Advancing Wastewater Surveillance: Development of High-Throughput Green Robotic SPE-UPLC-MS/MS Workflow for Monitoring of 27 Steroids and Hormones
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation
2.3. Outlines of Method Optimization and Validation
2.4. Mass Spectrometric and Liquid Chromatographic Method Optimization
2.5. Wastewater Matrix Blank Preparation Protocol
2.6. Evaluation of SPE Cartridges for Extraction Efficiency
2.7. Sample Extraction Automation
2.8. Estimation of Measurement Uncertainty (MU)
2.9. Data Analysis, Calculation, and Representations
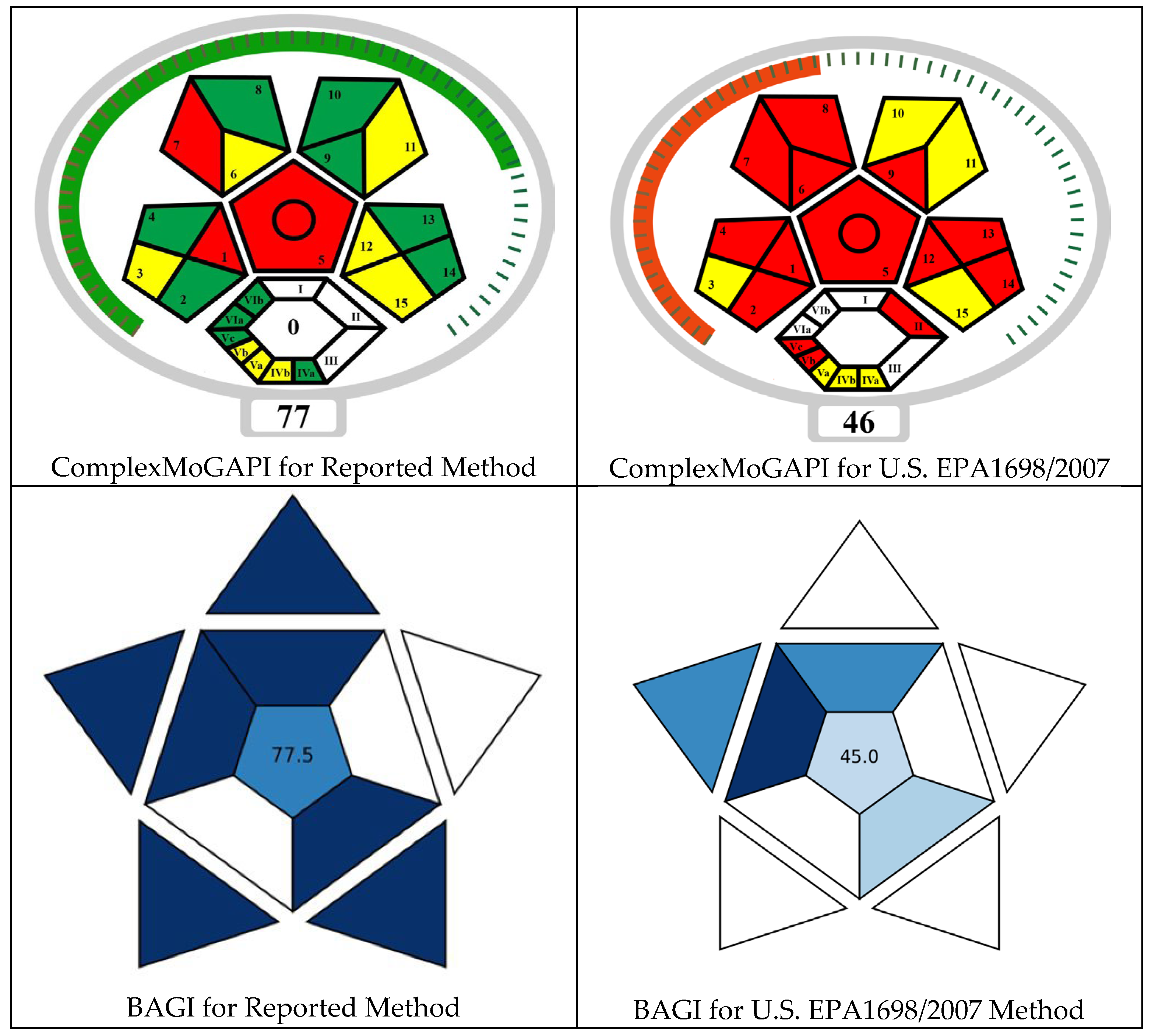
2.10. Evaluation of the Method Greenness and Its Applicability
3. Results and Discussion
3.1. Outcome of Method Optimization
3.2. Method Validation
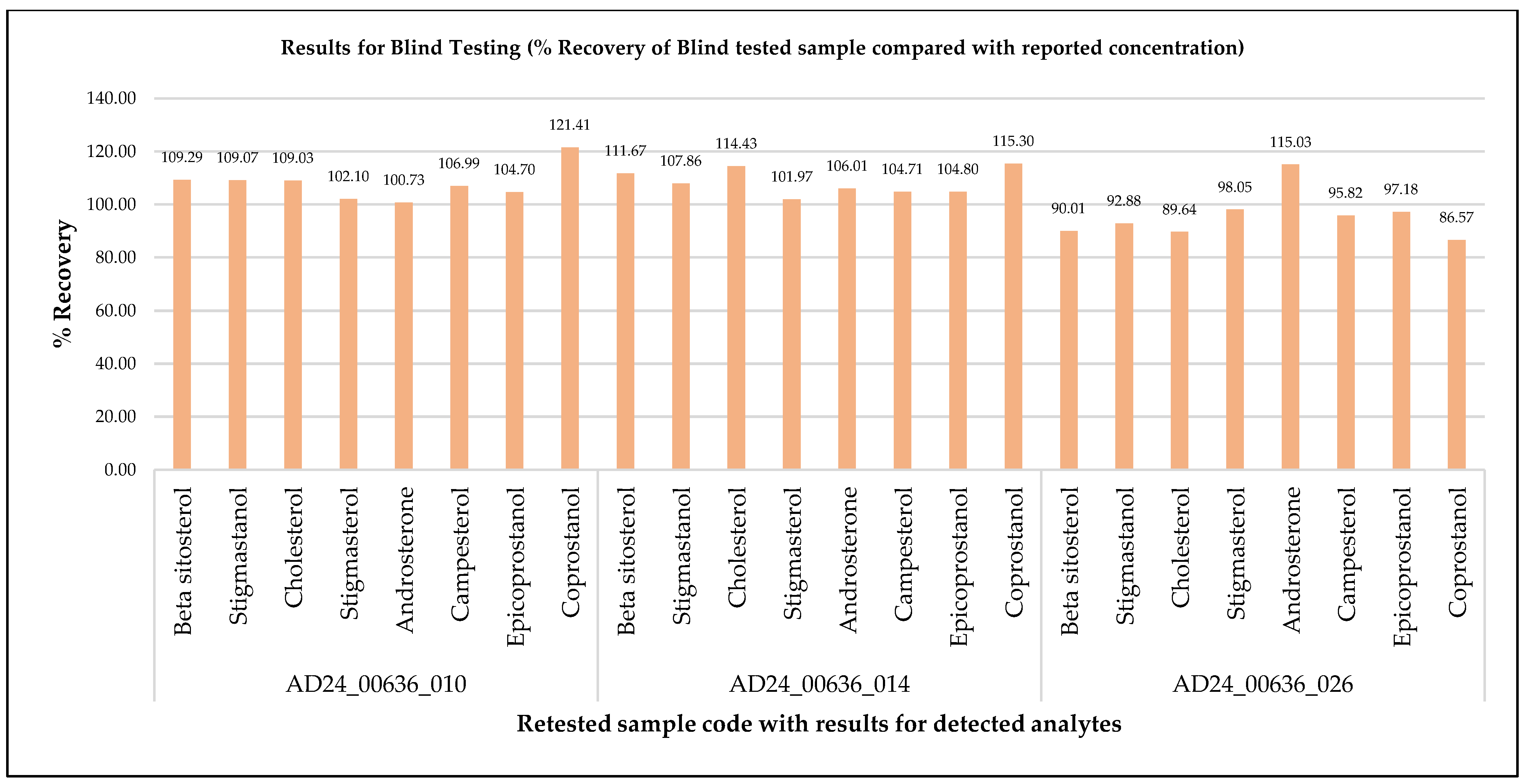
3.3. Comparative Evaluation of Method Greenness and Practicality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yazdan, M.M.S.; Kumar, R.; Leung, S.W. The Environmental and Health Impacts of Steroids and Hormones in Wastewater Effluent, as Well as Existing Removal Technologies: A Review. Ecologies 2022, 3, 206–224. [Google Scholar] [CrossRef]
- Aydoğan, D.; Yurdun, T. Determination of Selected Steroid Compounds in Sediment Samples from Golden Horn Estuary (the Sea of Marmara, Turkey) Using LC-ESI/MS-MS. J. Black Sea/Mediterr. Environ. 2021, 27, 342–364. Available online: https://blackmeditjournal.org/volumes-archive/vol-27-2021/vol-27-2021-no-3/determination-of-selected-steroid-compounds-in-sediment-samples-from-golden-horn-estuary-the-sea-of-marmara-turkey-using-lc-esi-ms-ms/ (accessed on 16 July 2025).
- Almazrouei, B.; Islayem, D.; Alskafi, F.; Catacutan, M.K.; Amna, R.; Nasrat, S.; Sizirici, B.; Yildiz, I. Steroid Hormones in Wastewater: Sources, Treatments, Environmental Risks, and Regulations. Emerg. Contam. 2023, 9, 100210. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; López de Alda, M.J.; López, R.; Barceló, D. Determination of Estrogens and Progestogens by Mass Spectrometric Techniques (GC/MS, LC/MS and LC/MS/MS). J. Mass Spectrom. 2003, 38, 917–923. [Google Scholar] [CrossRef]
- Naldi, A.C.; Fayad, P.B.; Prévost, M.; Sauvé, S. Analysis of Steroid Hormones and Their Conjugated Forms in Water and Urine by On-Line Solid-Phase Extraction Coupled to Liquid Chromatography Tandem Mass Spectrometry. Chem. Cent. J. 2016, 10, 30. [Google Scholar] [CrossRef]
- Goeury, K.; Vo Duy, S.; Munoz, G.; Prévost, M.; Sauvé, S. Assessment of Automated Off-Line Solid-Phase Extraction LC-MS/MS to Monitor EPA Priority Endocrine Disruptors in Tap Water, Surface Water, and Wastewater. Talanta 2022, 241, 123216. [Google Scholar] [CrossRef]
- Biswas, S.; Shapiro, C.A.; Kranz, W.L.; Mader, T.L.; Shelton, D.P.; Snow, D.D.; Bartelt-Hunt, S.L.; Tarkalson, D.D.; van Donk, S.J.; Zhang, T.C.; et al. Current Knowledge on the Environmental Fate, Potential Impact, and Management of Growth-Promoting Steroids Used in the US Beef Cattle Industry. J. Soil Water Conserv. 2013, 68, 325–336. [Google Scholar] [CrossRef]
- Liu, S.; Ying, G.-G.; Zhao, J.-L.; Zhou, L.-J.; Yang, B.; Chen, Z.-F.; Lai, H.-J. Occurrence and Fate of Androgens, Estrogens, Glucocorticoids and Progestagens in Two Different Types of Municipal Wastewater Treatment Plants. J. Environ. Monit. 2012, 14, 482–491. [Google Scholar] [CrossRef]
- Mouatassim-Souali, A.; Tamisier-Karolak, S.L.; Perdiz, D.; Cargouët, M.; Levi, Y. Validation of a Quantitative Assay Using GC/MS for Trace Determination of Free and Conjugated Estrogens in Environmental Water Samples. J. Sep. Sci. 2003, 26, 105–111. [Google Scholar] [CrossRef]
- Zamri, M.F.M.A.; Bahru, R.; Suja’, F.; Shamsuddin, A.H.; Pramanik, S.K.; Fattah, I.M.R. Treatment Strategies for Enhancing the Removal of Endocrine-Disrupting Chemicals in Water and Wastewater Systems. J. Water Process Eng. 2021, 41, 102017. [Google Scholar] [CrossRef]
- Yazdan, M.Md.S.; Ahad, M.T.; Mallick, Z.; Mallick, S.P.; Jahan, I.; Mazumder, M. An Overview of the Glucocorticoids’ Pathways in the Environment and Their Removal Using Conventional Wastewater Treatment Systems. Pollutants 2021, 1, 141–155. [Google Scholar] [CrossRef]
- Tölgyesi, Á.; Verebey, Z.; Sharma, V.K.; Kovacsics, L.; Fekete, J. Simultaneous Determination of Corticosteroids, Androgens, and Progesterone in River Water by Liquid Chromatography–Tandem Mass Spectrometry. Chemosphere 2010, 78, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Persistence and Impact of Steroidal Estrogens on the Environment and Their Laccase-Assisted Removal. Sci. Total Environ. 2019, 690, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Ojoghoro, J.O.; Scrimshaw, M.D.; Sumpter, J.P. Steroid Hormones in the Aquatic Environment. Sci. Total Environ. 2021, 792, 148306. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J. Clinical Use of Androgens. Annu. Rev. Med. 1984, 35, 207–217. [Google Scholar] [CrossRef]
- Rocha, M.; Rocha, E. Synthetic Progestins in Waste and Surface Waters: Concentrations, Impacts and Ecological Risk. Toxics 2022, 10, 163. [Google Scholar] [CrossRef]
- Kuster, M.; José López de Alda, M.; Barceló, D. Analysis and Distribution of Estrogens and Progestogens in Sewage Sludge, Soils and Sediments. TrAC Trends Anal. Chem. 2004, 23, 790–798. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Contaminant Candidate List 4-CCL. Available online: https://www.epa.gov/ccl/contaminant-candidate-list-4-ccl-4-0 (accessed on 19 December 2024).
- Liu, S.; Ying, G.-G.; Zhao, J.-L.; Chen, F.; Yang, B.; Zhou, L.; Lai, H.-J. Trace Analysis of 28 Steroids in Surface Water, Wastewater and Sludge Samples by Rapid Resolution Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1367–1378. [Google Scholar] [CrossRef]
- Snyder, S.A.; Keith, T.L.; Verbrugge, D.A.; Snyder, E.M.; Gross, T.S.; Kannan, K.; Giesy, J.P. Analytical Methods for Detection of Selected Estrogenic Compounds in Aqueous Mixtures. Environ. Sci. Technol. 1999, 33, 2814–2820. [Google Scholar] [CrossRef]
- Huang, C.-H.; Sedlak, D.L. Analysis of Estrogenic Hormones in Municipal Wastewater Effluent and Surface Water Using En-zyme-Linked Immunosorbent Assay and Gas Chromatography/Tandem Mass Spectrometry. Environ. Toxicol. Chem. 2001, 20, 133–139. [Google Scholar] [CrossRef]
- Ferguson, P.L.; Iden, C.R.; McElroy, A.E.; Brownawell, B.J. Determination of Steroid Estrogens in Wastewater by Immunoaffinity Extraction Coupled with HPLC−Electrospray-MS. Anal. Chem. 2001, 73, 3890–3895. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-P.; Lu, C.-C.; Liao, P.-C.; Chou, C.-H.; Sheu, H.-M.; Tsai, J.-C. Detection and Distribution of Endogenous Steroids in Human Stratum Corneum. Dermatol. Sin. 2014, 32, 19–24. [Google Scholar] [CrossRef]
- U.S. EPA Method 1698: Steroids and Hormones in Water, Soil, Sediment, and Biosolids by HRGC/HRMS. 2007. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/method_1698_2007.pdf (accessed on 19 December 2024).
- Bowden, J.A.; Colosi, D.M.; Mora-Montero, D.C.; Garrett, T.J.; Yost, R.A. Enhancement of Chemical Derivatization of Steroids by Gas Chromatography/Mass Spectrometry (GC/MS). J. Chromatogr. B 2009, 877, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Ammann, A.A.; Macíková, P.; Groh, K.J.; Schirmer, K.; Suter, M.J.F. LC-MS/MS Determination of Potential Endocrine Disruptors of Cortico Signalling in Rivers and Wastewaters. Anal. Bioanal. Chem. 2014, 406, 7653–7665. [Google Scholar] [CrossRef]
- Sapozhnikova, Y.; Hedgespeth, M.; Wirth, E.; Fulton, M. Analysis of Selected Natural and Synthetic Hormones by LC-MS-MS Using the US EPA Method 1694. Anal. Methods 2011, 3, 1079. [Google Scholar] [CrossRef]
- Ripollés, C.; Ibáñez, M.; Sancho, J.V.; López, F.J.; Hernández, F. Determination of 17β-Estradiol and 17α-Ethinylestradiol in Water at Sub-Ppt Levels by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Anal. Methods 2014, 6, 5028. [Google Scholar] [CrossRef]
- Tomšíková, H.; Aufartová, J.; Solich, P.; Nováková, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. High-Sensitivity Analysis of Female-Steroid Hormones in Environmental Samples. TrAC Trends Anal. Chem. 2012, 34, 35–58. [Google Scholar] [CrossRef]
- SANTE 11312/2021v2. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed, EURL for Residues of Pesticides. 2024. Available online: http://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 19 December 2024).
- Joint Committee for Guides in Metrology. Evaluation of Measurement Data Guide to the Expression of Uncertainty in Measurement. JCGM 2008, 100, 1–116. [Google Scholar] [CrossRef]
- Ellison, S.L.R.; Williams, A. (Eds.) EURACHEM/CITAC Guid: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; EURACHEM: Teddington, UK, 2012; Available online: https://www.eurachem.org/index.php/publications/guides/quam (accessed on 19 December 2024).
- Shinde, D.; Murugan, V.; Dhagat, U.; Gupta, P.; Tadala, R.; Karubothula, B.; Golebiewska, I.; Salem, S.B.; Elamin, W.; Brudecki, G. Introduction of a Small Volume Ethyl Acetate Based Liquid-Liquid Extraction Procedure for Analysis of Polycyclic Aromatic Hydrocarbons in Wastewater by Atmospheric Pressure Gas Chromatography-Mass Spectrometry and Evaluation of Method Greenness. J. Chromatogr. A 2025, 1740, 465563. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; International Organization for Standardization, 2017 General Requirements for the Competence of Testing and Calibration Laboratories. 3rd ed. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 19 December 2024).
- Mansour, F.R.; Omer, K.M.; Płotka-Wasylka, J. A Total Scoring System and Software for Complex Modified GAPI (Com-plexMoGAPI) Application in the Assessment of Method Greenness. Green Anal. Chem. 2024, 10, 100126. [Google Scholar] [CrossRef]
- Manousi, N.; Wojnowski, W.; Płotka-Wasylka, J.; Samanidou, V. Blue Applicability Grade Index (BAGI) and Software: A New Tool for the Evaluation of Method Practicality. Green Chem. 2023, 25, 7598–7604. [Google Scholar] [CrossRef]
- Fabregat-Safont, D.; Gracia-Marín, E.; Ibáñez, M.; Pitarch, E.; Hernández, F. Analytical Key Issues and Challenges in the LC-MS/MS Determination of Antibiotics in Wastewater. Anal. Chim. Acta 2023, 1239, 340739. [Google Scholar] [CrossRef]
- Jauković, Z.D.; Grujić, S.D.; Matić, I.V.; Laušević, M.D. Determination of Sterols and Steroid Hormones in Surface Water and Wastewater Using Liquid Chromatography-Atmospheric Pressure Chemical Ionization-Mass Spectrometry. Microchem. J. 2017, 135, 39–47. [Google Scholar] [CrossRef]
- ISO 13528:2022; Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparison. International Organi-zation for Standardization: Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/78879.html (accessed on 19 December 2024).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015; Available online: https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981 (accessed on 16 July 2025).
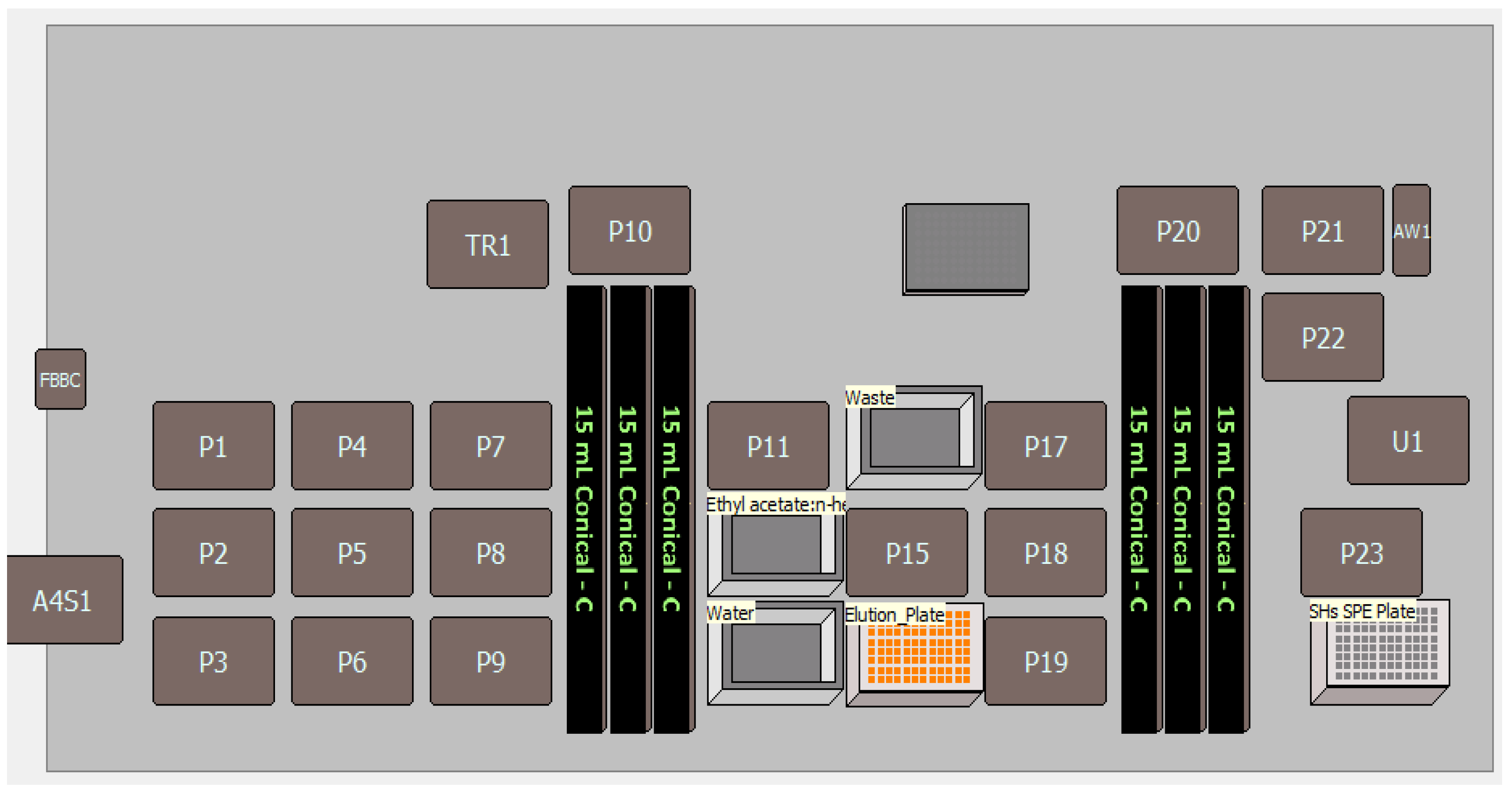
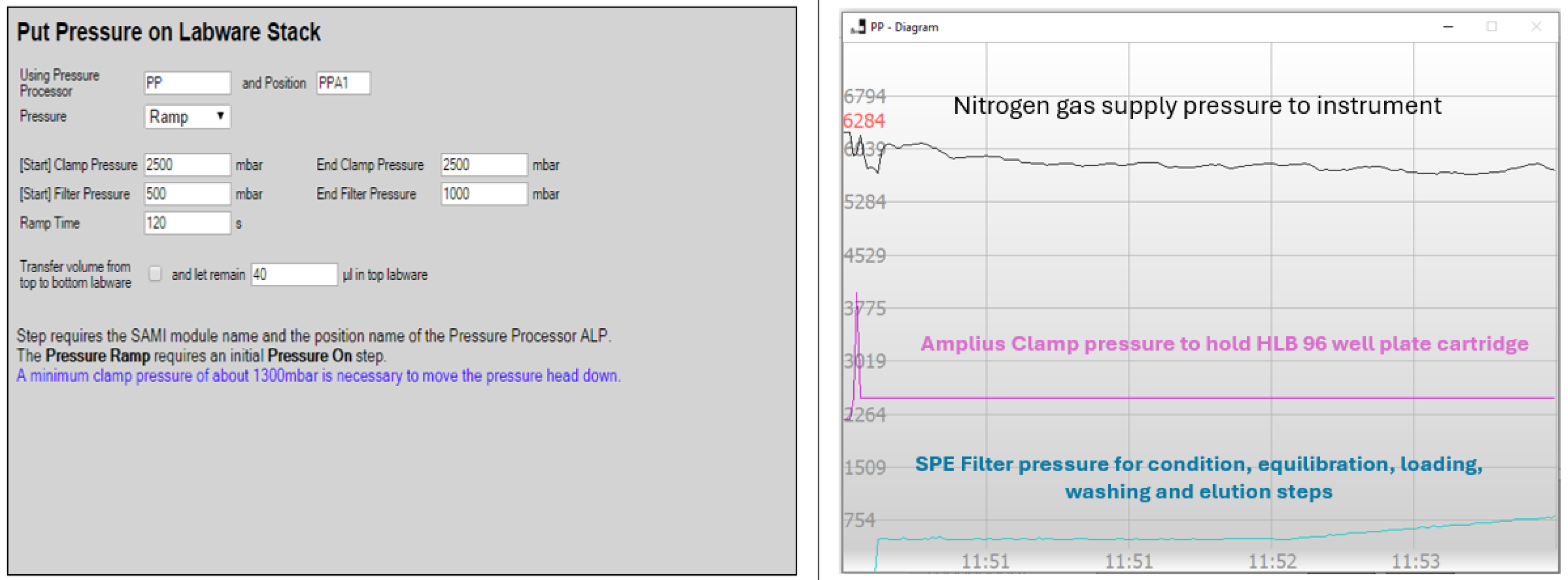
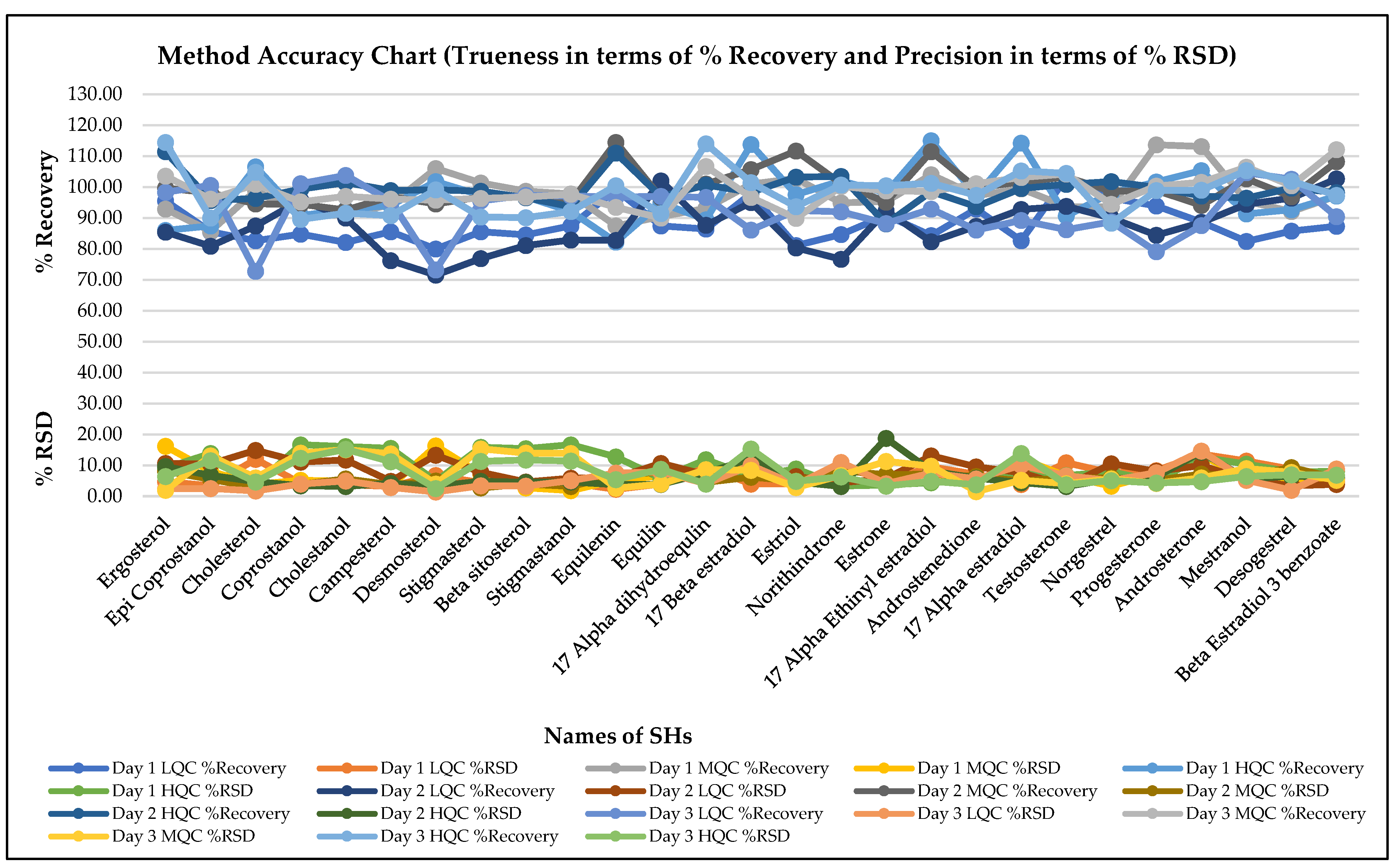
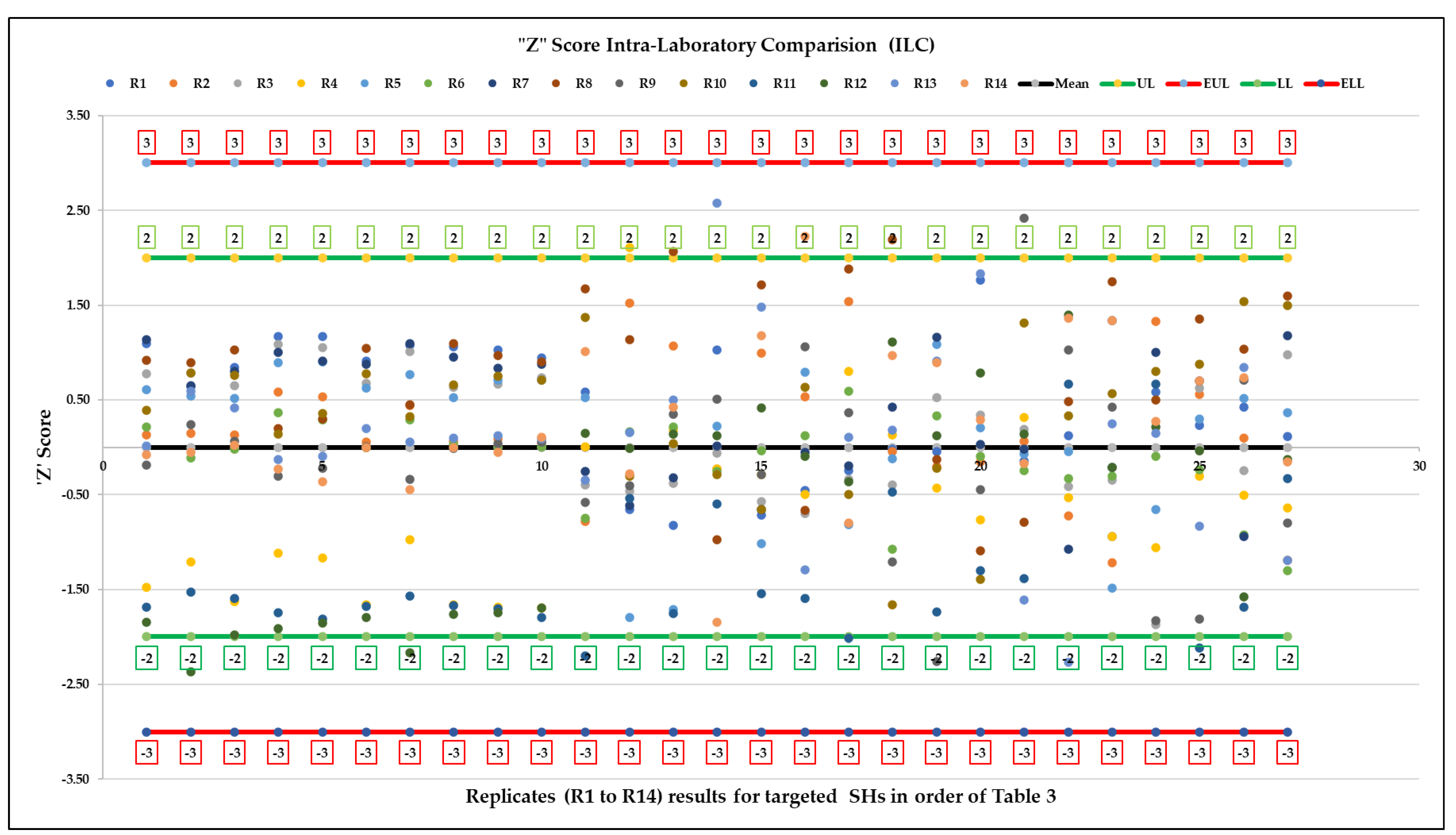
| Steps | Sample Preparation Task | ||
|---|---|---|---|
| Procedure for automated extraction of IDs in wastewater using Biomek i7 | |||
| Sample preparation | Unknown and spiked (QC) wastewater samples with ISTD were loaded in the 15 mL conical tube racks (10.0 mL samples) and precleared by centrifugation at 4500 (Eppendorf). | ||
| Conditioning | Condition the HLB 96-well plate cartridge with 1.0 mL 100% MeOH. | ||
| Equilibration | Equilibrate the cartridge with 1.0 mL of 100% ASTM Type I water. | ||
| Sample loading | Load 1.5 mL of samples and quality controls three times (total sample volume: 4.5 mL) on respective 96-well plate cartridge. | ||
| Washing | Wash the cartridge with 1.0 mL of 10% methanol in ASTM Type I water. Dry the cartridge at 1000 mbar for up to 10 min. | ||
| Elution | Elute cartridge two times with 1 mL of Ethyl acetate/n-hexane (70:30 v/v) in plate. | ||
| Dilution and well plate sealing | After drying (at 60 °C temperature under stream of nitrogen at gas flow of 60 L/m for approx. 30 min) completely, reconstitute the well plate with 0.200 mL of methanol/acetonitrile (70:30) v/v and 0.400 mL of ASTM Type I water and seal the cartridge plate by a4S 4titude automated-role heat sealer and vortex mixture at lower rpm. | ||
| LC-MS/MS detection | SHs: Load sealed 96-well plate cartridge autosampler and inject 10 μL of sample. | ||
| Equipment Details | |||
| Ion source | Z Spray XEVO Ion Source | ||
| Pump | Acquity UPLC I Class plus | ||
| Autosampler | FTN Sample Manager | ||
| Column oven | Acquity UPLC Column Heater | ||
| LC column | SHs: X Bridge premier BEH C18 Column, 2.5 µm, 2.1 mm × 100 mm | ||
| LC Parameters | |||
| Mobile phase A | 0.2 mM ammonium fluoride in water | ||
| Mobile phase B | 100% methanol | ||
| Sample purge | ACN/MeOH/IPA/Water: [1:1:1:1 with 0.1% formic acid v/v/v/v] | ||
| Sample wash | ACN/MeOH/IPA: [20:40:40 with 0.1% formic acid v/v/v] | ||
| Seal wash | MeOH/Water [90/10, v/v] | ||
| Flow rate | 0.40 mL/min | ||
| Column oven | 65 ± 5 °C | ||
| Sample manger | 8 ± 5 °C | ||
| Injection | 10.0 µL | ||
| LC Gradient | |||
| Flow (mL/min) | Time (Min) | Pump A% | Pump B% |
| 0.4 | Initial | 50 | 50 |
| 0.4 | 0.10 | 50 | 50 |
| 0.4 | 3.0 | 35 | 65 |
| 0.4 | 3.10 | 12 | 88 |
| 0.4 | 8.5 | 0 | 100 |
| 0.4 | 9.0 | 0 | 100 |
| 0.4 | 10.5 | 0 | 100 |
| 0.4 | 11 | 50 | 50 |
| 0.4 | 13.0 | 50 | 50 |
| MS Parameters | |||
| Mode and polarity | APCI +/− | ||
| Scan type | (MRM) | ||
| Source temperature (°C) | 500 | ||
| APCI Probe temp. (°C) | 500 | ||
| Desolvation gas flow (L/h) | 1000 | ||
| Cone gas flow (L/h) | 150 | ||
| Corona pin voltage (kV) | 3.0 kV (+tive mode of ionization) and 2.80 kV (−tive mode ionization) | ||
| Nebulizer gas flow (Bar) | 7 | ||
| Sr. No. | Name of the Steroids and Hormones (Molecular Weight in Da) | Details of MRM Parameters, Retention Time (RT), and Ion Ratios (SHs and IS) | Evaluation of SPE Efficiency of Targeted SHs (Average Area ± RSD at MQC, n = 4) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent Ion | Product Ion (Q1, Q2) | CV (V) | CE (Q1, Q2) (eV) | Within-Batch Stability (n = 26) | Details of the Internal Standard (IS) Used for Quantification | ||||||||||
| Ion Ratio (Q2/Q1 ± % RSD) | RT ± Stdev. | Name of IS | IS MRM Transition | RT ± Stdev. (IS) | CV (V) | CE (eV) | HLB | MCX | MAX | ||||||
| Steroids | |||||||||||||||
| 1 | Ergosterol (396) | 379.00 | 253.00, 295.00 | 20 | 15, 15 | 0.6619 ± 2.85 | 6.72 ± 0.02 | NA | NA | NA | NA | NA | 625,181 a ± 9.0 | 393,706 b ± 3.7 | 36,738 c ± 15.6 |
| 2 | Epi Coprostanol (388) | 371.10 | 95.00, 109.03 | 30 | 24, 24 | 0.4881 ± 3.30 | 7.06 ± 0.02 | NA | NA | NA | NA | NA | 463,104 a ± 4.1 | 58,545 b ± 4.8 | 76,129 b ± 11.2 |
| 3 | Cholesterol (386) | 369.40 | 95.20, 147.00 | 20 | 38, 38 | 0.2278 ± 2.26 | 7.11 ± 0.02 | Cholesterol d7 | 376.50 > 160.90 | 7.09 ± 0.02 | 20 | 22 | 3,419,103 a ± 4.4 | 633,836 b ± 0.6 | 808,024 b ± 10.8 |
| 4 | Coprostanol (388) | 371.21 | 95.00, 109.00 | 30 | 18, 24 | 0.7135 ± 3.24 | 7.34 ± 0.02 | NA | NA | NA | NA | NA | 483,266 a ± 4.5 | 98,376 b ± 2.8 | 122,289 b ± 12.7 |
| 5 | Cholestanol (388) | 371.40 | 95.00, 109.00 | 30 | 32, 30 | 0.5976 ± 2.15 | 7.34 ± 0.02 | NA | NA | NA | NA | NA | 655,427 a ± 4.5 | 136,824 b ± 2.2 | 170,979 b ± 12.5 |
| 6 | Campesterol (400) | 383.40 | 147.05, 94.99 | 25 | 30, 30 | 0.7288 ± 1.96 | 7.37 ± 0.02 | NA | NA | NA | NA | NA | 1,191,273 a ± 4.8 | 211,903 b ± 1.1 | 275,635 b ± 9.6 |
| 7 | Desmosterol (384) | 367.20 | 81.20, 95.30 | 20 | 50, 50 | 0.6954 ± 2.52 | 7.38 ± 0.02 | NA | NA | NA | NA | NA | 1,097,725 a ± 4.0 | 155,213 b ± 0.6 | 209,846 b ± 11.0 |
| 8 | Stigmasterol (412) | 395.40 | 81.10, 83.10 | 15 | 37, 17 | 0.7923 ± 3.71 | 7.39 ± 0.02 | NA | NA | NA | NA | NA | 983,741 a ± 4.9 | 122,557 b ± 1.1 | 155,351 b ± 8.4 |
| 9 | Beta sitosterol (414) | 397.30 | 147.05, 161.17 | 35 | 24, 24 | 1.0878 ± 2.47 | 7.63 ± 0.02 | NA | NA | NA | NA | NA | 2,638,114 a ± 5.1 | 468,256 b ± 1.9 | 606,609 b ± 9.9 |
| 10 | Stigmastanol (416) | 399.26 | 95.00, 109.04 | 35 | 20, 20 | 0.6754 ± 2.34 | 7.87 ± 0.02 | NA | NA | NA | NA | NA | 490,392 a ± 4.2 | 86,668 c ± 0.5 | 114,266 b ± 10.6 |
| Hormones | |||||||||||||||
| 11 | Equilenin (266) | 267.10 | 209.03, 194.01 | 8 | 34, 18 | 0.3385 ± 6.85 | 2.20 ± 0.01 | NA | NA | NA | NA | NA | 599,672 a ± 0.5 | 393,706 b ± 3.7 | 331,612 c ± 4.0 |
| 12 | Equilin (268)- | 266.95 | 142.88, 264.94 | 35 | 35, 35 | 0.6667 ± 4.64 | 2.37 ± 0.02 | NA | NA | NA | NA | NA | 364,391 a ± 1.1 | 204,613 b ± 3.4 | 171,654 c ± 3.1 |
| 13 | 17 Alpha dihydroequlin (270)- | 269.19 | 195.10, 181.05 | 20 | 50, 50 | 1.1271 ± 4.82 | 2.39 ± 0.02 | NA | NA | NA | NA | NA | 123,337 a ± 4.8 | 98,560 b ± 4.5 | 80,726 c ± 3.1 |
| 14 | 17 Beta estradiol (272)- | 271.00 | 144.89, 182.90 | 40 | 40, 40 | 1.0364 ± 5.55 | 2.43 ± 0.02 | 17 Beta estradiol d4 | 275.00 > 186.90 | 2.43 ± 0.02 | 40 | 40 | 297,140 a ± 1.4 | 218,896 b ± 4.7 | 180,570 c ± 2.8 |
| 15 | Estriol (288) | 271.07 | 132.99, 159.00 | 35 | 19, 19 | 0.7618 ± 7.89 | 2.51 ± 0.02 | NA | NA | NA | NA | NA | 2,715,259 a ± 1.0 | 1,541,987 b ± 2.5 | 1,271,781 c ± 2.9 |
| 16 | Norethindrone (298) | 299.10 | 109.00, 231.10 | 31 | 26, 20 | 0.274 ± 5.53 | 2.51 ± 0.02 | Norethindrone d6 | 305.15 > 237.35 | 2.48 ± 0.02 | 25 | 20 | 599,669 a ± 2.3 | 356,057 b ± 2.5 | 293,135 c ± 2.7 |
| 17 | Estrone (270)- | 269.00 | 144.90, 182.90 | 40 | 37, 35 | 0.1515 ± 4.94 | 2.51 ± 0.02 | Estrone 2,3,4 13 13C3 | 272.10 > 148.00 | 2.51 ± 0.02 | 40 | 40 | 1,422,038 a ± 1.1 | 796,730 b ± 2.8 | 644,303 c ± 3.2 |
| 18 | 17 Alpha Ethinyl estradiol (296) | 279.30 | 159.00, 132.90 | 20 | 8, 12 | 0.9835 ± 4.08 | 2.51 ± 0.02 | Ethinyl estradiol d4 | 299.00 > 146.76 | 2.49 ± 0.02 | 15 | 40 | 1,536,082 a ± 2.8 | 1,052,247 b ± 2.0 | 866,339 c ± 2.2 |
| 19 | Androstenedione (286) | 287.10 | 97.00, 109.00 | 40 | 22, 24 | 0.688 ± 7.31 | 2.57 ± 0.02 | Androstene 3,17 dione 13C3 | 290.14 > 100.03 | 2.57 ± 0.02 | 20 | 20 | 284,362 a ± 2.0 | 269,325 a ± 2.7 | 220,032 b ± 4.3 |
| 20 | 17 Alpha estradiol (272)- | 271.01 | 144.89, 182.91 | 40 | 40, 40 | 0.3092 ± 5.04 | 2.67 ± 0.02 | 17 Beta estradiol d4 | 275.00 > 186.90 | 2.43 ± 0.02 | 40 | 40 | 429,770 a ± 2.3 | 288,300 b ± 5.1 | 235,498 c ± 4.5 |
| 21 | Testosterone (288) | 289.00 | 97.03, 109.05 | 15 | 24, 24 | 0.8871 ± 5.92 | 2.82 ± 0.02 | Testosterone d3 | 292.16 > 97.03 | 2.80 ± 0.02 | 15 | 24 | 274,572 a ± 0.5 | 201,858 b ± 0.4 | 168,043 c ± 5.1 |
| 22 | Norgestrel (312) | 313.20 | 109.00, 245.10 | 38 | 26, 18 | 0.6239 ± 8.51 | 3.18 ± 0.02 | Norgestrel d6 | 319.30 > 251.12 | 3.15 ± 0.02 | 25 | 20 | 429,995 a ± 1.0 | 219,172 b ± 2.5 | 181,053 c ± 2.8 |
| 23 | Progesterone (314) | 315.20 | 97.00, 109.00 | 38 | 22, 24 | 0.8817 ± 9.15 | 3.85 ± 0.20 | Progesterone d9 | 324.23 > 99.87 | 3.84 ± 0.01 | 22 | 20 | 274,572 a ± 5.0 | 142,145 b ± 2.9 | 130,306 c ± 1.7 |
| 24 | Androsterone (290) | 273.05 | 255.02, 147.04 | 35 | 22, 15 | 0.3579 ± 5.80 | 3.90 ± 0.01 | Androsterone d4 | 295.40 > 259.30 | 3.89 ± 0.01 | 35 | 20 | 430,044 a ± 0.6 | 257,091 b ± 2.6 | 214,604 c ± 3.3 |
| 25 | Mestranol (310) | 311.20 | 121.00, 146.95 | 35 | 25, 25 | 0.1691 ± 9.09 | 4.09 ± 0.01 | NA | NA | NA | NA | NA | 755,652 a ± 1.7 | 340,778 b ± 3.3 | 321,768 b ± 1.7 |
| 26 | Desogestrel (310) | 293.00 | 146.95, 172.96 | 20 | 20, 20 | 0.7968 ± 6.93 | 4.09 ± 0.01 | NA | NA | NA | NA | NA | 5,527,326 a ± 1.0 | 2,279,912 b ± 2.7 | 2,154,891 c ± 1.6 |
| 27 | Beta Estradiol 3 benzoate (376) | 377.20 | 104.81, 76.63 | 30 | 45, 25 | 0.18 ± 9.48 | 4.35 ± 0.02 | NA | NA | NA | NA | NA | 507,504 a ± 2.0 | 68,375 b ± 1.9 | 79,020 b ± 4.6 |
| Sr. No. | Name of the SHs | Results for Method Validation | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Specificity (%) | Matrix Effect (%) | Linearity Range (µµg/L) | Coefficient of Determination (r2) and % Deviation from Back-Calculated Concentration of Linear Calibration Curve (Average of Results from Day 1, 2, and 3 Validation Trials) | E-LOD (µg/L) | E-LOQ (µg/L) | T-LOQ (µg/L) | MU (@Mean Calculated Concentration at LOQ ± MU) µg/L | ||||||||||
| r2 | L1 | L2 | L3 | L4 | L5 | L6 | L7 | L8 | |||||||||
| 1 | Ergosterol | 0.00 | −4.38 | 40.0–600.0 | 0.9631 | 13.32 | −24.45 | −7.81 | −2.36 | −0.35 | −4.34 | 16.94 | 9.19 | 5.916 | 38.235 | 40.000 | 39.891 ± 7.096 |
| 2 | Epi Coprostanol | 5.88 | 34.92 | 10.0–150.0 | 0.9673 | 12.02 | −22.09 | −3.91 | −8.34 | −9.52 | 4.62 | 13.59 | 9.54 | 0.965 | 8.555 | 10.000 | 8.184 ± 0.595 |
| 3 | Cholesterol | 17.71 | 8.73 | 40.0–600.0 | 0.9739 | 11.94 | −22.86 | −6.64 | 4.07 | −1.98 | −2.11 | 11.40 | 9.17 | 13.057 | 33.047 | 40.000 | 35.317 ± 9.396 |
| 4 | Coprostanol | 11.77 | 18.25 | 10.0–150.0 | 0.9678 | 11.54 | −19.48 | −8.99 | −6.01 | −7.56 | 3.37 | 13.65 | 9.91 | 1.18 | 8.476 | 10.000 | 7.529 ± 0.945 |
| 5 | Cholestanol | 11.69 | 18.74 | 10.0–150.0 | 0.9654 | 12.44 | −21.81 | −7.34 | −7.52 | −8.05 | 4.35 | 15.44 | 8.76 | 1.017 | 8.215 | 10.000 | 7.854 ± 0.845 |
| 6 | Campesterol | 3.07 | 0.76 | 20.0–300.0 | 0.9688 | 11.56 | −20.61 | −6.13 | −7.75 | −6.92 | 3.18 | 14.40 | 8.04 | 1.77 | 17.111 | 20.000 | 14.600 ± 4.778 |
| 7 | Desmosterol | 0.00 | 5.17 | 20.0–300.0 | 0.9803 | 10.87 | −21.23 | −4.61 | −2.65 | 0.65 | 5.21 | 3.57 | 7.73 | 3.551 | 15.994 | 20.000 | 15.406 ± 4.868 |
| 8 | Stigmasterol | 11.04 | 4.42 | 20.0–300.0 | 0.9690 | 11.63 | −20.62 | −6.00 | −7.08 | −7.37 | 1.80 | 13.12 | 10.90 | 2.457 | 17.481 | 20.000 | 16.719 ± 1.989 |
| 9 | Beta sitosterol | 11.25 | 5.22 | 20.0–300.0 | 0.9711 | 12.81 | −23.17 | −5.88 | −7.31 | −8.23 | 4.39 | 13.95 | 10.06 | 1.898 | 16.917 | 20.000 | 15.575 ± 3.646 |
| 10 | Stigmastanol | 11.04 | 4.42 | 20.0–300.0 | 0.9681 | 11.71 | −20.54 | −6.11 | −8.29 | −6.54 | 3.29 | 14.85 | 8.44 | 2.47 | 17.104 | 20.000 | 16.719 ± 1.989 |
| 11 | Equilenin | 0.00 | −3.35 | 1.0–15.0 | 0.9923 | 5.83 | −13.13 | 1.48 | −3.25 | 0.20 | −1.96 | 2.75 | 5.07 | 0.066 | 0.901 | 1.000 | 0.948 ± 0.081 |
| 12 | Equilin | 0.00 | −2.15 | 10.0–150.0 | 0.9839 | 7.65 | −13.47 | −2.51 | −9.45 | −3.50 | 6.83 | 4.14 | 7.26 | 1.151 | 8.749 | 10.000 | 9.283 ± 1.658 |
| 13 | 17 Alpha dihydroequlin | 0.00 | 3.33 | 10.0–150.0 | 0.9934 | 3.35 | −4.07 | −6.91 | 0.42 | 0.61 | 3.25 | −0.55 | 5.21 | 2.714 | 8.643 | 10.000 | 9.991 ± 1.243 |
| 14 | 17 Beta estradiol | 12.49 | 3.6 | 10.0–150.0 | 0.9926 | 2.37 | −4.33 | −1.43 | −0.36 | −7.42 | 5.38 | 1.60 | 2.03 | 1.288 | 9.791 | 10.000 | 8.439 ± 1.454 |
| 15 | Estriol | 0.00 | −4.39 | 0.2–3.0 | 0.9939 | 4.50 | −8.92 | −0.09 | −3.70 | −0.69 | −3.35 | 1.87 | 8.13 | 0.022 | 0.162 | 0.200 | 0.175 ± 0.022 |
| 16 | Norethindrone | 0.00 | −1.64 | 1.0–15.0 | 0.9954 | 2.37 | −6.77 | 3.20 | −1.83 | 3.57 | −0.16 | −1.20 | −0.12 | 0.275 | 0.847 | 1.000 | 0.851 ± 0.197 |
| 17 | Estrone | 0.00 | −2.49 | 10.0–150.0 | 0.9955 | 3.14 | −6.34 | −1.61 | 0.78 | 3.45 | 2.98 | 3.37 | −3.25 | 1.475 | 9.087 | 10.000 | 9.15 ± 1.125 |
| 18 | 17 Alpha Ethinyl estradiol | 0.00 | −3.77 | 20.0–300.0 | 0.9894 | 6.21 | −13.62 | 0.00 | −2.12 | 5.00 | −0.91 | 3.43 | 1.75 | 2.641 | 8.439 | 10.000 | 8.437 ± 1.969 |
| 19 | Androstenedione | 0.00 | −3.43 | 0.2–3.0 | 0.9935 | 4.00 | −9.75 | −0.62 | −0.07 | 2.67 | 5.52 | −0.75 | −2.87 | 0.043 | 0.186 | 0.200 | 0.186 ± 0.032 |
| 20 | 17 Alpha estradiol | 0.00 | 8.85 | 10.0–150.0 | 0.9911 | −0.20 | −1.81 | 6.76 | −0.86 | −5.36 | 3.37 | −2.04 | 0.08 | 1.055 | 8.264 | 10.000 | 8.306 ± 0.815 |
| 21 | Testosterone | 0.00 | 1.15 | 0.2–3.0 | 0.9921 | 4.33 | −10.50 | 1.11 | 2.00 | 3.64 | 3.00 | 2.31 | −4.12 | 0.074 | 0.206 | 0.200 | 0.208 ± 0.054 |
| 22 | Norgestrel | 0.00 | −1.51 | 1.0–15.0 | 0.9907 | 2.10 | −5.92 | −3.75 | 7.45 | 6.03 | 1.65 | −0.47 | −6.34 | 0.237 | 0.964 | 1.000 | 0.873 ± 0.172 |
| 23 | Progesterone | 0.00 | −1.42 | 0.2–3.0 | 0.9938 | 4.67 | −7.58 | −6.71 | −0.57 | −0.44 | 5.15 | 5.27 | −0.47 | 0.051 | 0.188 | 0.200 | 0.188 ± 0.037 |
| 24 | Androsterone | 0.00 | 2.23 | 1.0–15.0 | 0.9799 | 3.03 | −7.70 | 0.12 | 4.59 | 9.88 | −4.20 | −2.70 | 1.66 | 0.389 | 0.866 | 1.000 | 0.915 ± 0.056 |
| 25 | Mestranol | 0.00 | −3.89 | 20.0–300.0 | 0.9875 | 7.35 | −13.85 | −3.68 | −3.41 | 1.81 | −2.93 | 7.22 | 5.90 | 6.199 | 16.494 | 20.000 | 16.834 ± 2.976 |
| 26 | Desogestrel | 0.58 | −6.99 | 0.2–3.0 | 0.9870 | 7.33 | −14.00 | −1.20 | −8.27 | −1.87 | −0.55 | 3.52 | 10.42 | 0.05 | 0.172 | 0.200 | 0.172 ± 0.037 |
| 27 | Beta Estradiol 3 benzoate | 0.00 | −10.92 | 1.0–15.0 | 0.9602 | 13.33 | 9.42 | −15.35 | −15.98 | −5.75 | 0.31 | 13.39 | 17.26 | 0.134 | 0.874 | 1.000 | 0.804 ± 0.101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karubothula, B.; Devireddy, C.; Shinde, D.; Shukoor, R.; Hafez, G.; Tadala, R.; Salem, S.B.; Elamin, W.; Brudecki, G. Advancing Wastewater Surveillance: Development of High-Throughput Green Robotic SPE-UPLC-MS/MS Workflow for Monitoring of 27 Steroids and Hormones. Appl. Sci. 2025, 15, 10012. https://doi.org/10.3390/app151810012
Karubothula B, Devireddy C, Shinde D, Shukoor R, Hafez G, Tadala R, Salem SB, Elamin W, Brudecki G. Advancing Wastewater Surveillance: Development of High-Throughput Green Robotic SPE-UPLC-MS/MS Workflow for Monitoring of 27 Steroids and Hormones. Applied Sciences. 2025; 15(18):10012. https://doi.org/10.3390/app151810012
Chicago/Turabian StyleKarubothula, Bhaskar, Chaitanya Devireddy, Dnyaneshwar Shinde, Rizwan Shukoor, Ghenwa Hafez, Raghu Tadala, Samara Bin Salem, Wael Elamin, and Grzegorz Brudecki. 2025. "Advancing Wastewater Surveillance: Development of High-Throughput Green Robotic SPE-UPLC-MS/MS Workflow for Monitoring of 27 Steroids and Hormones" Applied Sciences 15, no. 18: 10012. https://doi.org/10.3390/app151810012
APA StyleKarubothula, B., Devireddy, C., Shinde, D., Shukoor, R., Hafez, G., Tadala, R., Salem, S. B., Elamin, W., & Brudecki, G. (2025). Advancing Wastewater Surveillance: Development of High-Throughput Green Robotic SPE-UPLC-MS/MS Workflow for Monitoring of 27 Steroids and Hormones. Applied Sciences, 15(18), 10012. https://doi.org/10.3390/app151810012








