Simulation Study on the Influence of Reactions Between Granitic Hot Dry Rock Minerals and Water on Rock Non-Closed Crack Compressive Shear Initiation
Abstract
1. Introduction
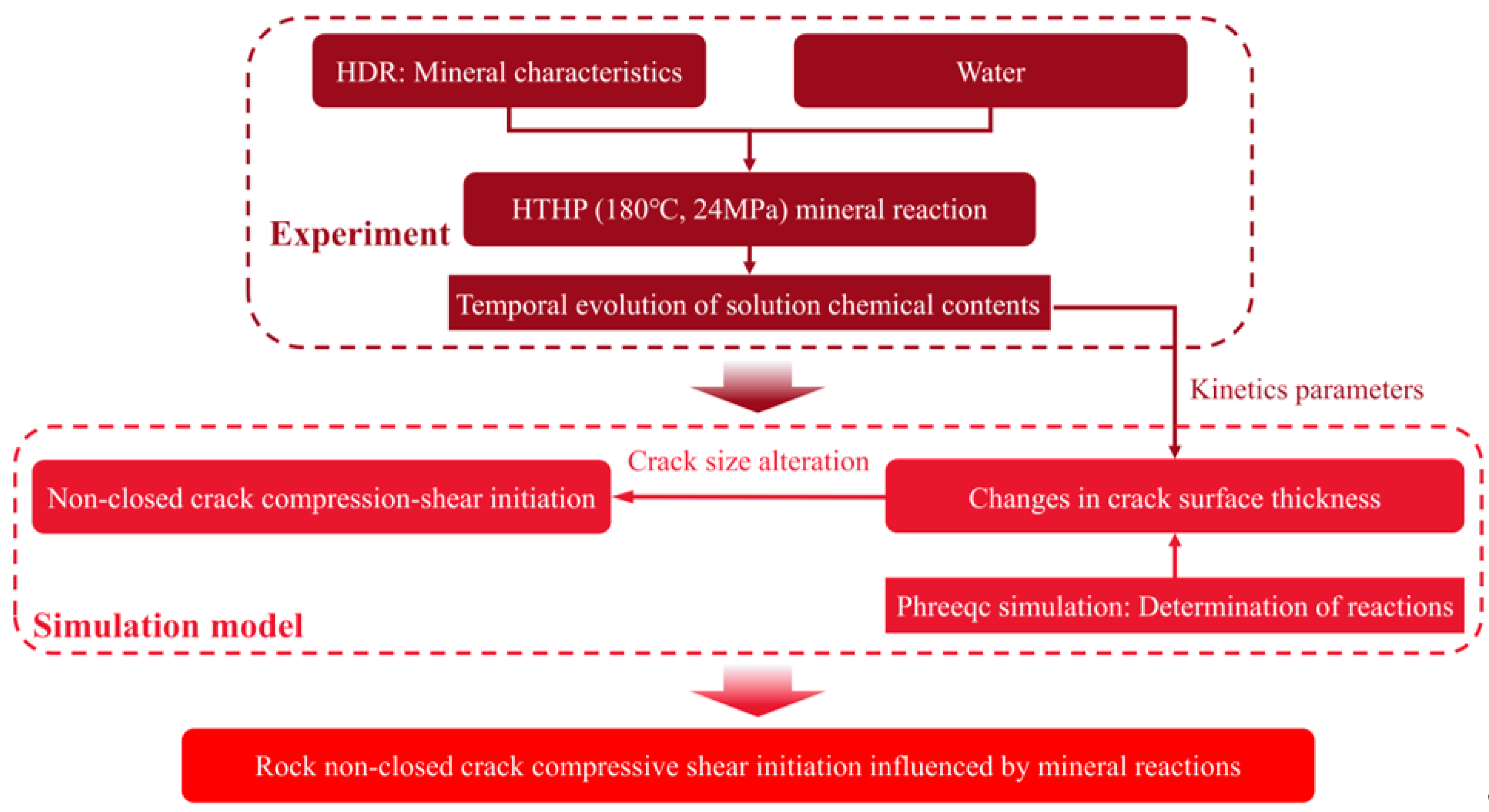
2. Materials and Methods
2.1. Materials
2.2. Tests and Experiments
2.2.1. Determination of the HDR Mineral Characteristics
2.2.2. HTHP Mineral Reaction Experiments
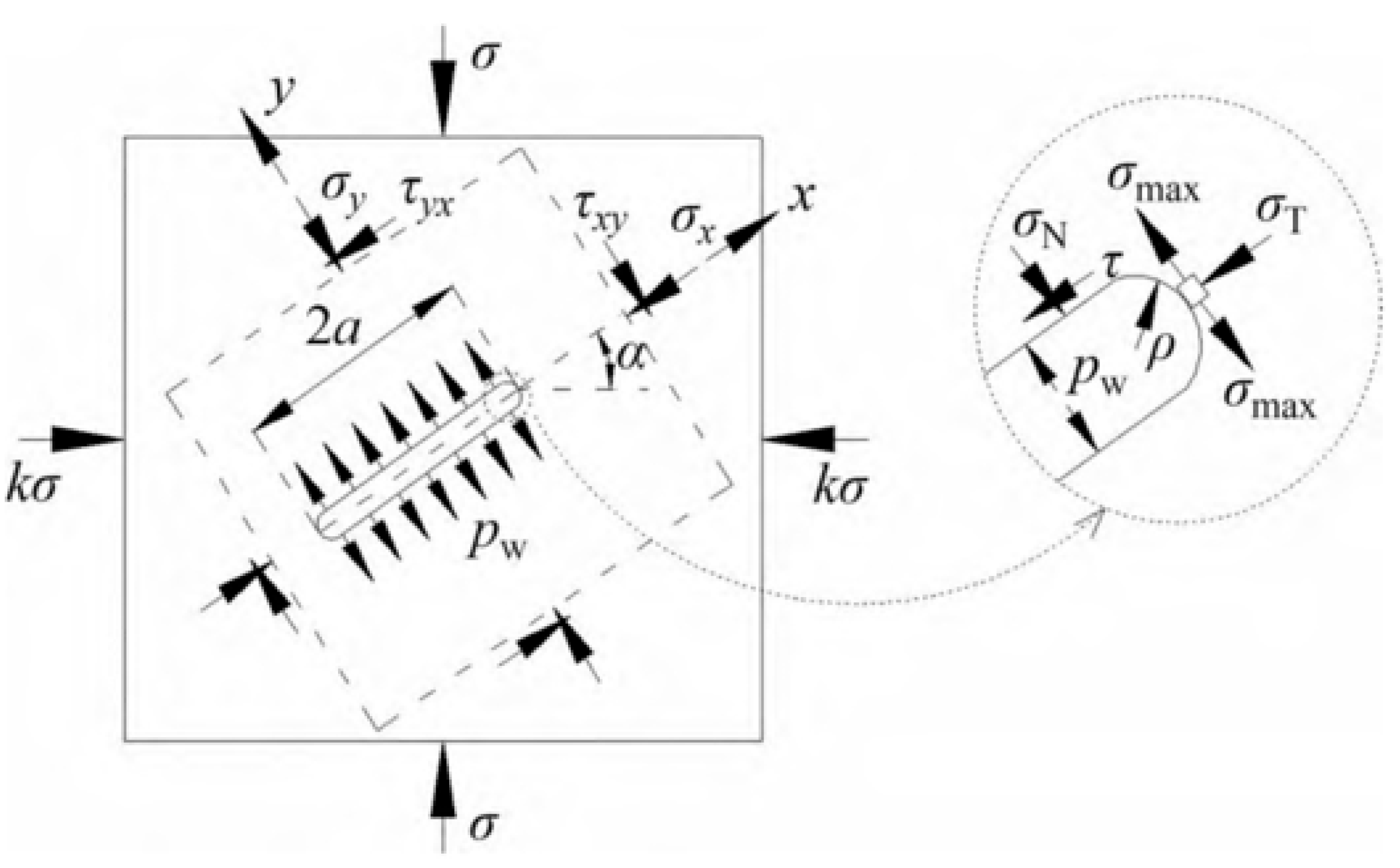
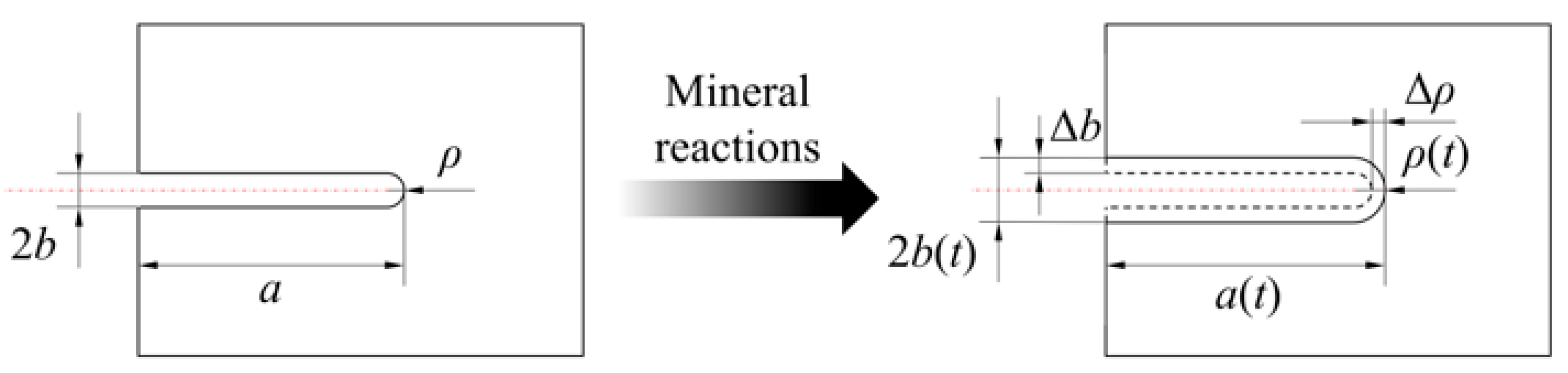
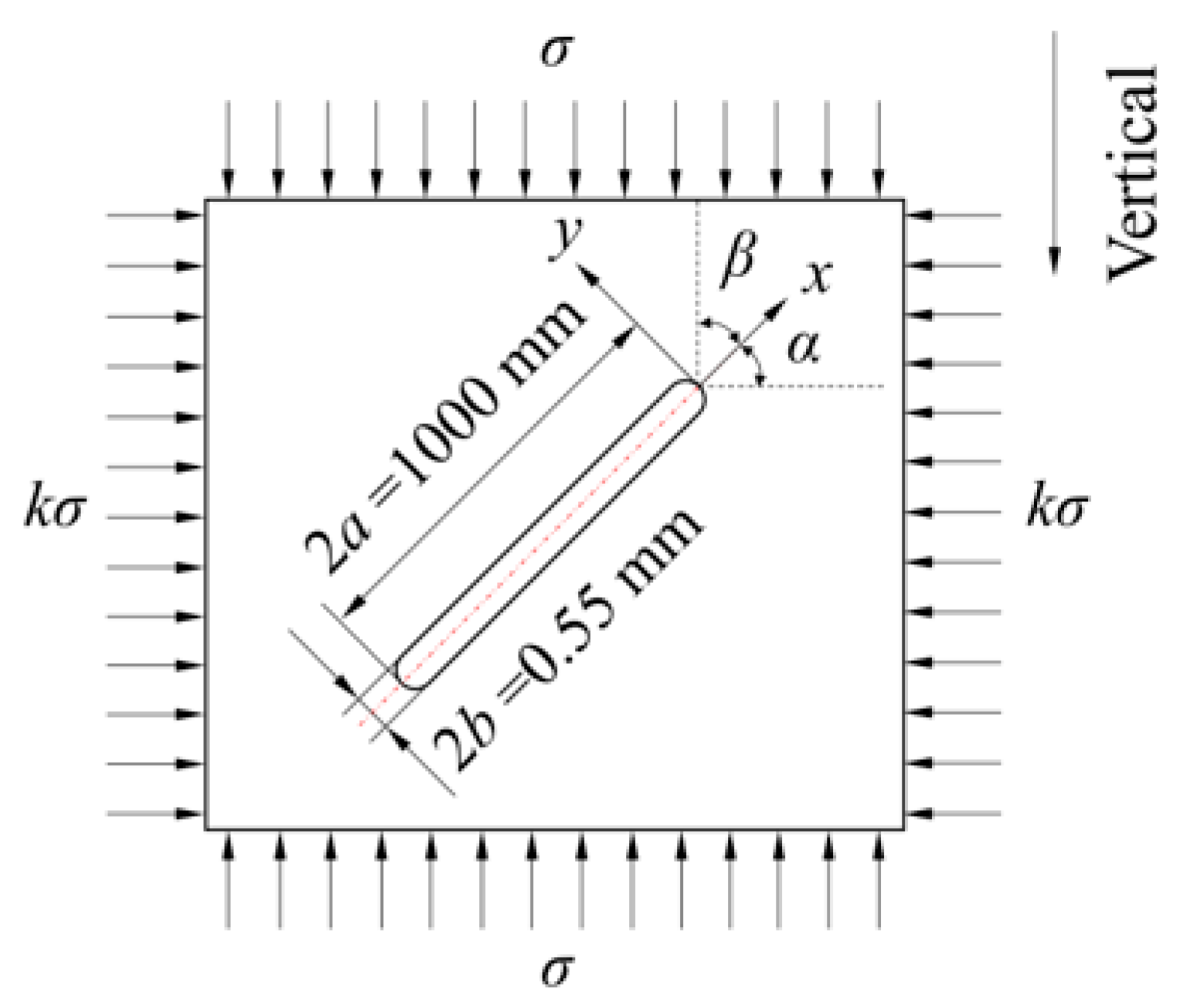
2.3. Model of Non-Closed Crack Compressive Shear Initiation Induced by Mineral Reactions
2.3.1. Determination of Mineral Reactions
2.3.2. Non-Closed Crack Compressive Shear Initiation Induced by Mineral Reactions
3. Results
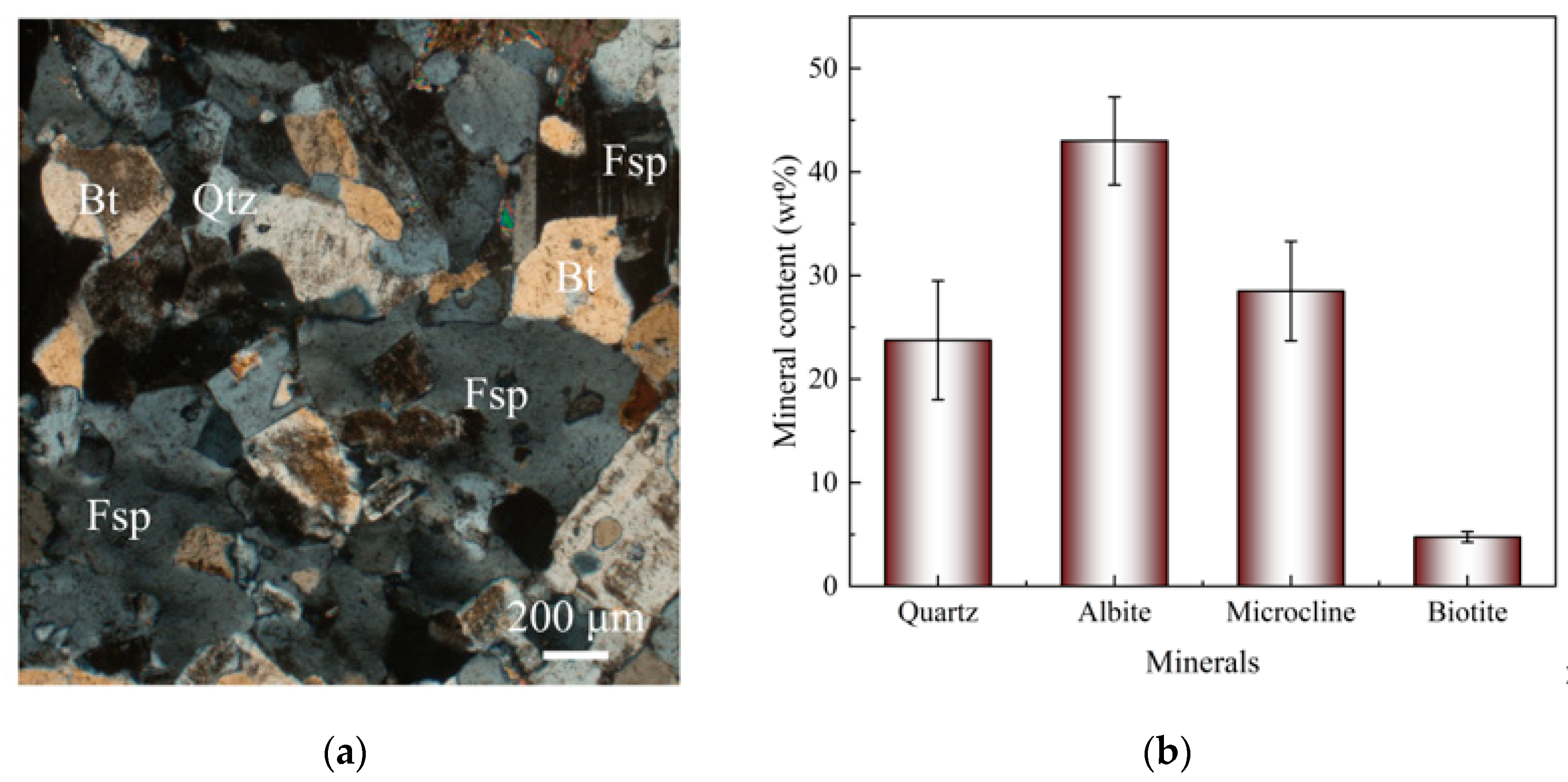
3.1. Mineral Characteristics of HDR
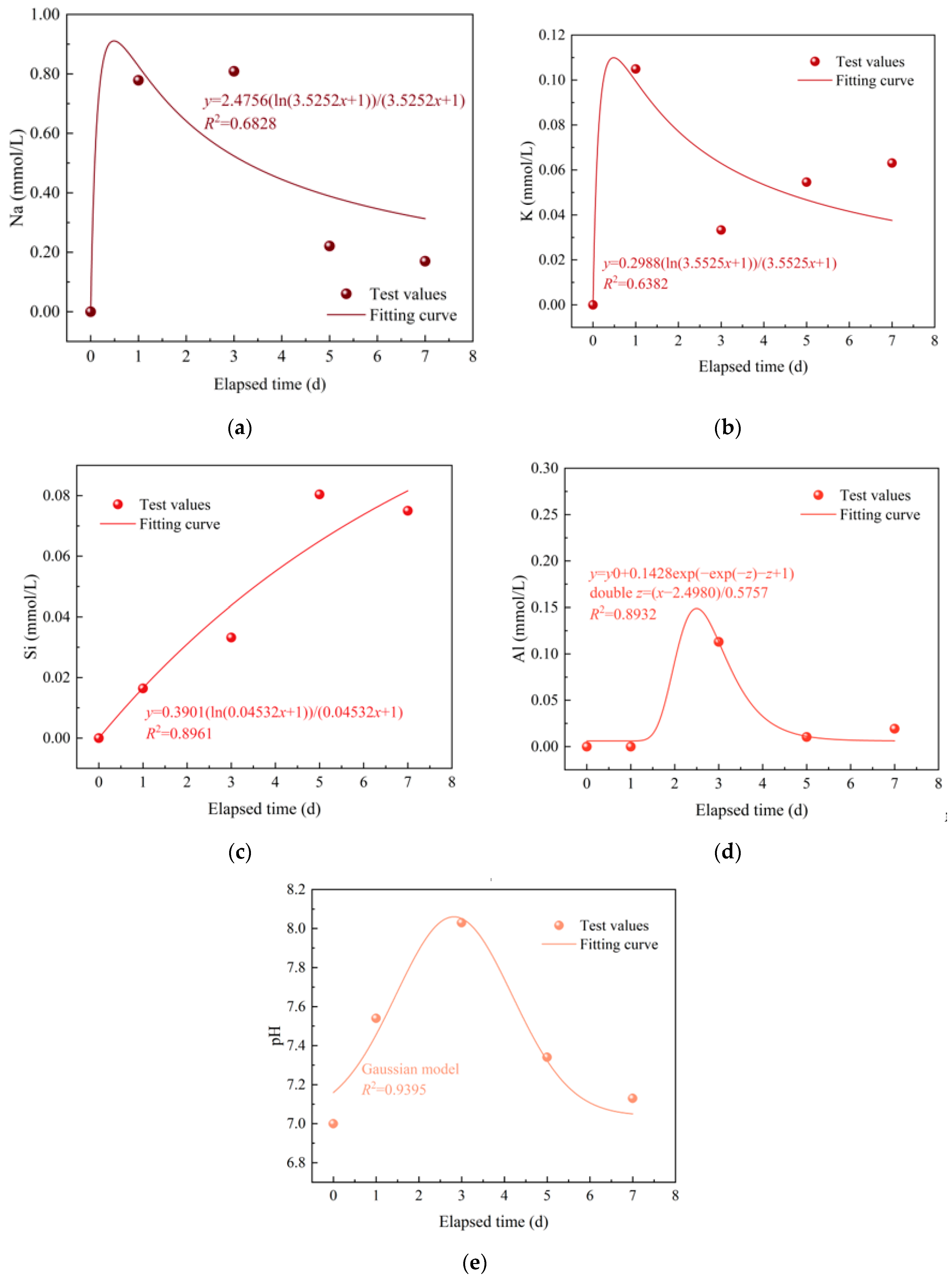
3.2. Temporal Evolution of the Solution Element Contents and pH
3.3. Simulation Results of Non-Closed Crack Compressive Shear Initiation Induced by Mineral Reactions
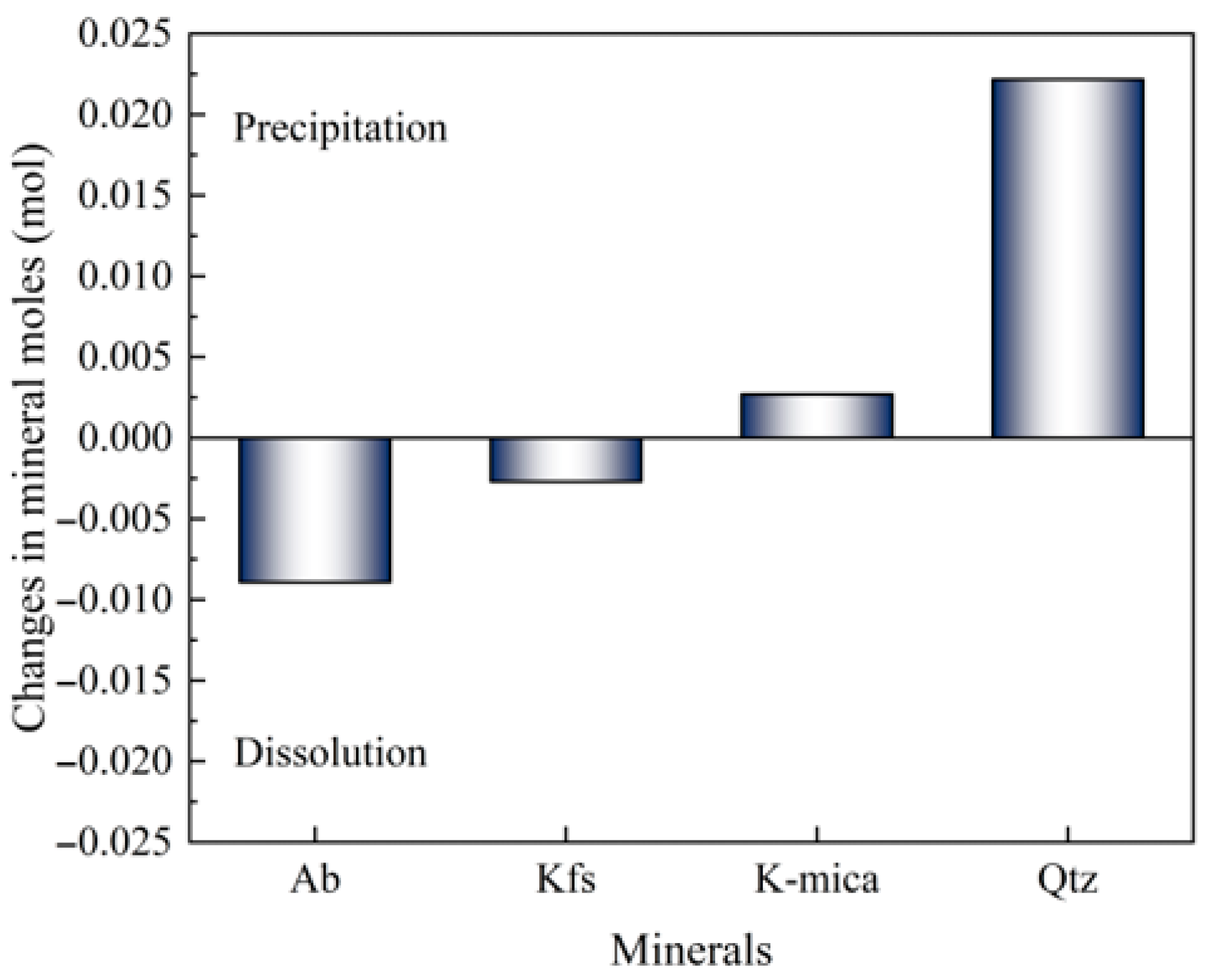
3.3.1. Simulation Results of Reaction Equilibrium
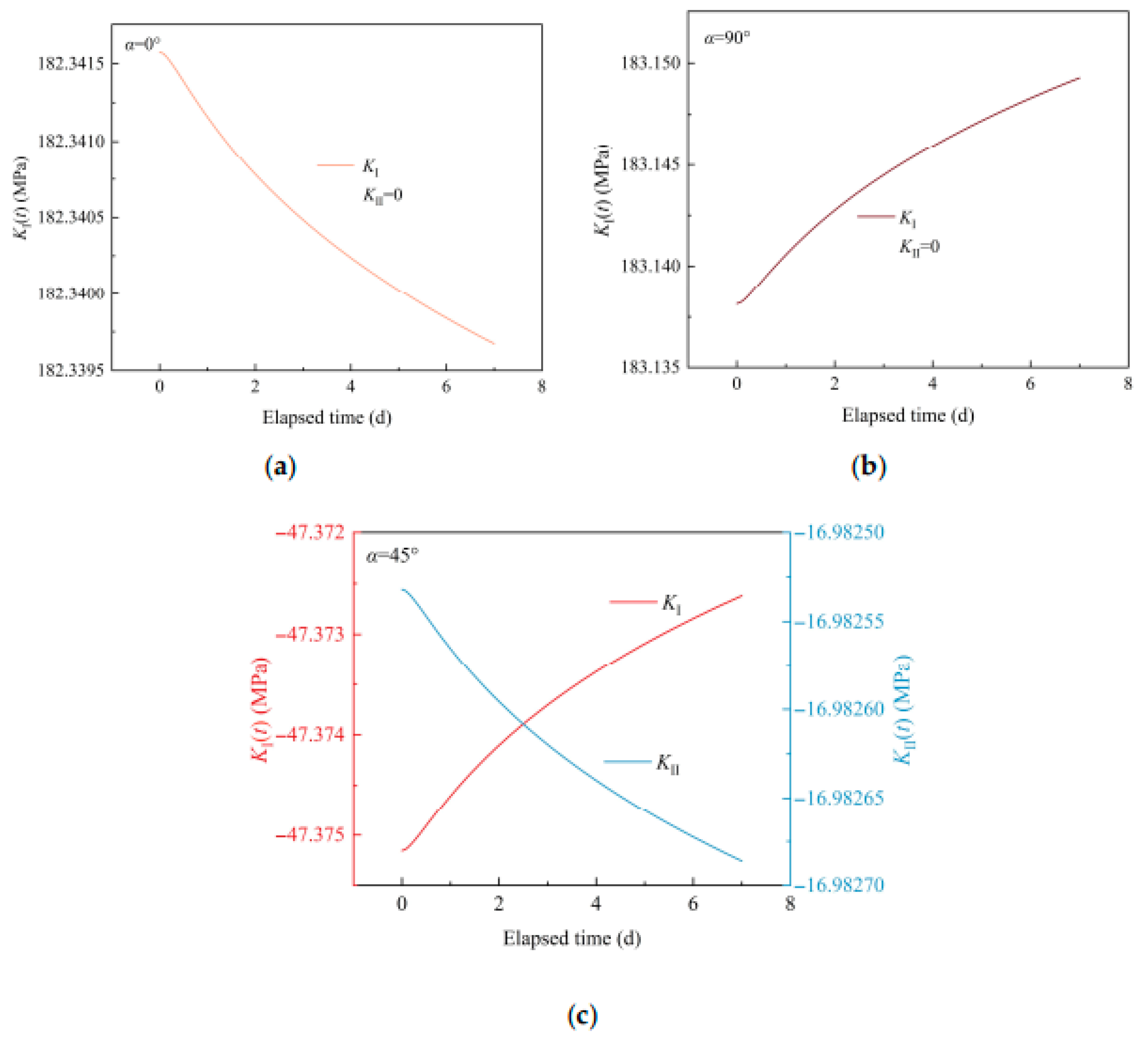
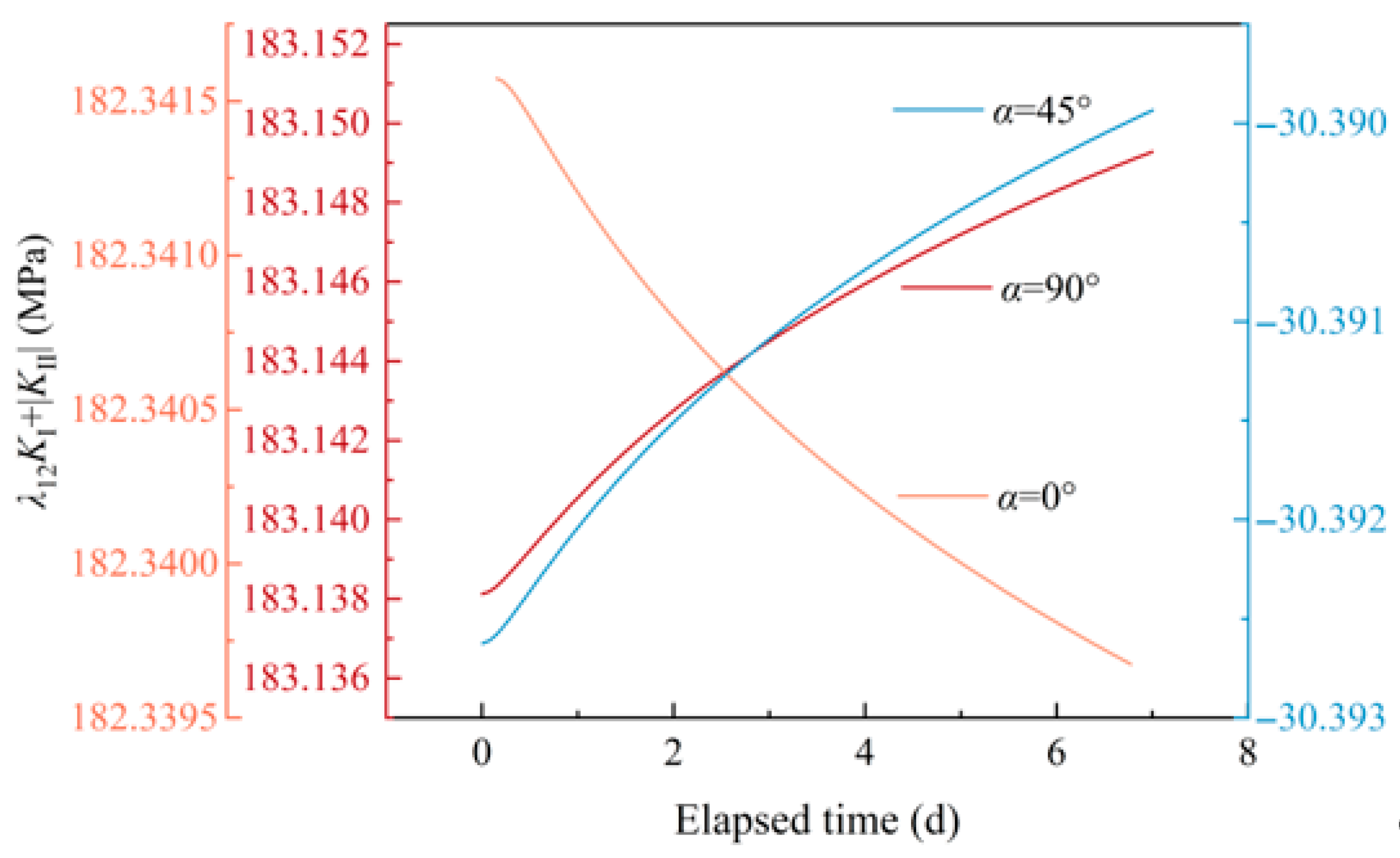
3.3.2. Simulation Results of the Non-Closed Crack Compressive Shear Initiation
4. Discussions
5. Conclusions
- The primary mineral reactions between granitic HDR minerals and water contain Ab and Mic (Fsp) dissolution, which enlarges cracks, as well as Qtz precipitation, which narrows cracks. The crack enlargement due to Fsp dissolution is significantly larger than the crack narrowing due to Qtz precipitation.
- The mineral reactions between granitic HDR minerals and water influence the rock non-closed crack compressive shear initiation by enlarging the length and aperture of a crack.
- For cracks with an inclination angle α of 0°, the crack tip blunting due to mineral reactions reduces the crack initiation potential, as the enlarged crack length is oriented vertically to the maximum principal stress direction, which inhibits stress concentration at the crack tip. For cracks with α of 45° and 90°, the crack length increase promotes the crack initiation potential.
- In this paper, feldspar dissolution and quartz precipitation were considered, whereas the formation of clay minerals and zeolite due to short-term HTHP mineral reactions is also possible [22]. As the mechanical properties of clay minerals and zeolite are distinct from the minerals in the granitic HDR, studying the variations in mineral compositions after HTHP mineral reactions is of crucial importance to further comprehend the influence of mineral reactions on rock non-closed crack compressive shear initiation.
- This paper elucidates the influences of mineral reactions on rock non-closed crack compressive shear initiation with consideration for HDR exploitation, which provides insights into HDR reservoir damages induced by working fluids. In future works, compressive shear cracking experiments on rock specimens with non-closed cracks before and after HTHP mineral reactions will be conducted to further investigate the influencing mechanisms of mineral reactions on non-closed crack compressive shear initiation.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDR | Hot dry rock |
| HTHP | High temperature and high pressure |
| XRD | X-ray diffraction |
| ICP-OES | Inductively coupled plasma optical emission spectrometer |
| SIF | Stress intensity factor |
| Fsp | Feldspar |
| Qtz | Quartz |
| Bt | Biotite |
| Ab | Albite |
| Mic | Microcline |
| Kfs | K-feldspar |
References
- Feng, B.; Song, D.; Fu, L.; Zhang, S.Q.; Chen, M.T.; Jiang, Z.J. Formation laws of hydrothermal alteration minerals and the genesis of travertine in the Zhacang geothermal field, Guide Basin. Nat. Gas Ind. 2019, 39, 133–142. [Google Scholar]
- Chen, J.Y.; Xu, T.F.; Jiang, Z.J.; Feng, B.; Xu, L. Reducing formation damage by artificially controlling the fluid-rock chemical interaction in a double-well geothermal heat production system. Renew. Energy 2020, 149, 455–467. [Google Scholar] [CrossRef]
- Yasuhara, H.; Kinoshita, N.; Ohfuji, H.; Lee, D.S.; Nakashima, S.; Kishida, K. Temporal alteration of fracture permeability in granite under hydrothermal conditions and its interpretation by coupled chemo-mechanical model. Appl. Geochem. 2011, 26, 2074–2088. [Google Scholar] [CrossRef]
- Hou, Z.M.; Wu, X.N.; Luo, J.S.; Zhang, L.H.; Li, Z.Y.; Cao, C.; Wu, L.; Chen, Q.J. Major challenges of deep geothermal systems and an innovative development mode of REGS integrated with energy storage. Coal Geol. Explor. 2024, 52, 1–13. [Google Scholar]
- Richards, H.G.; Savage, D.; Andrews, J.N. Granite-water reactions in an experimental Hot Dry Rock geothermal reservoir, Rosemanowes test site, Cornwall, U.K. Appl. Geochem. 1992, 7, 193–222. [Google Scholar] [CrossRef]
- He, M.; Gong, W.Z.; Xu, M.B.; Song, J.J. Research status and prospect analysis of hot dry rock development technology. Renew. Energy Resour. 2021, 39, 1447–1454. [Google Scholar]
- Edmunds, W.M.; Abdrews, J.N.; Darbyshire, D.P.F.; Hussain, N.; Savage, D.; Shepherd, T.J. Granite-water interactions in relation to Hot Dry Rock geothermal development. In Proceedings of the Fourth International Seminar on the Results of European Community Geothermal Energy Research and Demonstration, Florence, Italy, 27–30 April 1989. [Google Scholar]
- Tang, L.S.; Wang, S.J. Discussion on the mechanism and quantification method of rock hydrochemical damage. Chin. J. Rock Mech. Eng. 2002, 21, 314–319. [Google Scholar]
- Sanchez-Roa, C.; Saldi, G.D.; Mitchell, T.M.; Iacoviello, F.; Bailey, J.; Shearing, P.R.; Oelkers, E.H.; Meredith, P.G.; Jones, A.P.; Striolo, A. The role of fluid chemistry on permeability evolution in granite: Applications to natural and anthropogenic systems. Earth Planet. Sci. Lett. 2021, 553, 116641. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, W.Q. Finite element analysis of influence of pressure solution of fracture aperture on T-H-M coupling in dual-porosity medium. Rock Soil Mech. 2010, 31, 1269–1275. [Google Scholar]
- Yang, J.B.; Feng, X.T.; Pan, P.Z.; Shen, L.F. Aperture evolution of single fracture in granite under triaxial compressive stress and chemical solution seepage. Chin. J. Rock Mech. Eng. 2012, 31, 1869–1878. [Google Scholar]
- Yang, H.; Cao, P.; Jiang, X.L. Micromechanical model for equivalent crack propagation under chemical corrosion of water-rock interaction. Rock Soil Mech. 2010, 31, 2104–2110. [Google Scholar]
- Wang, Z.C.; Wei, R.L.; Bi, J.C.; Wu, Z.H. Characteristics of water-rock reaction and effect of Reynolds number on dissolution rate for fractures. J. Northeast. Univ. (Sci. Technol.) 2020, 41, 1174–1179. [Google Scholar]
- Miao, S.J.; Cai, M.F.; Ji, D.; Guo, Q.F.; Bai, Y.B. Aging features and mechanism of Granite’s damage under the action of acidic chemical solutions. J. China Coal Soc. 2016, 41, 1137–1144. [Google Scholar]
- Li, J.F. Simulation research and performance analysis of hot dry rock power generation technology. Shandong Chem. Ind. 2021, 50, 114–119. [Google Scholar]
- Zhao, Y.H.; Feng, B.; Zhang, G.B.; Shangguan, S.T.; Qi, X.F.; Li, X.; Qiao, Y.C.; Xu, J.N. Study of the interaction between the granitic hot-dry rock (HDR) and different injection water. Acta Geol. Sin. 2020, 94, 2115–2123. [Google Scholar]
- Chen, D.G.; Zhi, X.C.; Yang, H.T. Geochemistry, 2nd ed.; University of Science and Technology of China Press: Hefei, China, 2009; pp. 124–126. [Google Scholar]
- Hou, G.Y. Advanced Course in Rock Mechanics; Science Press: Beijing, China, 2018; pp. 374–375. [Google Scholar]
- Liu, H.Y.; Zhou, Y.Z.; Zhang, G.X.; Xue, L.; Zheng, X.H. Compression-shear initiation criterion for rockmass crack under water pressure. J. Cent. South Univ. (Sci. Technol.) 2023, 54, 920–929. [Google Scholar]
- Zhou, Q.L. Compressive shear fracture criterion of rock and its application. J. Geotech. Eng. 1987, 9, 33–37. [Google Scholar]
- Rimstidt, J.D.; Barnes, H.L. The kinetics of silica-water reactions. Geochim. Et Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar]
- Lin, W.J.; Wang, G.L.; Shao, J.L.; Gan, H.N.; Tan, X.F. Distribution and exploration of hot dry rock resources in China: Progress and inspiration. Acta Geol. Sin. 2021, 95, 1366–1381. [Google Scholar]
- Jiang, O.; Zheng, X.H.; Gong, Q.J.; Wu, H.D.; Chu, B.Z. Research on mass transfer mechanisms due to short-term water-rock interactions between granite cuttings and alkaline NaCl solution and their patterns. Geoenergy Sci. Eng. 2025, 245, 213512. [Google Scholar] [CrossRef]
- Savage, D.; Bateman, K.; Milodowski, A.E.; Hughes, C.R. An experimental evaluation of the reaction of granite with streamwater, seawater and NaCl solutions at 200 °C. J. Volcanol. Geotherm. Res. 1993, 57, 167–191. [Google Scholar] [CrossRef]
- Dove, P.M.; Crerar, D.A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Et Cosmochim. Acta 1990, 54, 955–969. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, R.C.; Lu, X.C.; Gao, J.F.; Xu, S.J. Experimental study on the low-temperature dissolution of microperthite in alkaline solution. Acta Mineral. Sin. 2003, 23, 333–340. [Google Scholar]
- Yang, F.J.; Wang, G.L.; Hu, D.W.; Zhou, H.; Tan, X.F. Influence of water-rock interaction on permeability and heat conductivity of granite under high temperature and pressure conditions. Geothermics 2022, 100, 102347. [Google Scholar] [CrossRef]
- Hellmann, R.; Tisserand, D. Dissolution kinetics as a function of the Gibbs free energy of reaction: An experimental study based on albite feldspar. Geochim. Et Cosmochim. Acta 2006, 70, 364–383. [Google Scholar] [CrossRef]
- Crundwell, F.K. The mechanism of dissolution of the feldspars: Part II dissolution at conditions close to equilibrium. Hydrometallurgy 2015, 151, 163–171. [Google Scholar] [CrossRef]
- Zhang, S.Y.; He, W.Y.; Gao, X.; Tian, C.H.; Xiao, Y.W. Alteration zonation and metallogenic mechanism of porphyry copper deposits: A case study of thermodynamic equilibrium simulation of fluid-rock interactions in Yulong deposit. Acta Petrol. Sin. 2024, 40, 1837–1852. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhang, Y.J.; Lei, Z.H.; Zhang, Q.; Zhang, S.Q.; Fu, L. Inversion analysis of 3D geostress field in GR2 well area of Gonghe hot rock geothermal well. Chin. Q. Mech. 2019, 40, 115–123. [Google Scholar] [CrossRef]
- Lei, Z.H.; Zhang, Y.J.; Zhang, S.Q.; Fu, L.; Hu, Z.J.; Yu, Z.W.; Li, L.Z.; Zhou, J. Electricity generation from a three-horizontal-well enhanced geothermal system in the Qiabuqia geothermal field, China: Slickwater fracturing treatments for different reservoir scenarios. Renew. Energy 2020, 145, 65–83. [Google Scholar] [CrossRef]
- Liu, R.C.; Yang, X.D.; Wang, Y.B.; Song, C.P. Study on fracture characteristics of fractured rock mass under unidirectional compression. Coal Geol. China 2025, 37, 65–71. [Google Scholar]
- Li, T.Y.; Zhao, Z.Y.; Wu, L. Study on the damage mode and law of single-fissure rock specimens under hydraulic pressure. J. Univ. South China (Sci. Technol.) 2024, 38, 50–57. [Google Scholar]
- Longval, R.; Meirbekova, R.; Fisher, J.; Maignot, A. An overview of silica scaling reduction technologies in the geothermal market. Energies 2024, 17, 4825. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wang, K.X.; Yao, X.L.; Liang, P.; Liu, X.X. Simulation experiment study on the effect of fracture geometry on rock strength. China Min. Mag. 2019, 28, 141–148. [Google Scholar]

| Minerals | Chemical Formulas | Cuttings Mass (g) | Molar Mass (g/mol) | Mineral Moles in the Cuttings (mol) |
|---|---|---|---|---|
| Ab | NaAlSi3O8 | 33.3439 | 262 | 5.472 × 10−2 |
| Kfs | KAlSi3O8 | 278 | 3.418 × 10−2 | |
| K-mica | KAl3Si3O10(OH)2 | 398 | 3.979 × 10−3 | |
| Qtz | SiO2 | 60 | 1.320 × 10−1 |
| Parameters | Units | Values | References |
|---|---|---|---|
| Maximum principal stress gradient | MPa·m−1 | 0.0186 | [31] |
| Minimum principal stress gradient | MPa·m−1 | 0.0071 | [31] |
| Crack depth | m | 2350 | |
| Lateral pressure coefficient, k | 0.38 | ||
| Crack aperture, 2b | m | 5.50 × 10−4 | [32] |
| Curvature radius at the crack tip, ρ | m | 2.75 × 10−4 | |
| Crack length, 2a | m | 1 | |
| Crack inclination angle | ° | 0, 45, 90 | |
| Water pressure, pw | MPa | 24 | |
| Shear compression coefficient, λ12 | 1 | [19] | |
| Specific surface area of the cuttings, SBET | m2/kg | 99.4 | [23] |
| Solution mass flow rate, F | kg·d−1 | 0.072 | |
| Temperature, T | K | 453.15 | |
| Molar volume of Ab, VAb | m3·mol−1 | 1.00 × 10−4 | [3] |
| Molar volume of Mic, VMic | m3·mol−1 | 1.09 × 10−4 | [3] |
| Molar volume of Qtz, VQtz | m3·mol−1 | 2.27 × 10−5 | [3] |
| Precipitation rate constant, k−,Qtz | mol·m−2·d−1 | 3.63 × 10−7 | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, O.; Zheng, X.; Liu, H.; Zhu, W.; Wu, H. Simulation Study on the Influence of Reactions Between Granitic Hot Dry Rock Minerals and Water on Rock Non-Closed Crack Compressive Shear Initiation. Appl. Sci. 2025, 15, 9695. https://doi.org/10.3390/app15179695
Jiang O, Zheng X, Liu H, Zhu W, Wu H. Simulation Study on the Influence of Reactions Between Granitic Hot Dry Rock Minerals and Water on Rock Non-Closed Crack Compressive Shear Initiation. Applied Sciences. 2025; 15(17):9695. https://doi.org/10.3390/app15179695
Chicago/Turabian StyleJiang, Ou, Xiuhua Zheng, Hongyan Liu, Wenxi Zhu, and Haidong Wu. 2025. "Simulation Study on the Influence of Reactions Between Granitic Hot Dry Rock Minerals and Water on Rock Non-Closed Crack Compressive Shear Initiation" Applied Sciences 15, no. 17: 9695. https://doi.org/10.3390/app15179695
APA StyleJiang, O., Zheng, X., Liu, H., Zhu, W., & Wu, H. (2025). Simulation Study on the Influence of Reactions Between Granitic Hot Dry Rock Minerals and Water on Rock Non-Closed Crack Compressive Shear Initiation. Applied Sciences, 15(17), 9695. https://doi.org/10.3390/app15179695






