Abstract
A reliable synthetic method based on the already known Blanc–Quelet methodology has been developed for upgrading bio-based phenols into valuable electrophilic mono-, di-, and trifunctional benzyl chlorides. These compounds show significant potential as building blocks for polymer production and the synthesis of specialty chemicals. As an example of their applicability, their direct interaction with formamide has been evaluated, obtaining an effective transformation towards the corresponding N-formylamides. These compounds represent versatile synthetic precursors to a variety of functionalized targets.
1. Introduction
Naturally occurring phenols represent a valuable source of aryl-cored compounds with significant potential for a wide range of applications in the chemical industry. Abundant lignocellulosic biomass serves as a major source of these phenolic compounds, which can be obtained through various thermochemical, chemical and/or biotechnological processes [1,2,3]. This renewable feedstock holds great promise for the synthesis of high-value building blocks, polymers, and other valuable materials [1,2,4], which take advantage of the structural stiffness of aromatic core. Biorenewable plant phenolics represent key intermediates with which to develop high-performance multifunctional polymers or composite materials [5].
However, to fully exploit their potential and integrate them into sustainable industrial practices, it is essential to develop efficient and environmentally friendly upgrading processes [1,5,6,7]. Such processes should focus on maximizing yield, minimizing waste, and using green chemistry principles to reduce energy consumption and reliance on non-renewable resources.
The presence of oxygen atoms bonded to the benzenoid nucleus in phenolic compounds imparts electron-rich characteristics, making them particularly reactive toward electrophilic aromatic substitution (Ar-SE) reactions. Aldehydes—either naturally occurring or readily synthesized from (bio)alcohols—act as effective electrophiles and are therefore expected to react efficiently with bio-sourced arenes. The reaction between aldehydes and phenolics is well established and frequently results in the coupling of two arene units, as the initial substitution product can further alkylate a second arene molecule. On this basis, phenolic resins or benzoxazines can be easily obtained by reaction with formaldehyde [5]. Moreover, other alkylants such as alkyl- or acyl-halides can transform phenolics into vinylic monomers by forming new ether or ester bonds with the phenolic oxygen atom, respectively. Epoxy monomers are also obtainable through interaction with epichlorohydrin, while polyurethanes can be prepared with isocyanates [5]. Some other specific processes such as bioconversion [8], continuous flow methods [9], or reductive catalytic technologies [10] have also been described.
Going back to the interaction between phenolics and formaldehyde, it is interesting to note that the outcome of the process can be profoundly changed by the presence of chloride ions. First described by Grassi-Cristaldi and Maselli in 1898 [11] and later expanded upon by Stephen [12], Blanc [13], and Quelet [14], this effect is attributed to a strong interaction between the halide and the aldehyde, leading to the formation of a chlorinated intermediate—such as a Cl,O-acetal [15]—which acts as the true electrophilic species in the reaction with the aromatic partner.
Despite the long history of the Blanc–Quelet reaction [16], its application to bio-based phenols has been the subject of only a few studies [17]. In this work, we investigated the application of the Blanc–Quelet procedure for the selective chloromethylation of representative bio-based phenols, including vanillin, salicylaldehyde, piceol, eugenol, guaiacol, and p-cresol. The resulting benzyl halides (2a–f, Figure 1) serve as valuable building blocks for the production of bio-based materials and chemicals [18,19,20]. Diarylmethanes, novolac-, resol-, or reosol-type oligomers and resins are some of the valuable derivatives obtainable from benzyl chlorides.
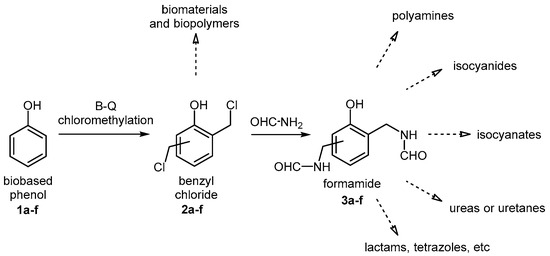
Figure 1.
Upgrading of biobased phenols through the Blanc–Quelet reaction and amidation of chloromethyl derivatives (2a–f) to versatile formamides (3a–f).
One interesting application of compounds 2a–f, which exemplify an extension of their synthetic versatility, is their amidation to form benzyl formamides (3a–f, Figure 1). These molecular targets exhibit good potential as a source of many other functionalized building blocks and materials. Formamides are highly versatile intermediates, serving as precursors to a variety of functional groups and scaffolds. They can be converted into amines [21,22,23] or isocyanides [24,25,26] and may also be transformed into isocyanates [27], which in turn enable the synthesis of urethanes [28], ureas [29,30], and thiocarbamates [31]. Additionally, specialized methods have been developed for coupling formamides with various synthons to generate valuable structures such as aminonitriles [32], tetrazoles [33], and lactams [34], which are often key structural motifs in low-molecular-weight active pharmaceutical ingredients.
This synthetic approach represents a novel and practical strategy for upgrading bio-based phenols into valuable chemical building blocks.
2. Materials and Methods
2.1. General Information
Reagents and solvents were reagent-grade products and were used without further purification. Vanillin (1a, Carlo Erba, Cornaredo (MI), Italy), salicylaldehyde (1b, Merk Life Science S.r.l., Milano, Italy), eugenol (BLD Pharmatech GmbH, Reinbek, Germany), guaiacol (Merk Life Science S.r.l., Milano, Italy), piceol (BLDpharm), p-cresol (TCI Europe N.V.), paraformaldehyde (PF, Merk Life Science S.r.l., Milano, Italy) and triethylamine (TEA, Merck Life Science S.r.l., Milano, Italy) were used as purchased.
Elemental analyses were performed with a FLASH 2000 CHNS/O elemental analyzer (Thermo Fisher Scientific Inc., Rodano (MI), Italy). 1H NMR and 13C NMR spectra were recorded on an Avance 600 spectrometer (Bruker, Billerica, MA, USA). Melting points were recorded on a MP30 melting point system (Mettler-Toledo S.p.A., Milano, Italy) with a 3 °C/min heating ramp. Most of the chloromethylated compounds decomposed upon heating before reaching their melting point. This behavior is attributed to their self-curing phenol-formaldehyde monomeric nature.
The compounds 2a [35], 2b [36], 2c [37], and 2f [38] are known and already characterized.
2.2. Chloromethylation
General procedure. The phenolic substrate (10 mmol) was mixed with PF (from 12 to 42 mmol, depending on the number of chloromethyl groups incorporated into the product) and aqueous 37% HCl (from 6.5 to 25.0 mL). Concentrated H2SO4 (96%, up to 1.0 mL) was also added in the case of electron-poor phenolics. In some cases, dichloromethane (DCM, up to 2.0 mL) was added as a co-solvent. The reaction mixture was stirred at a specific temperature (from 25 °C to 100 °C) for several hours until the disappearance of the substrate, which was evaluated through TLC. Filtration on a Gooch glass filter (P3) followed by aqueous 10% NaHCO3 (10 mL) and water washing (2 × 10 mL) allowed for the isolation of the product. Alternatively, extraction with DCM (3 × 10 mL) and washing of the organic phase with aq. 10% NaHCO3 (2 × 10 mL) and brine (2 × 10 mL) allowed for the isolation of the product after vacuum-drying the organic phase.
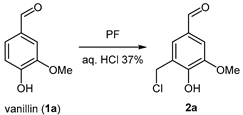
3-(Chloromethyl)-4-hydroxy-5-methoxybenzaldehyde (2a). Following the general procedure, vanillin (1a, 10 mmol) was stirred with PF (12 mmol), conc. HCl (37%, 6.5 mL), and conc. H2SO4 (0.5 mL) at 60 °C for 6 h. Filtration on a P3 glass filter allowed the isolation of the product as a brown solid in a 70% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 9.84 (s, 1H, Ar-C(=O) H), 7.53 (d, 1H, Ar–H), 7.40 (d, 1H, Ar–H), 6.43 (s, 1H, Ar–OH), 4.71 (s, 2H, Ar–CH2-Cl), 3.99 (s, 3H, O-CH3). 13C NMR (400 MHz, CDCl3) δ(ppm): 197.1 (C=O), 151.2 (C-Ar), 148.0 (C-Ar), 125.3 (C-Ar), 123.3 (C-Ar), 119.6 (C-Ar), 113.2 (C-Ar), 56.0 (OCH3), 40.2 (-CH2-). EA found: C 53.71%, H 4.69%; calcd for (C9H9O3Cl): C 53.88%, H 4.52%. Mp 116–119 °C (Lit.: 120–122 °C).
3,5-Bis(chloromethyl)-2-hydroxybenzaldehyde (2b). Following the general procedure, salicylaldehyde (1b, 10 mmol) was stirred with PF (25 mmol), conc. HCl (37%, 17 mL), and conc. H2SO4 (1.0 mL) at 70 °C for 20 h. After extraction with DCM, the product was obtained as a pale pink solid, in 99% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 11.49 (s, 1H, Ar-OH), 9.91 (s, 1H, Ar-C(=O)H), 7.68 (d, 1H, Ar–H), 7.59 (d, 1H, Ar–H), 4.68 (s, 2H, Ar-oCH2-Cl), 4.59 (s, 2H, Ar-pCH2-Cl). 13C NMR (600 MHz, CDCl3) δ(ppm): 196.3 (C=O), 159.4 (C-Ar), 137.8 (C-Ar), 134.1 (C-Ar), 129.3 (C-Ar), 126.8 (C-Ar), 120.4 (C-Ar), 45.1 (-CH2-), 39.6 (-CH2-). EA found: C 49.28%, H 3.71%; calcd for (C9H8O2Cl2): C 49.34%, H 3.68%. Mp 90–92 °C
1-(3-(Chloromethyl)-4-hydroxyphenyl)ethan-1-one (2c). Following the general procedure, piceol (1c, 10 mmol) was stirred with PF (12 mmol), conc. HCl (37%, 6.5 mL), and conc. H2SO4 (0.25 mL) at 40 °C for 6 h. Filtration on a P3 glass filter allowed the isolation of the product as a pink solid in 68% yield. 1H NMR (600 MHz, CDCl3) δ(ppm): 7.95 (d, 1H, Ar-H), 7.88 (dd, 8.4 Hz, 1H, Ar-H), 6.91 (d, 1H, 8.4 Hz, Ar-H), 5.81 (br s, 1H, Ar-OH), 4.70 (s, 2H, Ar-CH2-Cl), 2.57 (s, 3H, Ar-C(O)CH3). 13C NMR (400 MHz, CDCl3) δ(ppm): 196.6 (C=O), 158.5 (C-Ar), 131.6 (C-Ar), 131.4 (C-Ar), 130.8 (C-Ar), 123.9 (C-Ar), 116.3 (C-Ar), 41.8 (-CH2-), 26.6 (-CH3). EA found: C 58.60%, H 4.87%; calcd for (C9H9O2Cl): C 58.55%, H 4.91%. Mp n.d. (decomposed).
1-(3,5-Bis(chloromethyl)-4-hydroxyphenyl)ethan-1-one (2c2). Following the general procedure, piceol (1c, 10 mmol) was stirred with PF (25 mmol), conc. HCl (37%, 13 mL), and conc. H2SO4 (0.5 mL) at 100 °C for 6 h. Filtration on a P3 glass filter allowed the isolation of the product as a pink solid in 60% yield. 1H NMR (600 MHz, CDCl3) δ(ppm): 7.93 (s, 2H, Ar-H), 6.28 (s, 1H, Ar-OH), 4.72 (s, 4H, Ar-CH2-Cl), 2.58 (s, 3H, Ar-C(=O)-CH3). 13C NMR (600 MHz, CDCl3) δ(ppm): 196.2 (C=O), 157.5 (C-Ar), 131.8 (C-Ar), 130.5 (C-Ar), 124.7 (C-Ar), 42.0 (-CH2-), 26.5 (CH3). EA found: C 51.67%, H 4.15%; calcd for (C10H10O2Cl2): C 51.53%, H 4.32%. Mp n.d. (decomposed).
4-Allyl-2,3-bis(chloromethyl)-6-methoxyphenol (2d). Following the general procedure, eugenol (10 mmol) was stirred with PF (32 mmol), conc. HCl (37%, 20 mL), and DCM (2.0 mL) at 25 °C for 4 h. Following extraction with DCM, a highly impure material was obtained, contaminated by unreacted eugenol and diverse polycondensation byproducts. Several purification methods were attempted to obtain a purer product, without complete success (see Figure S4). The product was finally obtained as a viscous black liquid in 50% yield. 1H NMR (600 MHz, CDCl3) δ(ppm): 6.68 (s, 1H, Ar-H), 5.98 (m, 1H, -CH=C), 5.30 (s, 1H, Ar-OH), 5.10 (m, 1H, -C=CH2), 5.02 (m, 1H, -C=CH2) 4.87 (s, 2H, Ar-CH2-Cl), 4.74 (s, 2H, Ar-CH2-Cl), 3.89 (s, 2H, Ar-OCH3), 3.46 (s, 2H, Ar-CH2-C=C). 13C NMR (400 MHz, CDCl3) δ(ppm): 150.3 (C-Ar), 141.6 (C-Ar), 136.0 (-CH=), 129.8 (C-Ar), 127.6 (C-Ar), 125.9 (C-Ar), 116.3 (=CH2), 113.4 (C-Ar), 56.1 (OCH3), 39.9 (-CH2-), 38.5 (-CH2-), 37.7 (-CH2-). EA found: C 55.35%, H 5.22%; calcd for (C12H14O2Cl2): C 55.19%, H 5.40%.
2,3,4-Tris (chloromethyl)-6-methoxyphenol (2e). Following the general procedure, guaiacol acetate (1e, 10 mmol) was stirred with PF (42 mmol), conc. HCl (37%, 25 mL), and DCM (2.5 mL) at 40 °C for 6 h. After extraction with DCM, the product was obtained as a dark brown solid in 60% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 6.87 (s, H, Ar-H), 6.03 (s, H, Ar-OH), 4.85 (s, 2H, Ar-CH2-Cl), 4.84 (s, 2H, Ar-CH2-Cl), 4.68 (s, 2H, Ar-CH2-Cl), 3.93 (s, 3H, Ar-OCH3). 13C-NMR (600 MHz, CDCl3) δ(ppm): 146.8 (C-Ar) 145.1 (C-Ar), 128.9 (C-Ar), 128.7 (C-Ar), 123.4 (C-Ar), 112.9 (C-Ar), 56.3 (OCH3), 43.9 (CH2); 38.7 (CH2), 36.5 (CH2). EA found: C 44.65%, H 3.95%; calcd for (C10H11O2Cl3): C 44.56%, H 4.11%. Mp n.d. (decomposed).
2,6-Bis (chloromethyl)-4-methylphenol (2f). Following the general procedure, p-cresol (1f, 10 mmol) was stirred with PF (22 mmol), conc. HCl (37%, 5.4 mL), and DCM (0.5 mL) at 40 °C for 6 h. After extraction with DCM, the product was obtained as a white waxy solid, in 76% yield. 1H NMR (600 MHz, CDCl3) δ(ppm): 7.09 (s, 2H, mAr-H), 5.55 (br s, 1H, Ar-OH), 4.66 (s, 4H, Ar-CH2-Cl), 2.28 (s, 3H, Ar-CH3). 13C-NMR (400 MHz, CDCl3) δ(ppm): 36.5 (CH2), 56.0 (OCH3), 113.3 (C-Ar). EA found: C 52.88%, H 5.07%; calcd for (C9H10OCl2): C 52.71%, H 4.91%. Mp n.d. (decomposed).
2.3. Amidation
General procedure. The chloromethyl substrate (10 mmol), dissolved in tetrahydrofuran (THF, 10 mL), was added dropwise to formamide (FA) (20, 40, or 60 mmol, for one, two, or three chloromethyl groups, respectively), which was kept at 60 °C inside a round-bottom flask. The reaction mixture was then stirred at reflux for 3 h. Once cooled to RT, the mixture was washed with brine (3 × 10 mL) and dried over sodium sulfate. The product was obtained by vacuum-drying.
N-(5-formyl-2-hydroxy-3-methoxybenzyl)formamide (3a): Following the general procedure, 2a (10 mmol) and FA (20 mmol) afforded the monoamide product as a brown thick liquid, in 99% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 9.84 (s, 1H, Ar-C(=O)H), 8.17 (s, 1H, N-C(=O)H), 7.51 (d, 1H, Ar–H), 7.42 (d, 1H, Ar–H), 6.47 (br s, 1H, Ar–OH), 5.33 (s, 2H, Ar–CH2-N), 3.99 (s, 3H, O-CH3). EA found: C 57.54%, H 5.13%, N 6.83%; calcd for (C10H11NO4): C 57.41%, H 5.30%, N 6.70%.
N,N′-((5-formyl-4-hydroxy-1,3-phenylene)bis(methylene))diformamide (3b): Following the general procedure, 2b (10 mmol) and FA (40 mmol) afforded the diamide product as a pale yellow thick liquid, in 98% yield. 1H NMR (600 MHz, CDCl3) δ(ppm): 11.43 (s, 1H, Ar-OH), 9.92 (s, 1H, Ar-C(=O)H), 8.17 (s, H, o-N-C(=O)H), 8.13 (s, H, pC-N-C(=O)H), 7.65 (d, 1H, Ar–H), 7.61 (d, 1H, Ar–H), 5.31 (s, 2H, Ar–oCH2-N), 5.19 (s, 2H, Ar–pCH2-N). EA found: C 56.05%, H 5.21%, N 11.99%; calcd for (C11H12N2O4): C 55.93%, H 5.12%, N 11.86%.
N-(5-acetyl-2-hydroxybenzyl)formamide (3c): Following the general procedure, 2c (10 mmol) and FA (20 mmol) afforded the monoamide product as a pale pink thick liquid, in 97% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 8.16 (s, H, N-C(=O)H), 7.96 (d, 1H, Ar-H), 7.89 (dd, 1H, Ar-H), 6.97 (d, 1H, Ar-H), 5.87 (br s, 1H, Ar-OH), 5.27 (s, 2H, Ar-CH2-N), 2.56 (s, 3H, CH3-(C=O)-Ar). EA found: C 62.36%, H 5.65%, N 7.40%; calcd for (C10H11NO3): C 62.17%, H 5.74%, N 7.25%.
N,N′-((5-acetyl-2-hydroxy-1,3-phenylene)bis(methylene))diformamide (3c2): Following the general procedure, 2c2 (10 mmol) and FA (40 mmol) afforded the monoamide product as a pale pink thick liquid, in 92% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 8.16 (s, 2H, N-C(=O)H), 7.99 (s, 2H, Ar-H), 5.75 (br s, 1H, Ar-OH), 5.28 (d, 2H, Ar-CH2-N), 2.57 (s, 3H, CH3-(C=O)-Ar). EA found: C 57.43%, H 5.78%, N 11.02%; calcd for (C12H14N2O4): C 57.59%, H 5.64%, N 11.19%.
N,N′-((6-allyl-3-hydroxy-4-methoxy-1,2-phenylene)bis(methylene))diformamide (3d): Following the general procedure, 2d (10 mmol) and FA (20 mmol) afforded the diamide product as a dark thick liquid, in 94% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 8.10 (s, 1H, N-C(=O)H), 8.06 (s, 1H, N-C(=O)H), 6.75 (s, 1H, Ar-H), 5.91 (m, 1H, C-CH=C), 5.43 (s, 2H, Ar-CH2-N), 5.28 (s, 2H, Ar-CH2-N), 5.07 (dd, 1H, -C=CH2), 4.97 (dd, 1H, -C=CH2), 3.91 (s, 3H, Ar-OCH3), 3.44 (dt, 2H, Ar-CH2-C=C). EA found: C 61.03%, H 5.69%, N 9.96%; calcd for (C14H16N2O4): C 60.86%, H 5.84%, N 10.14%.
N,N′,N″-((4-hydroxy-5-methoxybenzene-1,2,3-triyl)tris(methylene))triformamide (3e). Following the general procedure, 2e (10 mmol) and FA (60 mmol) afforded the triamide product as a dark thick liquid, in 96% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 8.10 (s, H, N-C(=O)H), 8.09 (s, H, N-C(=O)H), 8.06 (s, H, N-C(=O)H), 6.98 (s, 1H, Ar-H), 6.14 (s, 1H, Ar-OH), 5.45 (s, 2H, Ar-CH2-N), 5.38 (s, 2H, Ar-CH2-N), 5.30 (s, 2H, Ar-CH2-N), 3.94 (s, 3H, Ar-OCH3). EA found: C 52.70%, H 5.73%, N 14.11%; calcd for (C13H17N3O5): C 52.88%, H 5.80%, N 14.23%.
N,N′-((2-hydroxy-5-methyl-1,3-phenylene)bis(methylene))diformamide (3f): Following the general procedure, 2f (10 mmol) and FA (40 mmol) afforded the diamide product as a whitish thick liquid, in 93% yield. 1H NMR (400 MHz, CDCl3) δ(ppm): 8.13 (s, 2H, N-C(=O)H), 7.70 (s, 1H, Ar-OH), 7.14 (s, 2H, Ar-H), 5.22 (s, 4H, Ar-CH2-Cl), 2.28 (s, 3H, Ar-CH3). EA found: C 59.60%, H 6.22%, N 12.58%; calcd for (C11H14N2O3): C 59.45%, H 6.35%, N 12.61%.
2.4. Other Synthetic Operations
Formamide (FA) was synthesized by means of a procedure from the literature [39], as follows. Ammonium formate (10 g, 158 mmol), placed in a round-bottom flask equipped with Dean–Stark apparatus on its top, was heated to 150 °C and then slowly to 180 °C. The volume of evolved water was used as a measure of process conversion. The heating was stopped when 2.8 mL (156 mmol) of water was collected in the Dean–Stark trap. The obtained product was used without further purification.
Guaiacol acetate (1e) was synthesized by means of a procedure from the literature [40]. Guaiacol (2.20 mL, 20 mmol), Ac2O (2.85 mL, 25 mmol), and TEA (3.48 mL, 25 mmol) were mixed at 0 °C, then the reaction mixture was brought to 60 °C and left stirring for three hours. The reaction mixture was diluted with distilled water (10 mmol) and left stirring vigorously for 10 min. The reaction mixture was diluted with ethyl acetate (20 mL), and the organic phase was washed with 2M aqueous H2SO4 (3 × 5 mL), 10% m/v aq. NaHCO3 solution (3 × 10 mL), and saturated NaCl aqueous solution (3 × 10 mL), before being anhydrified over MgSO4 and vacuum-dried. The product was then used without further purification as a pale yellow liquid. 1H NMR (400 MHz, CDCl3) δ(ppm): 7.20 (dq, 1.80 Hz, 7.50 Hz, 1H, Ar-H), 7.04 (dd, 1.68 Hz, 7.82 Hz, 1H, Ar-H), 6.96 (ddd, 1.20 Hz, 8.17 Hz, 15.76 Hz, 2H, Ar-H), 3.83 (s, 3H, Ar-OCH3), 2.32 (s, 3H, -OC(=O)CH3). EA found: C 64.93% H 5.92%; calcd for (C9H10O3): C 65.05% H 6.07%.
3. Results and Discussion
The chemical upgrading of biomass-sourced phenolics is a promising approach to obtaining industrially relevant building blocks with enhanced properties. To minimize exposure to harmful chemicals during the chloromethylation process, paraformaldehyde (PF) was selected as the alkylating agent instead of 37% aqueous formaldehyde. This substitution also reduced the amount of water in the reaction medium, resulting in a faster reaction.
Vanillin (1a) was chosen as the initial starting material, as it is one of the main phenolic monomers obtainable through the oxidation of lignin [41]. Initial experiments were conducted at 25 °C using one molar equivalent of PF, along with a small amount of DCM to improve the uniformity of the reaction medium. A five- to eight-fold molar excess of hydrochloric acid relative to PF was found to be necessary to obtain a chloromethylated product free of alcohol byproducts. Initially, substrate conversion was limited, reaching only 51%, with the selective formation of the monoalkylated product 2a (Table 1, entry 1) [35].

Table 1.
Chloromethylation of vanillin *.
Many established Blanc–Quelet procedures employ a metal salt catalyst, in addition to 37% aqueous HCl, to promote the reaction. A typical example is ZnCl2, which is particularly effective for deactivated substrates [42]. However, in light of sustainability considerations, we opted for a metal-free approach. Concentrated H2SO4, a cost-effective and efficient alternative, was introduced from entry 2 onward. Its addition significantly enhanced the formation of 2a, with further improvements observed by extending the reaction time (entry 3) or increasing the temperature (entry 4). Subsequent investigations (entries 5–7) revealed that two molar equivalents of H2SO4 yielded the best results. Notably, omitting DCM (entries 5–7) led to a faster reaction and slightly improved yields in a shorter time frame. Under vigorous stirring, the chloromethylation of vanillin can be efficiently performed in water at 60 °C, achieving completion in just 6 h. No dichloromethylated products were detected in any of the experiments, even when increased amounts of PF were used, suggesting a specific deactivation of the positions ortho to the aldehyde group.
The amidation of 2a was subsequently investigated through the N-alkylation of formamide. When carried out in a small amount of THF under reflux conditions and using an excess of formamide, a clean and complete conversion to compound 3a was achieved (Figure 2).
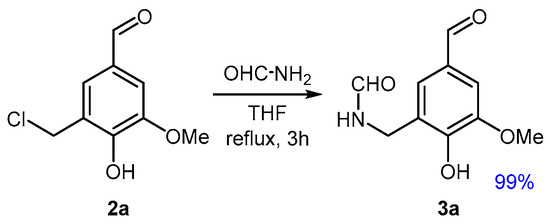
Figure 2.
Amidation of the chloromethylated derivative (2a) of vanillin (1a).
Continuing the investigation with salicylaldehyde (1b), which was selected as the second bio-based phenolic substrate, the application of the developed chloromethylation protocol resulted in incomplete conversions at 40 °C. This is likely due to the lower activation of the aromatic ring, attributed to the absence of the methoxy group present in vanillin. However, unlike the previous case, a mixture of mono- and dichloromethylated derivatives was obtained. Increasing the reaction temperature to 70 °C proved effective in achieving complete conversion and enhanced selectivity toward the dialkylated product 2b, a trend consistent with previous reports [36]. Some attempts to attain the selective preparation of a monoalkylated product by limiting the amount of PF were unsuccessful.
The converging directing effects of the substrate′s functional groups—ortho/para for the hydroxyl and meta for the aldehyde—facilitated electrophilic substitution at both positions with comparable speed (Figure 3).
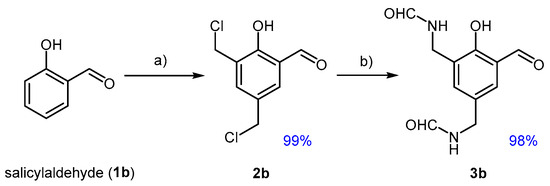
Figure 3.
Chloromethylation of salicylaldehyde towards dialkyl derivative 2b and conversion of this to the di-formamide 3b; (a) 1b (1.0 eq), PF (2.5 eq), HCl (16.0 eq), H2SO4 (0.9 eq), DCM, 70 °C, 16 h; (b) FA (4.0 eq), THF, reflux, 3 h.
The amidation of 2b cleanly afforded the diamide 3b under conditions analogous to those previously established.
Piceol, or 4-hydroxyacetophenone (1c), a bio-based phenolic compound found in the needles and mycorrhizal roots of Norway spruces [43], was selected as the third case study. Its substitution pattern suggests a reactivity profile similar to that of 1b, with a synergistic directing effect exerted by the carbonyl and hydroxyl functional groups. Indeed, its chloromethylation proceeded toward both mono- and dialkylation; however, unlike the previous case, both products could be obtained with excellent selectivity. This is likely due to the less deactivating nature of the ketone group in 1c, compared to the aldehyde in 1b, which allowed the formation of the monochloromethylated product 2c (Figure 4) at temperatures as low as 40 °C. In contrast, higher amounts of PF and higher temperatures were necessary to obtain the doubly alkylated product 2c2.
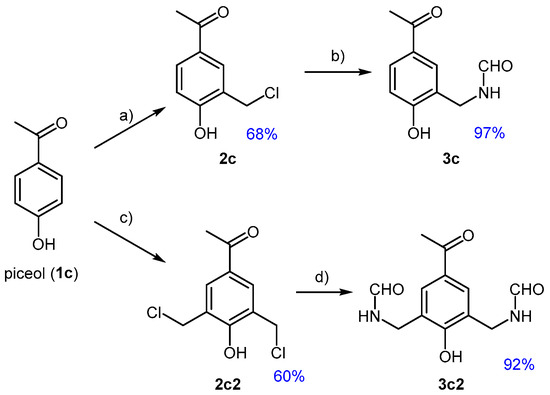
Figure 4.
Chloromethylation of piceol (1c) towards 2c or 2c2. Conversion of these benzyl chlorides to the corresponding formamides; (a) 1c, PF (1.2 eq), 37% HCl (8.0 eq), H2SO4 (0.5 eq) 40 °C, 6 h; (b) 2c, FA (2.0 eq), THF, reflux, 3 h; (c) 1c, PF (2.5 eq), 37% HCl (16.0 eq) H2SO4 (0.9 eq), 100 °C, 6 h; (d) 2c2, FA (4.0 eq), THF, reflux, 3 h.
Amidation of the monochloro-(2c) and dichloro-(2c2) compounds occurred with high selectivity, using a molar excess of formamide (2.0 and 4.0 eq., respectively) and operating in refluxing THF, in accordance with the previously established conditions.
When considering bio-based phenolics, it is important to note that most of them lack electron-withdrawing groups, making them more prone to Ar-SE reactions. On the one hand, this favors chloromethylation, which could proceed even without sulphuric acid activation. On the other hand, it increases the likelihood of undesired coupling between two arene units and PF. For instance, eugenol (1d) is known to undergo condensation with formaldehyde and phosphoric acid, resulting in the formation of a bis-eugenol adduct [44]. As expected, applying the developed chloromethylation conditions to 1d (6 h, 25 °C) led to a complex mixture of chloroalkylated products, alongside a minor amount of coupling products. Therefore, the removal of H2SO4 was considered, resulting in improved selectivity towards chloromethylation products. However, the limited water solubility of eugenol led to the formation of a heterogeneous system, characterized by a sticky organic phase, complicating process understanding. This resulted in yields that were highly sensitive to minor experimental variations, along with the production of undesired by-products. This issue prompted the addition of a small amount of DCM, which significantly improved the repeatability of the experiments and allowed better control over the reaction outcome. Similar to the reaction on 1b, selective monoalkylation of eugenol was not achieved, even with substoichiometric amounts of PF. Instead, selective dialkylation toward 2d was observed when more than 2.5 equivalents of aldehyde were used (Figure 5). Amidation of 2d, conducted under the same conditions as those for previous cases, proceeded without issues.
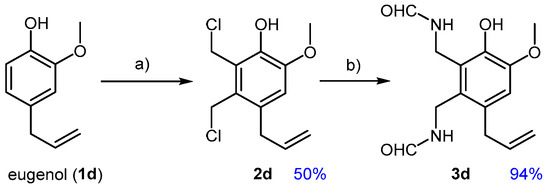
Figure 5.
Chloromethylation of eugenol (1d) towards dialkyl derivative 2d and conversion of this to the di-formamide 3d; (a) 1d, PF (3.2 eq), CH2Cl2, 37% HCl (16 eq), 25 °C, 4 h; (b) 2d, FA (4.0 eq), THF, reflux, 3 h.
Guaiacol is a major product of the hydrogenolysis of lignin [45] and wood pyrolysis [46]. Several studies have documented guaiacol′s tendency to undergo condensation with formaldehyde [47], a property often exploited for the production of bio-based thermosets [48]. Despite successfully applying a H2SO4-free protocol with eugenol, chloromethylation of guaiacol proved to be more challenging. The significant activation of the aromatic ring towards Ar-SE reactions, partially enhanced by its small steric hindrance and compounded with the peculiar water solubility, led to very unselective conversion. Mixtures of polyalkylated products, along with minor amounts of condensation byproducts, were consistently obtained, regardless of PF equivalents or the use of low temperatures (0 °C).
To partially deactivate the aromatic ring and adjust the substrate solubility away from the aqueous phase (similar to the behavior of eugenol), guaiacol was first converted to its O-acyl derivative using Ac2O (as detailed in the Materials and Methods section). The chloromethylation of this derivative was then investigated in the presence of DCM. Guaiacol acetate (1e) exhibited a strong tendency to form polyalkylated products, even when substoichiometric amounts of PF were used. However, to achieve better conversions of 1e, more than 2.5 equivalents of PF were required, resulting in the exclusive formation of the trialkylated product 2e. We soon realized that simultaneous deprotection of the acetate group occurred, which can be attributed to the aqueous acidic environment of the chloromethylation process. It is likely that the first alkylation step proceeded faster than the deprotection, generating a more apolar intermediate capable of undergoing further alkylations in the organic phase. As a result, complete conversion of 1e into 2e was achieved using 4.0 equivalents of PF (Figure 6).
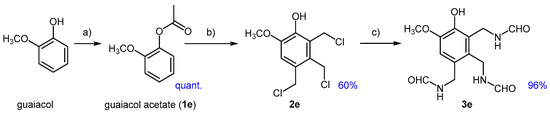
Figure 6.
Chloromethylation of guaiacol acetate (1e) towards trialkyl derivative 2e and conversion of this to the tri-formamide 3e; (a) guaiacol, Ac2O (1.3 eq), TEA (1.1 eq), 60 °C, 3 h; (b) 1e, PF (4.0 eq), CH2Cl2, 37% HCl (20 eq), 25 °C, 4 h; (c) 2e, FA (6.0 eq), THF, reflux, 3 h.
In addition to the expected activation of the para- positions relative to both the acetoxy and methoxy groups, the observed selectivity is explained by the significantly higher propensity for alkylation ortho- to the hydroxy group, rather than ortho- to the methoxy group. Indeed, no isomers of 2e were detected in any of the experiments. The first introduced para-CH2Cl substituent, exhibiting ′chameleon-like’ behavior [49], likely contributed to slowing the substitution at the position adjacent to the methoxy group. Transformation of 2e into the triamide 3e proceeded smoothly, following the same method applied to other benzyl chlorides.
p-Cresol (1f) is commonly extracted from coal tar but can also be obtained through biomass hydrothermal decomposition [50] and is present in tobacco smoke [51]. It is used in the production of antioxidants, such as di-tert-butylhydroxy toluene [52], and in the production of novolac resins where ortho-methylol groups are first introduced by reaction with formaldehyde under alkaline conditions [53]. As a ′moderately activated′ bio-based phenolic, and given that chloromethylation of this substrate has been scarcely investigated [38], we decided to include it within our studies. Despite its significantly lower activation compared to guaiacol, p-cresol showed a strong tendency for dichloromethylation, with high selectivity for product 2f (Figure 7).
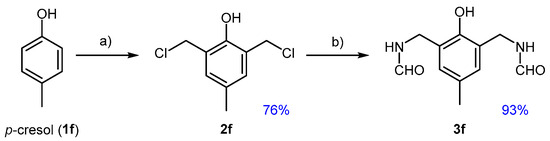
Figure 7.
Chloromethylation of p-cresol (1f) towards dialkyl derivative 2f and conversion of this to the di-formamide 3f; (a) 1f, PF (2.2 eq), CH2Cl2, 37% HCl (16 eq), 25 °C, 4 h; (b) 2f, FA (4.0 eq), THF, reflux, 3 h.
Several attempts to obtain monoalkylated products by reducing reaction time, temperature, or PF equivalents resulted in unsatisfactory selectivity and partial conversions of 1f. This behavior is similar to that of eugenol, not only in terms of its propensity for dialkylation but also in the beneficial effect of adding a small amount of DCM. This addition improved repeatability and reduced contamination from byproducts. The amidation of 2f successfully yielded the diamide 3f, following the same protocol developed for other benzyl chlorides.
4. Conclusions
In this paper, the chloromethylation of a series of bio-based phenolics was developed, demonstrating a practical and straightforward approach. Most chloromethylations on solid substrates can occur “on water”, i.e., without the need for organic solvents. Moreover, as most chloromethyl products are solid, their isolation can often be achieved through a simple (solventless) filtration. In other cases, such as liquid or substrates with low water solubility (e.g., 1d, 1e, 1f), the system benefits from the addition of small amount of DCM, resulting in satisfactory selectivity and repeatability.
The success of the chloromethylation and its selectivity depend on the modulation of the chloromethylating reagent (PF:37% HCl ~ 1:6) in relation to the substitution pattern of the substrate, which governs its activation towards Ar-SE processes. Phenolics devoid of deactivating groups (1d, 1e, 1f) were promptly chloromethylated by the standard PF:37% HCl (~1:6) reagent at 25 °C, although highly activated substrates often attain limited selectivity (as observed for 2d and 2e). Instead, deactivated phenolics, such as those containing one aldehyde (1a, 1b) or ketone (1c) function, require reagent activation, obtained through the addition of H2SO4 (~0.9 eq with respect to PF) and, usually, a higher operating temperature.
The number of chloromethyl groups incorporated into the product is primarily determined by the nature of the substrate. For instance, deactivated substrates such as 1a and 1c favor the formation of monoalkylated derivatives, whereas in other cases, such as 1b, high selectivity for the dialkylated product is observed. Piceol (1c), characterized by a balanced activation for Ar-SE, was the only case where a control of mono- vs. dialkylation was obtained by setting the process temperature. Most of the activated substrates investigated—such as 1d and 1f—exhibited a strong tendency toward dialkylation. However, substrates with excessive activation, such as guaiacol, led to complex mixtures containing significant amounts of condensation byproducts. A controlled process for the formation of the trialkylated product 2e was only achieved by reducing the reactivity of guaiacol through conversion into its O-acyl derivative (1e).
The developed method, working with low solvent amounts, is suitable for upscaling. Considering the case of piceol, the production of 2c was characterized by a volume-time output (VTO) of 83 L·h/kg, a PMI of around 8, and an E factor around 7. The obtained benzyl chlorides (2a–f), some of those previously not described, are characterized by a broad synthetic versatility and therefore represent valuable electrophilic building blocks for the chemical industry. Various applications in synthesis as well as in materials chemistry can be suggested based on the literature describing the reactivity of benzyl chlorides. As an application, their selective conversion to the corresponding N-formylamides (3a–f) was proven, through which the synthetic usefulness of the chloromethylated intermediates could be extended (see Figure 1 and the related description). Thanks to the high electrophilicity typical of these benzyl chlorides, the amidation process seamlessly proceeded to a clean and selective conversion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15147876/s1, Figure S1: 1H NMR of compound 2a, Figure S2: 1H NMR of compound 2b, Figure S3: 1H NMR of compound 2c, Figure S4: 1H NMR of compound 2c2, Figure S5: 1H NMR of compound 2d, Figure S6: 1H NMR of compound 2e, Figure S7: 1H NMR of compound 2f, Figure S8: 1H NMR of compound 3a, Figure S9: 1H NMR of compound 3b, Figure S10: 1H NMR of compound 3c, Figure S11: 1H NMR of compound 3c2, Figure S12: 1H NMR of compound 3d, Figure S13: 1H NMR of compound 3e, Figure S14: 1H NMR of compound 3f.
Author Contributions
Conceptualization, F.R. and N.P.; methodology, N.P., B.A.; software, A.U.; validation, N.P. and A.U.; investigation, M.G., M.F. and N.P.; resources, F.R.; data curation, N.P. and B.A.; writing—original draft preparation, N.P. and F.R.; writing—review and editing, F.R., N.P. and B.A.; visualization, M.F.; supervision, F.R.; project administration, N.P.; funding acquisition, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, W.; Ding, H.; Puglia, D.; Kenny, J.M.; Liu, T.; Guo, J.; Wang, Q.; Ou, R.; Xu, P.; Ma, P.; et al. Bio-Renewable Polymers Based on Lignin-Derived Phenol Monomers: Synthesis, Applications, and Perspectives. SusMat 2022, 2, 535–568. [Google Scholar] [CrossRef]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent Advances in the Catalytic Depolymerization of Lignin towards Phenolic Chemicals: A Review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef] [PubMed]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-Based Alternatives to Phenol and Formaldehyde for the Production of Resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Lochab, B.; Shukla, S.; Varma, I.K. Naturally Occurring Phenolic Sources: Monomers and Polymers. RSC Adv. 2014, 4, 21712–21752. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, J.; Yang, X.; Ke, Y.; Ou, R.; Wang, Y.; Madbouly, S.A.; Wang, Q. From Plant Phenols to Novel Bio-Based Polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Wan, J.; Zhao, J.; Zhang, X.; Fan, H.; Zhang, J.; Hu, D.; Jin, P.; Wang, D.-Y. Epoxy Thermosets and Materials Derived from Bio-Based Monomeric Phenols: Transformations and Performances. Prog. Polym. Sci. 2020, 108, 101287. [Google Scholar] [CrossRef]
- Martínková, L.; Grulich, M.; Pátek, M.; Křístková, B.; Winkler, M. Bio-Based Valorization of Lignin-Derived Phenolic Compounds: A Review. Biomolecules 2023, 13, 717. [Google Scholar] [CrossRef]
- Brown, E.E. Minireview: Recent Efforts toward Upgrading Lignin-Derived Phenols in Continuous Flow. J. Flow Chem. 2023, 13, 91–102. [Google Scholar] [CrossRef]
- Lang, M.; Li, H. Toward Mild Synthesis of Functional Chemicals from Lignin-Derived Phenolics via Emerging Catalytic Technology. Chem Catal. 2023, 3, 100609. [Google Scholar] [CrossRef]
- Grassi, G.; Maselli, C. Su alcuni derivati clorurati del triossimetilene. Gazz. Chim. Ital. 1898, 28, 477–500. [Google Scholar]
- Stephen, H.; Short, W.F.; Gladding, G. LIV.—The Introduction of the Chloromethyl Group into the Aromatic Nucleus. J. Chem. Soc. Trans. 1920, 117, 510–527. [Google Scholar] [CrossRef]
- Blanc, G.L. Sur La Préparation de Dérivés Chlorométhyléniques Aromatiques. Bull. Soc. Chim. 1923, 33, 313–319. [Google Scholar]
- Quelet, R. Preparation d’un Derive Chloro-Methyl Du Para-Bromo-Anisol (Methoxy-2 Bromo-2 α-Chlorotoluene). Compt. Rend. 1932, 195, 155. [Google Scholar]
- Olah, G.A.; Beal, D.A.; Olah, J.A. Aromatic Substitution. XXXVIII. Chloromethylation of Benzene and Alkylbenzenes with Bis(Chloromethyl)Ether, 1,4-Bis(Chloromethoxy)Butane, 1-Chloro-4-Chloromethoxybutane, and Formaldehyde Derivatives. J. Org. Chem. 1976, 41, 1627–1631. [Google Scholar] [CrossRef]
- Moulay, S. Towards Halomethylated Benzene-Bearing Monomeric and Polymeric Substrates. Des. Monomers Polym. 2011, 14, 179–220. [Google Scholar] [CrossRef]
- Kadwa, E.; Friedrich, H.B.; Bala, M.D. Structural Identification of Products from the Chloromethylation of Salicylaldehyde. Synlett 2018, 30, 44–48. [Google Scholar] [CrossRef]
- Jin, H.; Chen, X.; Qian, C.; Ge, X.; Zhou, S. Transition-Metal-Free, General Construction of Thioamides from Chlorohydrocarbon, Amide and Elemental Sulfur. Eur. J. Org. Chem. 2021, 2021, 3403–3406. [Google Scholar] [CrossRef]
- Roncaglia, F.; Ughetti, A.; Porcelli, N.; Anderlini, B.; Severini, A.; Rigamonti, L. Light on the Sustainable Preparation of Aryl-Cored Dibromides. Beilstein J. Org. Chem. 2024, 20, 1076–1087. [Google Scholar] [CrossRef]
- Zhu, L.; Cheng, P.; Xiao, Z.; Lu, C.; Li, B.; Jiang, X.; Shen, Z.; Qian, N.; Zhong, W.; He, Y. Incorporation of Phenolic Skeleton into Imidazolium Ionic Polymers as Recyclable Catalysts for Efficient Fixation of CO2 into Cyclic Carbonates. Chem. Eng. J. 2024, 481, 148359. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Alawaed, A.A.; Singh, A. Titanium-Mediated Reduction of Carboxamides to Amines with Borane–Ammonia. Molecules 2023, 28, 4575. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, C.; Wen, D.; Zheng, Q.; Tu, B.; Tu, T. Nickel-Catalyzed Amination of Aryl Chlorides with Amides. Org. Lett. 2021, 23, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Rauch, M.; Kumar, A.; Leitus, G.; Ben-David, Y.; Milstein, D. Selective Room-Temperature Hydrogenation of Amides to Amines and Alcohols Catalyzed by a Ruthenium Pincer Complex and Mechanistic Insight. ACS Catal. 2020, 10, 5511–5515. [Google Scholar] [CrossRef]
- Waibel, K.A.; Nickisch, R.; Möhl, N.; Seim, R.; Meier, M.A.R. A More Sustainable and Highly Practicable Synthesis of Aliphatic Isocyanides. Green Chem. 2020, 22, 933–941. [Google Scholar] [CrossRef]
- Patil, P.; Ahmadian-Moghaddam, M.; Dömling, A. Isocyanide 2.0. Green Chem. 2020, 22, 6902–6911. [Google Scholar] [CrossRef]
- Cerra, B.; Blondeau, C.; Cannalire, R.; Giustiniano, M.; Shandiz, S.T.; Gioiello, A. Isocyanide Chemistry Enabled by Continuous Flow Technology. React. Chem. Eng. 2023, 8, 656–660. [Google Scholar] [CrossRef]
- Bruffaerts, J.; von Wolff, N.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Formamides as Isocyanate Surrogates: A Mechanistically Driven Approach to the Development of Atom-Efficient, Selective Catalytic Syntheses of Ureas, Carbamates, and Heterocycles. J. Am. Chem. Soc. 2019, 141, 16486–16493. [Google Scholar] [CrossRef]
- Townsend, T.M.; Bernskoetter, W.H.; Hazari, N.; Mercado, B.Q. Dehydrogenative Synthesis of Carbamates from Formamides and Alcohols Using a Pincer-Supported Iron Catalyst. ACS Catal. 2021, 11, 10614–10624. [Google Scholar] [CrossRef]
- Owen, A.E.; Preiss, A.; McLuskie, A.; Gao, C.; Peters, G.; Bühl, M.; Kumar, A. Manganese-Catalyzed Dehydrogenative Synthesis of Urea Derivatives and Polyureas. ACS Catal. 2022, 12, 6923–6933. [Google Scholar] [CrossRef]
- Langsted, C.R.; Paulson, S.W.; Bomann, B.H.; Suhail, S.; Aguirre, J.A.; Saumer, E.J.; Baclasky, A.R.; Salmon, K.H.; Law, A.C.; Farmer, R.J.; et al. Isocyanate-Free Synthesis of Ureas and Polyureas via Ruthenium Catalyzed Dehydrogenation of Amines and Formamides. J. Appl. Polym. Sci. 2022, 139, 52088. [Google Scholar] [CrossRef]
- Waibel, K.A.; Barther, D.; Malliaridou, T.; Moatsou, D.; Meier, M.A.R. One-Pot Synthesis of Thiocarbamates. Eur. J. Org. Chem. 2021, 2021, 4508–4516. [Google Scholar] [CrossRef]
- Yan, F.; Huang, Z.; Du, C.-X.; Bai, J.-F.; Li, Y. Iron-Catalyzed Reductive Strecker Reaction. J. Catal. 2021, 395, 188–194. [Google Scholar] [CrossRef]
- Ishihara, K.; Ishihara, K.; Tanaka, Y.; Shioiri, T.; Matsugi, M. Practical Synthesis of Tetrazoles from Amides and Phosphorazidates in the Presence of Aromatic Bases. Tetrahedron 2022, 108, 132642. [Google Scholar] [CrossRef]
- Li, H.; Wu, H.; Zhang, H.; Su, Y.; Yang, S.; Hensen, E.J.M. A Facile Direct Route to N-(Un)Substituted Lactams by Cycloamination of Oxocarboxylic Acids without External Hydrogen. ChemSusChem 2019, 12, 3778–3784. [Google Scholar] [CrossRef]
- Profft, E.; Kŕause, W. Über Die Chlormethylierung Des O- Und Novo-vanillins Und Die Gewinnung von 4-Hydroxy-5-alkoxy-isophthalaldehyden. Arch. Pharm. 1965, 298, 148–162. [Google Scholar] [CrossRef]
- Wang, Q.; Wilson, C.; Blake, A.J.; Collinson, S.R.; Tasker, P.A.; Schröder, M. The One-Pot Halomethylation of 5-Substituted Salicylaldehydes as Convenient Precursors for the Preparation of Heteroditopic Ligands for the Binding of Metal Salts. Tetrahedron Lett. 2006, 47, 8983–8987. [Google Scholar] [CrossRef]
- Harenberg, J.H.; Reddy Annapureddy, R.; Karaghiosoff, K.; Knochel, P. Continuous Flow Preparation of Benzylic Sodium Organometallics. Angew. Chem. Int. Ed. 2022, 61, e202203807. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lu, M.; Ge, Q.; Cheng Wang, P.; Zhang, S.B.; Lu, T.T. An Inexpensive and Convenient for Chloromethylation of Aromatic Hydrocarbons by Phase Transfer Catalysis in Aqueous Media. J. Chil. Chem. Soc. 2010, 55, 97–102. [Google Scholar] [CrossRef]
- Pietrucci, F.; Saitta, A.M. Formamide Reaction Network in Gas Phase and Solution via a Unified Theoretical Approach: Toward a Reconciliation of Different Prebiotic Scenarios. Proc. Natl. Acad. Sci. USA 2015, 112, 15030–15035. [Google Scholar] [CrossRef]
- Takashima, Y.; Isogawa, Y.; Tsuboi, A.; Ogawa, N.; Kobayashi, Y. Synthesis of a TNF Inhibitor, Flurbiprofen and an i-Pr Analogue in Enantioenriched Forms by Copper-Catalyzed Propargylic Substitution with Grignard Reagents. Org. Biomol. Chem. 2021, 19, 9906–9909. [Google Scholar] [CrossRef]
- Popescu, A.E.P.; Torralba, J.; Bonet, J.; Llorens, J. Vanillin Production from Lignin: Rigorous Process Simulation Results for Ethyl Acetate versus Aliphatic-Alcohol-Specific Process Designs. Clean. Eng. Technol. 2021, 4, 100133. [Google Scholar] [CrossRef]
- Fuson, R.C.; McKeever, C.H. Chloromethylation of Aromatic Compounds. In Organic Reactions; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 63–90. ISBN 978-0-471-26418-7. [Google Scholar]
- Løkke, H. Picein and Piceol Concentrations in Norway Spruce. Ecotoxicol. Environ. Saf. 1990, 19, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, L.; Zhang, Y.; Liu, Q.; Ru, C.; Zhang, W.; Zhao, C. Novel Biobased Epoxy Resin Thermosets Derived from Eugenol and Vanillin. Polym. Degrad. Stab. 2019, 160, 45–52. [Google Scholar] [CrossRef]
- Midhun Kumar, M.; Gurrala, L.; Paek, C.; Vinu, R. Selective Production of Guaiacol from Lignin via Catalytic Transfer Hydrogenolysis Using Ru-Cu/Zirconia. Mol. Catal. 2022, 530, 112532. [Google Scholar] [CrossRef]
- Möck, D.M.J.; Riegert, C.; Radtke, S.; Appelt, J. Process Optimization and Extraction of Acids, Syringols, Guaiacols, Phenols and Ketones from Beech Wood Slow Pyrolysis Liquids with Supercritical Carbon Dioxide at Different Densities. J. Supercrit. Fluids 2023, 199, 105937. [Google Scholar] [CrossRef]
- Enjoji, M.; Yamamoto, A.; Shibata, M. Wood-Derived Phenol Novolaks and Their Wood/Epoxy Biocomposites. J. Appl. Polym. Sci. 2015, 132, 41347. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Kotzebue, L.R.V.; Ribeiro, F.W.M.; Mota, B.C.; Zampieri, D.; Mazzetto, S.E.; Ishida, H.; Lomonaco, D. Microwave-Assisted Solvent-Free Synthesis of Novel Benzoxazines: A Faster and Environmentally Friendly Route to the Development of Bio-Based Thermosetting Resins. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3534–3544. [Google Scholar] [CrossRef]
- Bruckner, R.; Harmata, M. Kinetic Aspects of Ar-SE Reactions: Reactivity and Regioselectivity in Reactions of Electrophiles with Substituted Benzenes. In Organic Mechanism—Reactions, Stereochemistry and Synthesis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 201–257. ISBN 978-3-642-03650-7. [Google Scholar]
- Nonaka, H.; Funaoka, M. Decomposition Characteristics of Softwood Lignophenol under Hydrothermal Conditions. Biomass Bioenergy 2011, 35, 1607–1611. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; Van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous Compounds in Tobacco Smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Kondamudi, K.; Elavarasan, P.; Dyson, P.J.; Upadhyayula, S. Alkylation of p-Cresol with Tert-Butyl Alcohol Using Benign Bronsted Acidic Ionic Liquid Catalyst. J. Mol. Catal. Chem. 2010, 321, 34–41. [Google Scholar] [CrossRef]
- Yang, J.; Cong, J.; Cong, P.; Yu, S. Synthesis of 2,6-Dihydroxymethyl-4-Methyl Phenol and Its Application in Novolac Resins. J. Photopolym. Sci. Technol. 2014, 27, 545–551. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).