Effects of Rapid Maxillary Expansion on Pulmonary Function in Adolescents: A Spirometric Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
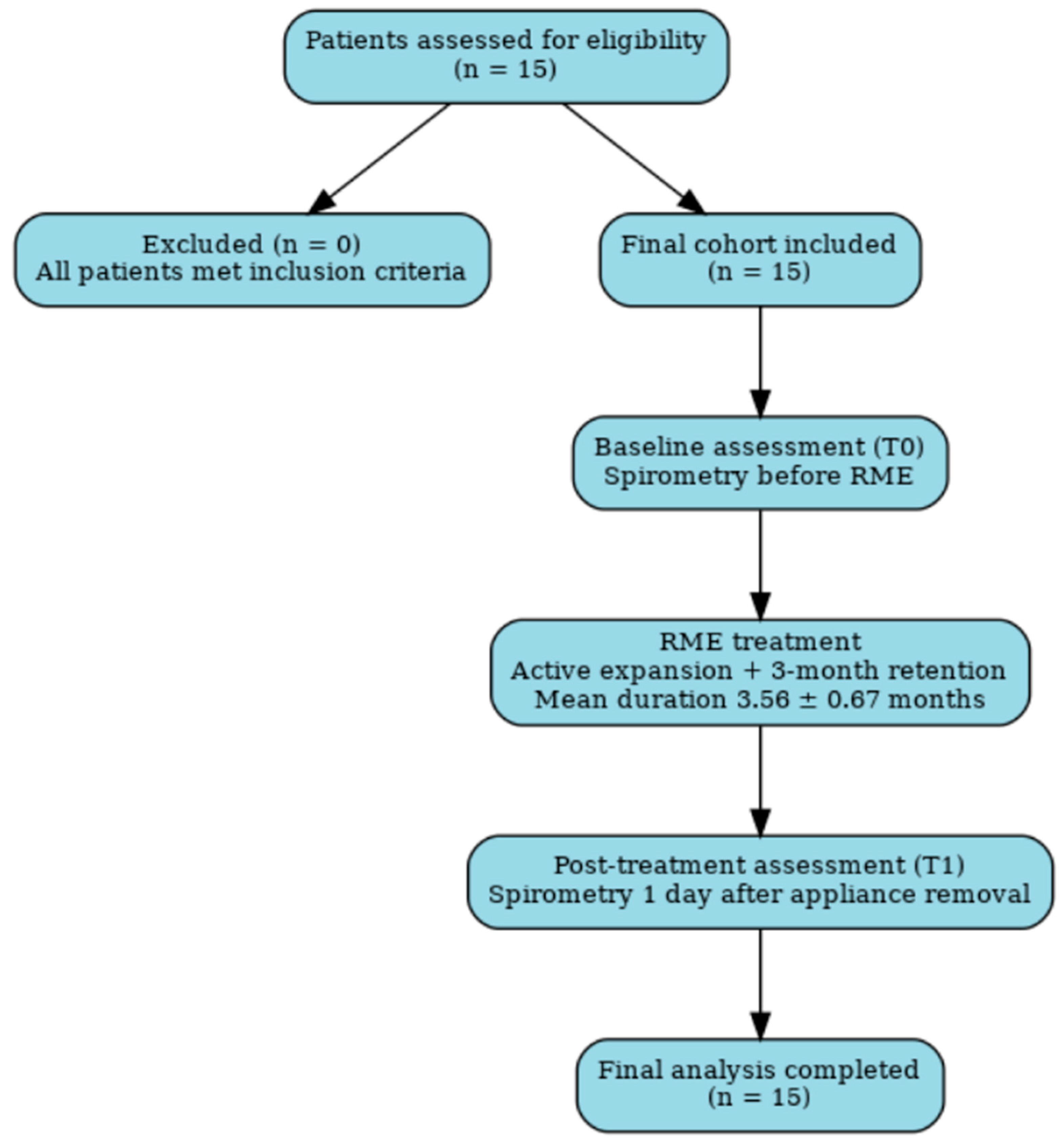
2.2. Participants and Eligibility Criteria
2.3. Intervention: Appliance Design and Activation Protocol
2.4. Outcomes and Measurements
2.5. Efforts to Reduce Bias
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Seif-Eldin, N.F.; Elkordy, S.A.; Fayed, M.S.; Elbeialy, A.R.; Eid, F.H. Transverse Skeletal Effects of Rapid Maxillary Expansion in Pre and Post Pubertal Subjects: A Systematic Review. Open Access Maced. J. Med. Sci. 2019, 7, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Ferrara, I.; Viapiano, F.; Netti, A.; Campanelli, M.; Buongiorno, S.; Latini, G.; Carpentiere, V.; Ciocia, A.M.; Ceci, S.; et al. Rapid Maxillary Expansion on the Adolescent Patient: Systematic Review and Case Report. Children 2022, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, L.; Qabool, H.; Idrees, W.; Sukhia, R.H.; Fida, M. Skeletal and dental changes after bone-borne versus tooth-borne surgically assisted rapid palatal expansion in subjects with maxillary transverse deficiency: A systematic review and meta-analysis. Dent. Med. Probl. 2025, 62, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, F.; Solidoro, L.; Bartolucci, M.L.; Incerti Parenti, S.; Paganelli, C.; Alessandri-Bonetti, G. Skeletal and dental effects of surgically assisted rapid palatal expansion: A systematic review of randomized controlled trials. Eur. J. Orthod. 2020, 42, 434–440. [Google Scholar] [CrossRef]
- McNamara, J.A.; Lione, R.; Franchi, L.; Angelieri, F.; Cevidanes, L.H.S.; Darendeliler, M.A.; Cozza, P. The role of rapid maxillary expansion in the promotion of oral and general health. Prog. Orthod. 2015, 16, 33. [Google Scholar] [CrossRef]
- Kan, H.; Sözen, T.; Öğretmenoğlu, O.; Ciğer, S. Evaluation of the Effects of Orthopedic Treatment on the Dentofacial Structure and Upper Airway of Subjects with Skeletal Class III Malocclusion. Turk. J. Orthod. 2024, 37, 153–161. [Google Scholar] [CrossRef]
- Yalcin-Gungor, A.; Turkkahraman, H.; Baykul, T.; Alkis, H. Comparison of the Effects of Rapid Maxillary Expansion and Surgically Assisted Rapid Maxillary Expansion in the Sagittal, Vertical, and Transverse Planes. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e311–e319. [Google Scholar] [CrossRef]
- Militi, A.; Maio, A.; Nucera, R.; Bellocchio, A.M.; Fiorillo, L.; Galletti, F.; Portelli, M. Effects of rapid maxillary expansion in pediatric patients affected by obstructive sleep apnea syndrome: A literature review. Minerva Dent. Oral Sci. 2025, 74, 114–119. [Google Scholar] [CrossRef]
- Piełunowicz, M.; Kotuła, J.; Kotuła, K.; Więckiewicz, M.; Lis, J.; Kawala, B.; Kuc, A.E.; Sarul, M. Effects of rapid maxillary expansion and functional orthodontic treatment in children with sleep disordered breathing: A systematic review. BMC Oral Health 2025, 25, 1059. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, L.; Lu, Y. The role of rapid maxillary expansion in pediatric obstructive sleep apnea: Efficacy, mechanism and multidisciplinary collaboration. Sleep Med. Rev. 2023, 67, 101733. [Google Scholar] [CrossRef]
- Ugolini, A.; Abate, A.; Donelli, M.; Gaffuri, F.; Bruni, A.; Maspero, C.; Lanteri, V. Spontaneous Mandibular Dentoalveolar Changes after Rapid Maxillary Expansion (RME), Slow Maxillary Expansion (SME), and Leaf Expander-A Systematic Review. Children 2024, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; D’Anto, V.; Rongo, R.; Valletta, R.; Martina, R.; Michelotti, A. Dental and skeletal effects of palatal expansion techniques: A systematic review of the current evidence from systematic reviews and meta-analyses. J. Oral Rehabil. 2016, 43, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Ugolini, A.; Bruni, A.; Abate, A.; Pistoni, F.; Donelli, M.; Quinzi, V.; Silvestrini Biavati, F.; Lanteri, V. Effects On Palatal Surface Area In Mixed Dentition Patients Treated With Leaf Expander And Rapid Palatal Expander, Compared To Untreated Subjects: A Randomised Clinical Trial. Eur. J. Paediatr. Dent. 2025, 26, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Ugolini, A.; Bruni, A.; Quinzi, V.; Lanteri, V. Three-dimensional assessment on digital cast of spontaneous upper first molar distorotation after Ni-ti leaf springs expander and rapid maxillary expander: A two-centre randomized controlled trial. Orthod. Craniofac Res. 2025, 28, 104–115. [Google Scholar] [CrossRef]
- Warren, D.W.; Hershey, G.; Turvey, T.A.; Hinton, V.A.; Hairfield, W.M. The nasal airway following maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 111–116. [Google Scholar] [CrossRef]
- Sökücü, O.; Doruk, C.; Uysal, Ö.İ. Comparison of the effects of RME and fan-type RME on nasal airway by using acoustic rhinometry. Angle Orthod. 2010, 80, 870–875. [Google Scholar] [CrossRef]
- Almuzian, M.; Ju, X.; Almukhtar, A.; Ayoub, A.; Al-Muzian, L.; McDonald, J.P. Does rapid maxillary expansion affect nasopharyngeal airway? A prospective Cone Beam Computerised Tomography (CBCT) based study. Surgeon 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Iwasaki, T.; Saitoh, I.; Takemoto, Y.; Inada, E.; Kakuno, E.; Kanomi, R.; Hayasaki, H.; Yamasaki, Y. Tongue posture improvement and pharyngeal airway enlargement as secondary effects of rapid maxillary expansion: A cone-beam computed tomography study. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 235–245. [Google Scholar] [CrossRef]
- Panetti, B.; Federico, C.; Sferrazza Papa, G.F.; Di Filippo, P.; Di Ludovico, A.; Di Pillo, S.; Chiarelli, F.; Scaparrotta, A.; Attanasi, M. Three Decades of Managing Pediatric Obstructive Sleep Apnea Syndrome: What’s Old, What’s New. Children 2025, 12, 919. [Google Scholar] [CrossRef]
- Adoni, V.V.; Indrakumar, H.S.; Venkatesh, D.; Kashyap, R.; Jayanthi, D.; Prakash, N. Spirometric Assessment of Impact of Complete Dentures on Respiratory Performance: An in vitro Study. J. Contemp. Dent. Pract. 2018, 19, 177–180. [Google Scholar] [CrossRef]
- Weber Santos, B.; Scalco, J.C.; Parazzi, P.L.F.; Schivinski, C.I.S. Compatibility of the global lung function 2012 spirometry reference values in children, adolescents and young adults: A systematic review. Expert Rev. Respir. Med. 2024, 18, 883–892. [Google Scholar] [CrossRef]
- Agusti, A.; Fabbri, L.M.; Baraldi, E.; Celli, B.; Corradi, M.; Faner, R.; Martinez, F.D.; Melén, E.; Papi, A. Spirometry: A practical lifespan predictor of global health and chronic respiratory and non-respiratory diseases. Eur. J. Intern. Med. 2021, 89, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Neves, B.R.; Leao, R.S.; da Silva, A.S.C.; Junior, J.F.S.; do Egito Vasconcelos, B.C.; Pellizzer, E.P.; Moraes, S.L.D. Influence of Complete Denture Use on Respiratory Capacity: A Systematic Review. J. Contemp. Dent. Pract. 2021, 22, 1197–1205. [Google Scholar] [PubMed]
- Foltan, R.; Hoffmannova, J.; Pavlikova, G.; Hanzelka, T.; Klima, K.; Horka, E.; Adamek, S.; Sedy, J. The influence of orthognathic surgery on ventilation during sleep. Int. J. Oral. Maxillofac. Surg. 2011, 40, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, N.; Whitefield, S.; Kleinman, S.; Ianculovici, C.; Feldman, S.; Peleg, O. Posterior airway compromise following orthognathic surgery in skeletal class III patient—A systematic review and meta-analysis. Sleep Med. 2025, 129, 192–199. [Google Scholar] [CrossRef]
- Segna, E.; Goker, F.; Tirelli, G.; Del Fabbro, M.; Giannì, A.B.; Beltramini, G.A.; Rossi, D.S. Maxillomandibular Advancement with the Use of Virtual Surgical Planning and the CAD/CAM Technology in OSA Surgery: Volumetric Analysis of the Posterior Airway Space. Medicina 2025, 61, 179. [Google Scholar] [CrossRef]
- Alan, A.; Ugurlu, M.; Bayrakdar, İ.S.; Gonuldas, F.; de Castro Lopes, S.L.P.; Ferreira Costa, A.L.; Orhan, K. Evaluation of Respiratory Conditions in Individuals Undergoing Rapid Maxillary Expansion: A Computational Fluid Dynamics Study. Diagnostics 2025, 15, 527. [Google Scholar] [CrossRef]
- Diab, A.M.I.; Mohammed, B.B.H.; Ghoneim, M.M.; Ali, M.A.M.; Özdemir, S.; Shendy, M.A.M.; Boufahja, F.; Ali, M.M.M. Effect of Slow Maxillary Expansion and Alternative Rapid Maxillary Expansion Protocols on Airway Volume in Cleft Palate Cases: A Cone Beam Computed Tomography Based Study. Cureus 2024, 16, e59534. [Google Scholar] [CrossRef]
- Pirelli, P.; Fiaschetti, V.; Mampieri, G.; Condo, R.; Ubaldi, N.; Pachi, F.; Giancotti, A. Effect of rapid maxillary expansion on nasomaxillary structure and sleep disordered breathing in children with obstructive sleep apnoea. Aust. Dent. J. 2024, 69, S112–S120. [Google Scholar] [CrossRef]
- Schiavi, E.; Ryu, M.H.; Martini, L.; Balasubramanian, A.; McCormack, M.C.; Fortis, S.; Regan, E.A.; Bonini, M.; Hersh, C.P. Application of the European Respiratory Society/American Thoracic Society Spirometry Standards and Race-Neutral Equations in the COPDGene Study. Am. J. Respir. Crit. Care Med. 2024, 210, 1317–1328. [Google Scholar] [CrossRef]
- Zreaqat, M.; Hassan, R.; Alforaidi, S.; Kassim, N.K. Effects of rapid maxillary expansion on upper airway parameters in OSA children with maxillary restriction: A CBCT study. Pediatr. Pulmonol. 2024, 59, 2490–2498. [Google Scholar] [CrossRef]
- Shah Bukhari, J.A.; Sudan, S.; Bangar, B.; Kumar, N.; Bhatia, P.; Duggal, R. Assessment of the Effect of Complete Dentures on Respiratory Performance: A Spirometric Analysis. J. Pharm. Bioallied Sci. 2021, 13, S440–S443. [Google Scholar] [CrossRef]
- Abate, A.; Cavagnetto, D.; Fama, A.; Matarese, M.; Lucarelli, D.; Assandri, F. Short term effects of rapid maxillary expansion on breathing function assessed with spirometry: A case-control study. Saudi Dent. J. 2021, 33, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Monini, S.; Malagola, C.; Villa, M.P.; Tripodi, C.; Tarentini, S.; Malagnino, I.; Marrone, V.; Lazzarino, A.I.; Barbara, M. Rapid Maxillary Expansion for the Treatment of Nasal Obstruction in Children Younger Than 12 Years. Arch. Otolaryngol.—Head Neck Surg. 2009, 135, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Chang, E.T.; Song, S.A.; Abdullatif, J.; Zaghi, S.; Pirelli, P.; Certal, V.; Guilleminault, C. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Laryngoscope 2017, 127, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Gokce, G.; Gode, S.; Ozturk, A.; Kirazli, T.; Veli, I. Evaluation of the effects of different rapid maxillary expansion appliances on airway by acoustic rhinometry: A randomized clinical trial. Int. J. Pediatr. Otorhinolaryngol. 2022, 155, 111074. [Google Scholar] [CrossRef]
- Caruso, S.; Lisciotto, E.; Caruso, S.; Marino, A.; Fiasca, F.; Buttarazzi, M.; Sarzi Amade, D.; Evangelisti, M.; Mattei, A.; Gatto, R. Effects of Rapid Maxillary Expander and Delaire Mask Treatment on Airway Sagittal Dimensions in Pediatric Patients Affected by Class III Malocclusion and Obstructive Sleep Apnea Syndrome. Life 2023, 13, 673. [Google Scholar] [CrossRef]
- Smith, T.; Ghoneima, A.; Stewart, K.; Liu, S.; Eckert, G.; Halum, S.; Kula, K. Three-dimensional computed tomography analysis of airway volume changes after rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 618–626. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Polizzi, A.; Lagravere, M.; Flores-Mir, C.; Isola, G.; Ronsivalle, V.; Leonardi, R. Changes in upper airway airflow after rapid maxillary expansion considering normal craniofacial development as a factor: A retrospective study using computer fluid dynamics. Eur. J. Orthod. 2024, 47, cjae077. [Google Scholar] [CrossRef]
- Echarri-Nicolas, J.; Gonzalez-Olmo, M.J.; Echarri-Labiondo, P.; Romero, M. Short-term outcomes in the upper airway with tooth-bone-borne vs bone-borne rapid maxillary expanders. BMC Oral Health 2023, 23, 714. [Google Scholar] [CrossRef]
- Gokce, S.M.; Gorgulu, S.; Gokce, H.S.; Bengi, O.; Sabuncuoglu, F.; Ozgen, F.; Bilgic, H. Changes in posterior airway space, pulmonary function and sleep quality, following bimaxillary orthognathic surgery. Int. J. Oral Maxillofac. Surg. 2012, 41, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Papageorgiou, S.N.; Yamasaki, Y.; Ali Darendeliler, M.; Papadopoulou, A.K. Nasal ventilation and rapid maxillary expansion (RME): A randomized trial. Eur. J. Orthod. 2021, 43, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, F.; Knode, V.; Plaksin, A.; Magnuson, A.; Ludwig, B. Three-dimensional comparison of tooth-borne and tooth-bone-borne RME appliances: A randomized controlled trial with 5-year follow-up. Eur. J. Orthod. 2023, 45, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Røsland, A.; Bertelsen, R.J.; Bunæs, D.F.; Drengenes, C.; Engström, G.; Klinge, B.; Lie, S.A.; Nilsson, P.M.; Jönsson, D.; Malinovschi, A. Periodontitis is associated with airflow obstruction in the Malmö Offspring Dental Study. J. Clin. Periodontol. 2023, 51, 86–96. [Google Scholar] [CrossRef]
- Aksilp, C.; Pechpongsai, P.; Intakorn, P.; Chaweewannakorn, C.; Boonpratham, S.; Satravaha, Y.; Anuwongnukroh, N.; Peanchitlertkajorn, S. A randomized controlled trial comparing treatment efficacy between rapid maxillary expansion and adenotonsillectomy in pediatric obstructive sleep apnea. Sleep Breath. 2025, 29, 256. [Google Scholar] [CrossRef]
- Caprioglio, A.; Meneghel, M.; Fastuca, R.; Zecca, P.A.; Nucera, R.; Nosetti, L. Rapid maxillary expansion in growing patients: Correspondence between 3-dimensional airway changes and polysomnography. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 23–27. [Google Scholar] [CrossRef]
- Hariharan, A.; Muwaquet Rodriguez, S.; Hijazi Alsadi, T. The Role of Rapid Maxillary Expansion in the Management of Obstructive Sleep Apnoea: Monitoring Respiratory Parameters-A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 14, 116. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| Number of subjects (n) | 15 |
| Gender (Female/Male) | 8/7 |
| Mean age ± SD (years) | 13.93 ± 2.89 |
| Mean height ± SD (cm) | 154.00 ± 12.07 |
| Mean weight ± SD (kg) | 48.33 ± 11.93 |
| Body mass index (BMI) ± SD (kg/m2) | 20.18 ± 3.39 |
| Mean RME treatment time ± SD (months) | 3.56 ± 0.67 |
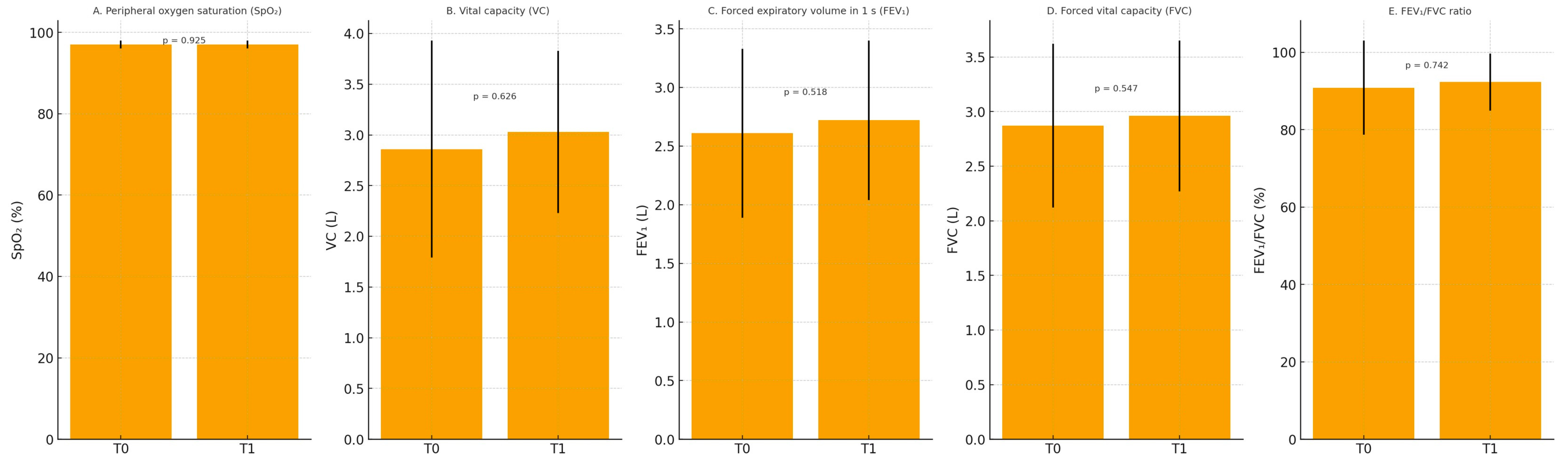
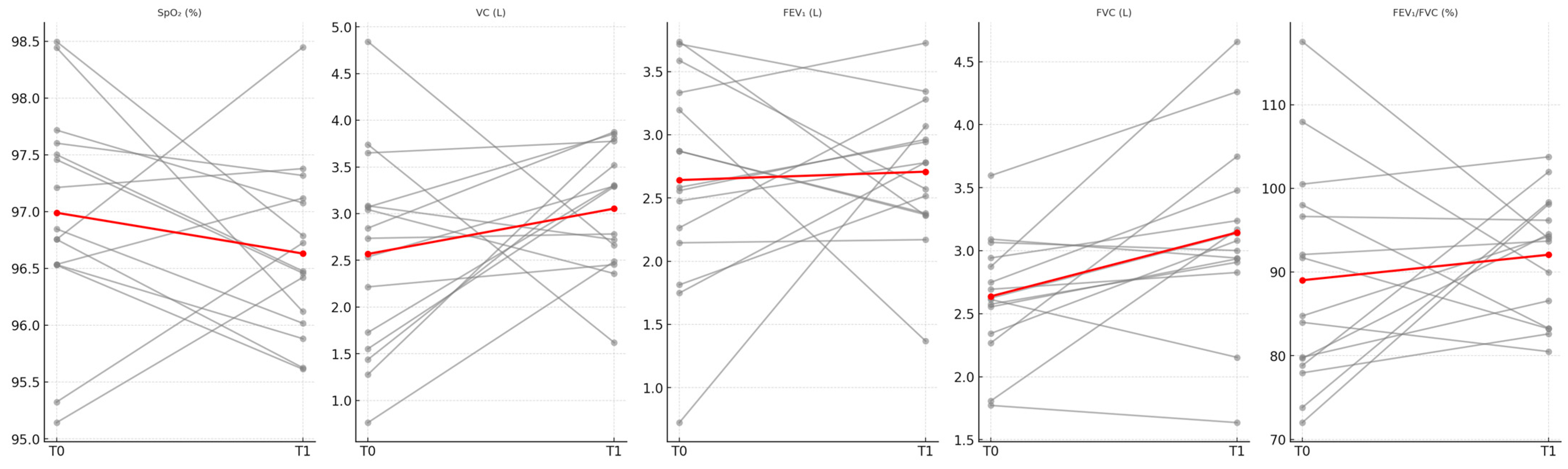
| Variable | T0 Mean ± SD | T1 Mean ± SD | p Value * |
|---|---|---|---|
| SpO2 (%) | 96.98 ± 0.96 | 97.01 ± 0.98 | 0.925 |
| VC (L) | 2.86 ± 1.07 | 3.03 ± 0.80 | 0.626 |
| FEV1 (L) | 2.61 ± 0.72 | 2.72 ± 0.68 | 0.518 |
| FVC (L) | 2.87 ± 0.75 | 2.96 ± 0.69 | 0.547 |
| FEV1/FVC (%) | 90.88 ± 12.17 | 92.34 ± 7.37 | 0.742 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbulut, Y.; Oksayan, R.; Sokucu, O.; Isman, N.E.; Demir, T. Effects of Rapid Maxillary Expansion on Pulmonary Function in Adolescents: A Spirometric Evaluation. Appl. Sci. 2025, 15, 10189. https://doi.org/10.3390/app151810189
Akbulut Y, Oksayan R, Sokucu O, Isman NE, Demir T. Effects of Rapid Maxillary Expansion on Pulmonary Function in Adolescents: A Spirometric Evaluation. Applied Sciences. 2025; 15(18):10189. https://doi.org/10.3390/app151810189
Chicago/Turabian StyleAkbulut, Yasin, Rıdvan Oksayan, Oral Sokucu, Nurettin Eren Isman, and Tuncer Demir. 2025. "Effects of Rapid Maxillary Expansion on Pulmonary Function in Adolescents: A Spirometric Evaluation" Applied Sciences 15, no. 18: 10189. https://doi.org/10.3390/app151810189
APA StyleAkbulut, Y., Oksayan, R., Sokucu, O., Isman, N. E., & Demir, T. (2025). Effects of Rapid Maxillary Expansion on Pulmonary Function in Adolescents: A Spirometric Evaluation. Applied Sciences, 15(18), 10189. https://doi.org/10.3390/app151810189






