In Vitro Photoprotective and Skin Aging-Related Enzyme In-Hibitory Activities of Cylindrospermum alatosporum (NR125682) and Loriellopsis cavernicola (NR117881) Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Total Sulfhydryl Content
2.2.2. In Vitro Anti-Aging Enzyme Assays
Collagenase Inhibitory Assay
Elastase Inhibitory Assay
Hyaluronidase Inhibitory Assay
Tyrosinase Inhibitory Assay
2.2.3. In Vitro Photoprotective Activity
In Vitro UVB Photoprotection
In Vitro UVA Photoprotection
2.2.4. Statistical Analysis
3. Results
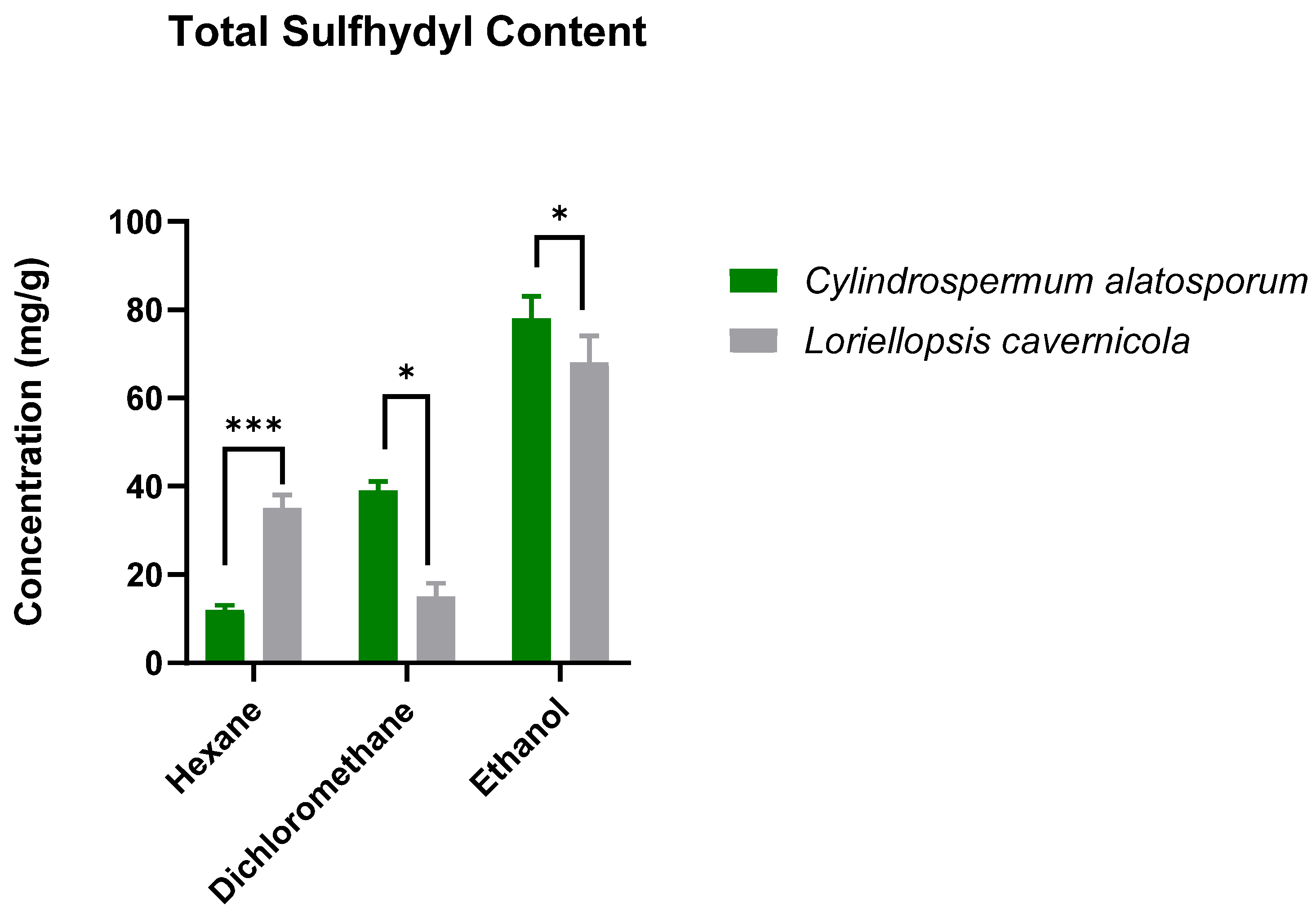
3.1. Total Sulfhydryl Content
3.2. Enzyme-Inhibitory Activity of the Extracts
3.2.1. Inhibitory Effect on Collagenase and Elastase
3.2.2. Inhibitory Effect on Hyaluronidase and Tyrosinase
3.3. In Vitro Photoprotective Activity
3.3.1. In Vitro UVB Photoprotection
3.3.2. In Vitro UVA Photoprotection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| UVR | Ultraviolet radiation |
| ROS | Reactive oxygen species |
| MMP | Matrix metalloproteinase |
| FALGPA | N-(3-[2-Furyl] acryloyl)-Leu-Gly-Pro-Ala |
| SANA | N-succinyl-Ala-Ala-Ala-p-nitroanilide |
| DOPA | 3,4-dihydroxyphenylalanine |
References
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.I.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V. Promising natural products in new drug design, development, and therapy for skin disorders: An overview of scientific evidence and understanding their mechanism of action. Drug Des. Dev. Ther. 2023, 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.; Liceaga, A. Identification of chia seed (Salvia hispanica L.) peptides with enzyme inhibition activity towards skin-aging enzymes. Amino Acids 2020, 52, 1149–1159. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin ageing: Pathophysiology and current market treatment approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef]
- Mohiuddin, A.K. Skin aging & modern age anti-aging strategies. Int. J. Clin. Dermatol. Res 2019, 7, 209–240. [Google Scholar]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent advances in herbal-derived products with skin anti-aging properties and cosmetic applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef]
- Sriram, R.; Gopal, V. Aging Skin and Natural Bioactives that Impede Cutaneous Aging: A Narrative Review. Indian J. Dermatol. 2023, 68, 414–424. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory molecules associated with ultraviolet radiation-mediated skin aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Fisher, J.F.; Mobashery, S. β-Lactams against the Fortress of the Gram-Positive Staphylococcus aureus Bacterium. Chem. Rev. 2020, 121, 3412–3463. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic ingredients as emerging pollutants of environmental and health concern. A mini-review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaur, R.; Bansal, A.; Kapur, S.; Sundaram, S. Chapter 8—Biotechnological exploitation of cyanobacteria and microalgae for bioactive compounds. In Biotechnological Production of Bioactive Compounds; Verma, M.L., Chandel, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–259. [Google Scholar]
- Orfanoudaki, M.; Hartmann, A.; Karsten, U.; Ganzera, M. Chemical profiling of mycosporine-like amino acids in twenty-three red algal species. J. Phycol. 2019, 55, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.-L.; Phang, S.-M. Bioactive compounds from microalgae and their potential applications as pharmaceuticals and nutraceuticals. In Grand Challenges in Algae Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 429–469. [Google Scholar]
- Khan, F.; Akhlaq, A.; Rasool, M.H.; Srinuanpan, S. Cyanobacterial Bioactive compounds: Synthesis, extraction, and applications. In Pharmaceutical and Nutraceutical Potential of Cyanobacteria; Springer: Berlin/Heidelberg, Germany, 2024; pp. 215–243. [Google Scholar]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria and microalgae bioactive compounds in skin-ageing: Potential to restore extracellular matrix filling and overcome hyperpigmentation. J. Enzym. Inhib. Med. Chem. 2021, 36, 1829–1838. [Google Scholar] [CrossRef]
- Nowruzi, B.; Sarvari, G.; Blanco, S. The cosmetic application of cyanobacterial secondary metabolites. Algal Res. 2020, 49, 101959. [Google Scholar] [CrossRef]
- Ručová, D.; Vilková, M.; Sovová, S.; Vargová, Z.; Kostecká, Z.; Frenák, R.; Routray, D.; Bačkor, M. Photoprotective and antioxidant properties of scytonemin isolated from Antarctic cyanobacterium Nostoc commune Vaucher ex Bornet & Flahault and its potential as sunscreen ingredient. J. Appl. Phycol. 2023, 35, 2839–2850. [Google Scholar] [CrossRef]
- Jerez-Martel, I.; García-Poza, S.; Rodríguez-Martel, G.; Rico, M.; Afonso-Olivares, C.; Gómez-Pinchetti, J.L. Phenolic profile and antioxidant activity of crude extracts from microalgae and cyanobacteria strains. J. Food Qual. 2017, 2017, 2924508. [Google Scholar] [CrossRef]
- Gao, X.; Jing, X.; Liu, X.; Lindblad, P. Biotechnological production of the sunscreen pigment scytonemin in cyanobacteria: Progress and strategy. Mar. Drugs 2021, 19, 129. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Vicente, A.A.; Vasconcelos, V. Cyanobacteria-based bioprocess for cosmetic products—Cyanobium sp. as a novel source of bioactive pigments. Phycology 2023, 3, 47–64. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wu, X.; Chen, L.; Li, X.; Wang, G. UV-B radiation increased the sensitivity of Tibetan soil cyanobacterium Loriellopsis cavernicola to the herbicide glyphosate. Chemosphere 2023, 335, 139141. [Google Scholar] [CrossRef]
- Ikhane, A.O.; Sithole, S.Z.; Cele, N.D.; Osunsanmi, F.O.; Mosa, R.A.; Opoku, A.R. In Vitro Antioxidant and In Silico Evaluation of the Anti-β-Lactamase Potential of the Extracts of Cylindrospermum alatosporum NR125682 and Loriellopsis cavenicola NR117881. Antioxidants 2024, 13, 608. [Google Scholar] [CrossRef]
- Cohn, V.H.; Lyle, J. A fluorometric assay for glutathione. Anal. Biochem. 1966, 14, 434–440. [Google Scholar] [CrossRef]
- Van Wart, H.E.; Steinbrink, D.R. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal. Biochem. 1981, 113, 356–365. [Google Scholar] [CrossRef]
- Kraunsoe, J.A.; Claridge, T.D.; Lowe, G. Inhibition of human leukocyte and porcine pancreatic elastase by homologues of bovine pancreatic trypsin inhibitor. Biochemistry 1996, 35, 9090–9096. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, J.C.; Bandi, A.K.R.; Hyun, C.-G.; Lee, N.H. Compounds with elastase inhibition and free radical scavenging activities from Callistemon lanceolatus. J. Med. Plants Res. 2009, 3, 914–920. [Google Scholar]
- Tadtong, S.; Amornrat, V.; Suwanna, V.; Sathaporn, N.; Sunit, S. Antityrosinase and antibacterial activities of mangosteen pericarp extract. J. Health Res. 2009, 23, 99–102. [Google Scholar]
- Mansur, J.d.S.; Breder, M.; Mansur, M.; Azulay, R.D. Determination of sun protection factor by spectrophotometry. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Detoni, C.B.; Souto, G.D.; Da Silva, A.L.M.; Pohlmann, A.R.; Guterres, S.S. Photostability and skin penetration of different e-resveratrol-loaded supramolecular structures. Photochem. Photobiol. 2012, 88, 913–921. [Google Scholar] [CrossRef]
- Schalka, S.; Reis, V.M.S.d. Sun protection factor: Meaning and controversies. An. Bras. Dermatol. 2011, 86, 507–515. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Beck, F.; Ilie, N. Antioxidants and collagen-crosslinking: Benefit on bond strength and clinical applicability. Materials 2020, 13, 5483. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef]
- Altyar, A.E.; Ashour, M.L.; Youssef, F.S. Premna odorata: Seasonal metabolic variation in the essential oil composition of its leaf and verification of its anti-ageing potential via in vitro assays and molecular modelling. Biomolecules 2020, 10, 879. [Google Scholar] [CrossRef]
- Bose, B.; Choudhury, H.; Tandon, P.; Kumaria, S. Studies on secondary metabolite profiling, anti-inflammatory potential, in vitro photoprotective and skin-aging related enzyme inhibitory activities of Malaxis acuminata, a threatened orchid of nutraceutical importance. J. Photochem. Photobiol. B Biol. 2017, 173, 686–695. [Google Scholar] [CrossRef]
- Chaiyana, W.; Chansakaow, S.; Intasai, N.; Kiattisin, K.; Lee, K.-H.; Lin, W.-C.; Lue, S.-C.; Leelapornpisid, P. Chemical constituents, antioxidant, anti-MMPs, and anti-hyaluronidase activities of Thunbergia laurifolia Lindl. leaf extracts for skin aging and skin damage prevention. Molecules 2020, 25, 1923. [Google Scholar] [CrossRef]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure–activity relationship. J. Enzym. Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef] [PubMed]
- Panzella, L.; Napolitano, A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: Recent advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- LaBerge, G.S.; Duvall, E.; Grasmick, Z.; Haedicke, K.; Galan, A.; Leverett, J.; Baswan, S.; Yim, S.; Pawelek, J. Recent advances in studies of skin color and skin cancer. Yale J. Biol. Med. 2020, 93, 69. [Google Scholar]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Prade, R.A.; Terenzi, H.F. Role of sulfhydryl compounds in the control of tyrosinase activity in Neurospora crassa. Biochem. Genet. 1982, 20, 1235–1243. [Google Scholar] [CrossRef]
- Svobodová, A.; Vostálová, J. Solar radiation induced skin damage: Review of protective and preventive options. Int. J. Radiat. Biol. 2010, 86, 999–1030. [Google Scholar] [CrossRef]
- Sutar, M.P.; Chaudhari, S.R. Screening of in vitro sun protection factor of some medicinal plant extracts by ultraviolet spectroscopy method. J. Appl. Biol. Biotechnol. 2020, 8, 48–53. [Google Scholar] [CrossRef]
- Nascimento, L.B.d.S.; Tattini, M. Beyond photoprotection: The multifarious roles of flavonoids in plant terrestrialization. Int. J. Mol. Sci. 2022, 23, 5284. [Google Scholar] [CrossRef]
- Nunes, A.R.; Vieira, Í.G.; Queiroz, D.B.; Leal, A.L.A.B.; Maia Morais, S.; Muniz, D.F.; Calixto-Junior, J.T.; Coutinho, H.D.M. Use of flavonoids and cinnamates, the main photoprotectors with natural origin. Adv. Pharmacol. Pharm. Sci. 2018, 2018, 5341487. [Google Scholar] [CrossRef] [PubMed]

| IC50 (µg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hexane | Dichloromethane | Ethanol | Standards | ||||||
| HA | HB | DA | DB | EA | EB | EC | UA | KA | |
| Collagenase | 50 ± 1.50 | 70 ± 3.44 ** | 70 ± 5.93 ** | 70 ± 6.23 ** | 50 ± 6.34 | 60 ± 5.34 ** | 50 ± 2.30 | N/D | N/D |
| Elastase | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| Hyaluronidase | 6 ± 0.27 | 6 ± 0.25 | 6 ± 0.55 | 6 ± 0.12 | 6 ± 0.45 | 6 ± 0.70 | N/D | N/D | N/D |
| Tyrosinase | N/D | N/D | N/D | N/D | N/D | N/D | N/D | N/D | 9 ± 0.50 |
| Concentration (µg/mL) | HA | HB | DA | DB | EA | EB | Protection Level UV-B Blocking |
|---|---|---|---|---|---|---|---|
| 120 | 11.65 ± 0.04 | 31.12 ± 0.36 | 11.25 ± 0.00 | 49.98 ± 0.70 | 17.14 ± 0.05 | 14.67 ± 0.72 | Low to moderate |
| 240 | 21.30 ± 0.28 | 31.92 ± 0.11 | 14.96 ± 0.26 | 51.74 ± 0.31 | 21.28 ± 0.36 | 22.93 ± 1.06 | Moderate |
| 480 | 39.04 ± 0.03 | 33.39 ± 0.74 | 20.58 ± 0.51 | 52.04 ± 0.02 | 22.88 ± 0.01 | 25.12 ± 0.00 | Moderate to high |
| 960 | 59.76 ± 0.01 | 49.3 ± 0.18 | 26.24 ± 0.36 | 78.96 ± 10.90 | 53.20 ± 0.70 | 27.30 ± 1.47 | Moderate to High |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithole, S.Z.; Ikhane, A.O.; Osunsanmi, F.O.; Mosa, R.A.; Opoku, A.R. In Vitro Photoprotective and Skin Aging-Related Enzyme In-Hibitory Activities of Cylindrospermum alatosporum (NR125682) and Loriellopsis cavernicola (NR117881) Extracts. Appl. Sci. 2025, 15, 9718. https://doi.org/10.3390/app15179718
Sithole SZ, Ikhane AO, Osunsanmi FO, Mosa RA, Opoku AR. In Vitro Photoprotective and Skin Aging-Related Enzyme In-Hibitory Activities of Cylindrospermum alatosporum (NR125682) and Loriellopsis cavernicola (NR117881) Extracts. Applied Sciences. 2025; 15(17):9718. https://doi.org/10.3390/app15179718
Chicago/Turabian StyleSithole, Siphesihle Z., Albert O. Ikhane, Foluso O. Osunsanmi, Rebamang A. Mosa, and Andrew R. Opoku. 2025. "In Vitro Photoprotective and Skin Aging-Related Enzyme In-Hibitory Activities of Cylindrospermum alatosporum (NR125682) and Loriellopsis cavernicola (NR117881) Extracts" Applied Sciences 15, no. 17: 9718. https://doi.org/10.3390/app15179718
APA StyleSithole, S. Z., Ikhane, A. O., Osunsanmi, F. O., Mosa, R. A., & Opoku, A. R. (2025). In Vitro Photoprotective and Skin Aging-Related Enzyme In-Hibitory Activities of Cylindrospermum alatosporum (NR125682) and Loriellopsis cavernicola (NR117881) Extracts. Applied Sciences, 15(17), 9718. https://doi.org/10.3390/app15179718







