Pro-Angiogenic and Profibrotic Phenotypes in Human Endothelial Cells. J. Clin. Med. 2024, 13, 5913. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Fu, J.; Zhuo, R.; Xu, J.; Liu, L.; Dai, M.; Li, Z. Oxidative Balance Score and the Potential for Suffering Rheumatoid Arthritis: A Cross-Sectional Study. Front. Immunol. 2024, 15, 1454594. [Google Scholar] [CrossRef]
- Mo, B.; Ding, Y.; Ji, Q. NLRP3 Inflammasome in Cardiovascular Diseases: An Update. Front. Immunol. 2025, 16, 1550226. [Google Scholar] [CrossRef]
- Jurcau, M.-C.; Jurcau, A.; Diaconu, R.-G. Oxidative Stress in the Pathogenesis of Neurodegenerative Diseases. Stresses 2024, 4, 827–849. [Google Scholar] [CrossRef]
- Castelli, S.; Carinci, E.; Baldelli, S. Oxidative Stress in Neurodegenerative Disorders: A Key Driver in Impairing Skeletal Muscle Health. Int. J. Mol. Sci. 2025, 26, 5782. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Luo, F. The Role of Reactive Oxygen Species in Alzheimer’s Disease: From Mechanism to Biomaterials Therapy. Adv. Healthc. Mater. 2024, 13, 2304373. [Google Scholar] [CrossRef] [PubMed]
- Foret, M.K.; Orciani, C.; Welikovitch, L.A.; Huang, C.; Cuello, A.C.; Do Carmo, S. Early Oxidative Stress and DNA Damage in Aβ-Burdened Hippocampal Neurons in an Alzheimer’s-like Transgenic Rat Model. Commun. Biol. 2024, 7, 861. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling Pathways in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Xiao, B.; Kuruvilla, J.; Tan, E.-K. Mitophagy and Reactive Oxygen Species Interplay in Parkinson’s Disease. Npj Park. Dis. 2022, 8, 135. [Google Scholar] [CrossRef]
- Iftikhar, S.; Sameer, H.M.; Zainab. Significant Potential of Melatonin Therapy in Parkinson’s Disease—A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2023, 14, 1265789. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Montezinho, L.; Simões, R.F.; Carvalho, M.; Ferreiro, E.; Silva, F.S.G. Mitochondria: A Promising Convergent Target for the Treatment of Amyotrophic Lateral Sclerosis. Cells 2024, 13, 248. [Google Scholar] [CrossRef] [PubMed]
- Tarot, P.; Lasbleiz, C.; Liévens, J.-C. NRF2 Signaling Cascade in Amyotrophic Lateral Sclerosis: Bridging the Gap between Promise and Reality. Neural Regen. Res. 2024, 19, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Y.; Deng, M. New Developments and Opportunities in Drugs Being Trialed for Amyotrophic Lateral Sclerosis from 2020 to 2022. Front. Pharmacol. 2022, 13, 1054006. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Hermans, N.; Cos, P.; Maes, L.; De Bruyne, T.; Vanden Berghe, D.; Vlietinck, A.J.; Pieters, L. Challenges and Pitfalls in Antioxidant Research. Curr. Med. Chem. 2007, 14, 417–430. [Google Scholar] [CrossRef]
- Steinhubl, S.R. Why Have Antioxidants Failed in Clinical Trials? Am. J. Cardiol. 2008, 101, S14–S19. [Google Scholar] [CrossRef]
- Held, J.M. Redox Systems Biology: Harnessing the Sentinels of the Cysteine Redoxome. Antioxid. Redox Signal. 2020, 32, 659–676. [Google Scholar] [CrossRef]
- Huang, J.; Co, H.K.; Lee, Y.; Wu, C.; Chen, S. Multistability Maintains Redox Homeostasis in Human Cells. Mol. Syst. Biol. 2021, 17, e10480. [Google Scholar] [CrossRef]
- Pérez-Sala, D.; Quinlan, R.A. The Redox-Responsive Roles of Intermediate Filaments in Cellular Stress Detection, Integration and Mitigation. Curr. Opin. Cell Biol. 2024, 86, 102283. [Google Scholar] [CrossRef]
- Yousef, H.; Feng, S.F.; Jelinek, H.F. Exploratory Risk Prediction of Type II Diabetes with Isolation Forests and Novel Biomarkers. Sci. Rep. 2024, 14, 14409. [Google Scholar] [CrossRef]
- Zhu, H.-M.; Liu, N.; Sun, D.-X.; Luo, L. Machine-Learning Algorithm-Based Prediction of a Diagnostic Model Based on Oxidative Stress-Related Genes Involved in Immune Infiltration in Diabetic Nephropathy Patients. Front. Immunol. 2023, 14, 1202298. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, H.; Slabu, I.; Liehn, E.A.; Rusu, M. Vitamin C in Cardiovascular Disease: From Molecular Mechanisms to Clinical Evidence and Therapeutic Applications. Antioxidants 2025, 14, 506. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial Effects from a Mechanistic Perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, O.; Huber, B.T.; Meydani, S.N. Vitamin E-Enhanced IL-2 Production in Old Mice: Naive But Not Memory T Cells Show Increased Cell Division Cycling and IL-2-Producing Capacity. J. Immunol. 2001, 167, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.; Shahraki Jazinaki, M.; Aliakbarian, M.; Rashidmayvan, M.; Golafrouz, H.; Rahnama, I.; Khodashahi, R.; Malekahmadi, M. Propolis Supplementation on Inflammatory and Oxidative Stress Biomarkers in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2025, 12, 1542184. [Google Scholar] [CrossRef]
- Dabbaghi Varnousfaderani, S.; Musazadeh, V.; Ghalichi, F.; Kavyani, Z.; Razmjouei, S.; Faghfouri, A.H.; Ahrabi, S.S.; Seyyed Shoura, S.M.; Dehghan, P. Alleviating Effects of Coenzyme Q10 Supplements on Biomarkers of Inflammation and Oxidative Stress: Results from an Umbrella Meta-Analysis. Front. Pharmacol. 2023, 14, 1191290. [Google Scholar] [CrossRef]
- Cepeda, V.; Ródenas-Munar, M.; García, S.; Bouzas, C.; Tur, J.A. Unlocking the Power of Magnesium: A Systematic Review and Meta-Analysis Regarding Its Role in Oxidative Stress and Inflammation. Antioxidants 2025, 14, 740. [Google Scholar] [CrossRef]
- Wei, H.; Ye, L.; Li, M.; Huang, S.; Mo, Z. Effect of GLP-1 RA and SGLT2I on Biomarkers of Oxidative Stress in T2DM: A Systematic Review and Meta-Analysis. J. Endocr. Soc. 2025, 9, bvaf075. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Zhang, Y.; Wang, Y.; Qiao, X.; Sun, C.; Chen, Y.; Guo, M.; Han, W.; Ye, A.; et al. Precision Redox: The Key for Antioxidant Pharmacology. Antioxid. Redox Signal. 2021, 34, 1069–1082. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox Regulation: Mechanisms, Biology and Therapeutic Targets in Diseases. Signal Transduct. Target. Ther. 2025, 10, 72. [Google Scholar] [CrossRef]
- Murillo-Sanjuán, L.; Balmaña, J.; de Pablo García-Cuenca, A.; Lorente Guerrero, J.; Uria Oficialdegui, M.L.; Carrasco, E.; Diaz-de-Heredia, C. Post-Hematopoietic Stem Cell Transplant Squamous Cell Carcinoma in Patients with Fanconi Anemia: A Dreadful Enemy. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2022, 24, 388–392. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent Advances in the Redox-Responsive Drug Delivery Nanoplatforms: A Chemical Structure and Physical Property Perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hou, X.; Li, L.; Guo, J.; Jiang, W.; Shang, W. Application of Metal-Based Nanozymes in Inflammatory Disease: A Review. Front. Bioeng. Biotechnol. 2022, 10, 920213. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, X.; Ma, X.; Jiang, G.; Song, Q.; Guo, R.; Wang, S.; Gao, X.; Lu, L. Steroid-Loaded Reconstituted High-Density Lipoprotein Nanocarrier: A New Treatment for Systemic Lupus Erythematosus. J. Biomater. Appl. 2023, 37, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Deidda Tarquini, G.; Urbani, M.; Bejarano, I.; Traversa, E.; Ghibelli, L. The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox? Nanomaterials 2023, 13, 2803. [Google Scholar] [CrossRef]

| Method | Analyte | Sensitivity 1 | Specificity | Sample Type |
|---|---|---|---|---|
| TBARS 2 assay | MDA 3 | Low– moderate | Low (cross-reactivity with other aldehydes) | Plasma, serum, and tissue homogenates |
| HPLC 4 | MDA, 4-HNE 5, and other aldehydes | Moderate | Moderate–high (depends on derivatization) | Plasma, urine, and tissue |
| LC-MS/MS 6 | MDA, 4-HNE, and F2-isoprostanes | High | Very high | Plasma, urine, tissue, and exhaled breath condensate |
| GC-MS 7 | Volatile or derivatized products (e.g., MDA and isoprostanes) | High | High | Plasma, urine, and tissue |
| Immunoassays (ELISA) | 4-HNE adducts and F2-isoprostanes | Moderate | Moderate (depends on antibody) | Plasma, urine, and cell lysates |
| F2-isoprostanes (LC-MS/MS) | F2-isoprostanes | Very high | Very high | Plasma, urine, CSF 8, and tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Llorens, G.; El Ouardi, M.; Valls-Belles, V. Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases. Appl. Sci. 2025, 15, 10191. https://doi.org/10.3390/app151810191
Garcia-Llorens G, El Ouardi M, Valls-Belles V. Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases. Applied Sciences. 2025; 15(18):10191. https://doi.org/10.3390/app151810191
Chicago/Turabian StyleGarcia-Llorens, Guillem, Meryem El Ouardi, and Victoria Valls-Belles. 2025. "Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases" Applied Sciences 15, no. 18: 10191. https://doi.org/10.3390/app151810191
APA StyleGarcia-Llorens, G., El Ouardi, M., & Valls-Belles, V. (2025). Oxidative Stress Fundamentals: Unraveling the Pathophysiological Role of Redox Imbalance in Non-Communicable Diseases. Applied Sciences, 15(18), 10191. https://doi.org/10.3390/app151810191





