Effects of Deoxygenated Packaging and Super-Chilled Storage on Yellowtail (Seriola quinqueradiata) Quality Deterioration
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Storage Conditions
2.2. VBC Analysis
2.3. Biochemical Analysis
2.4. Browning Index
2.5. Sensory Test
2.6. NGS for Microbial Analysis and Data Processing
2.7. VC Analysis
2.8. Data Analysis
3. Results and Discussion
3.1. VBCs
3.2. TVB-N
3.3. TMA
3.4. TBARS
3.5. Browning Degree
3.6. Sensory Test
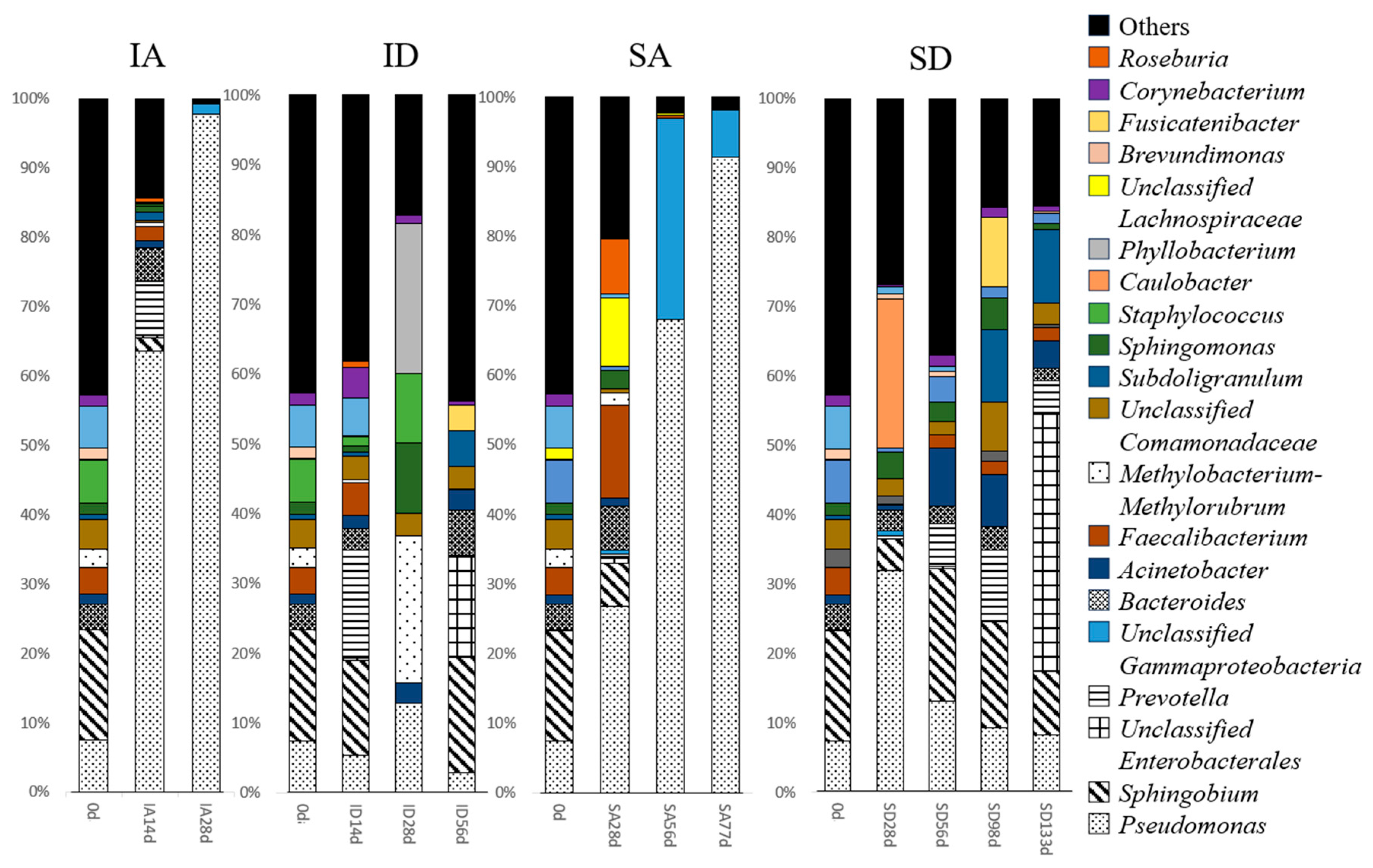
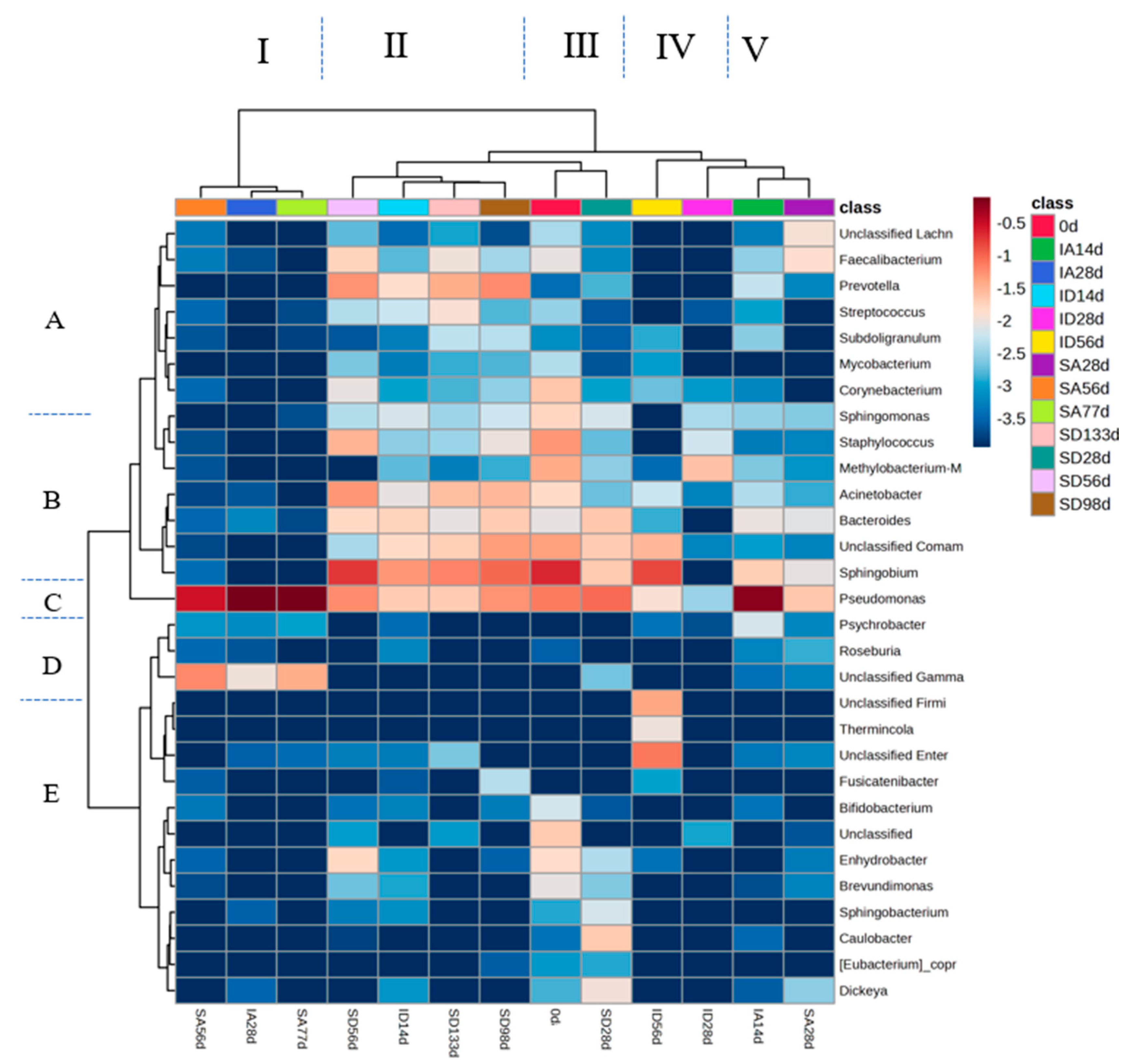
3.7. Microbial Community Analysis
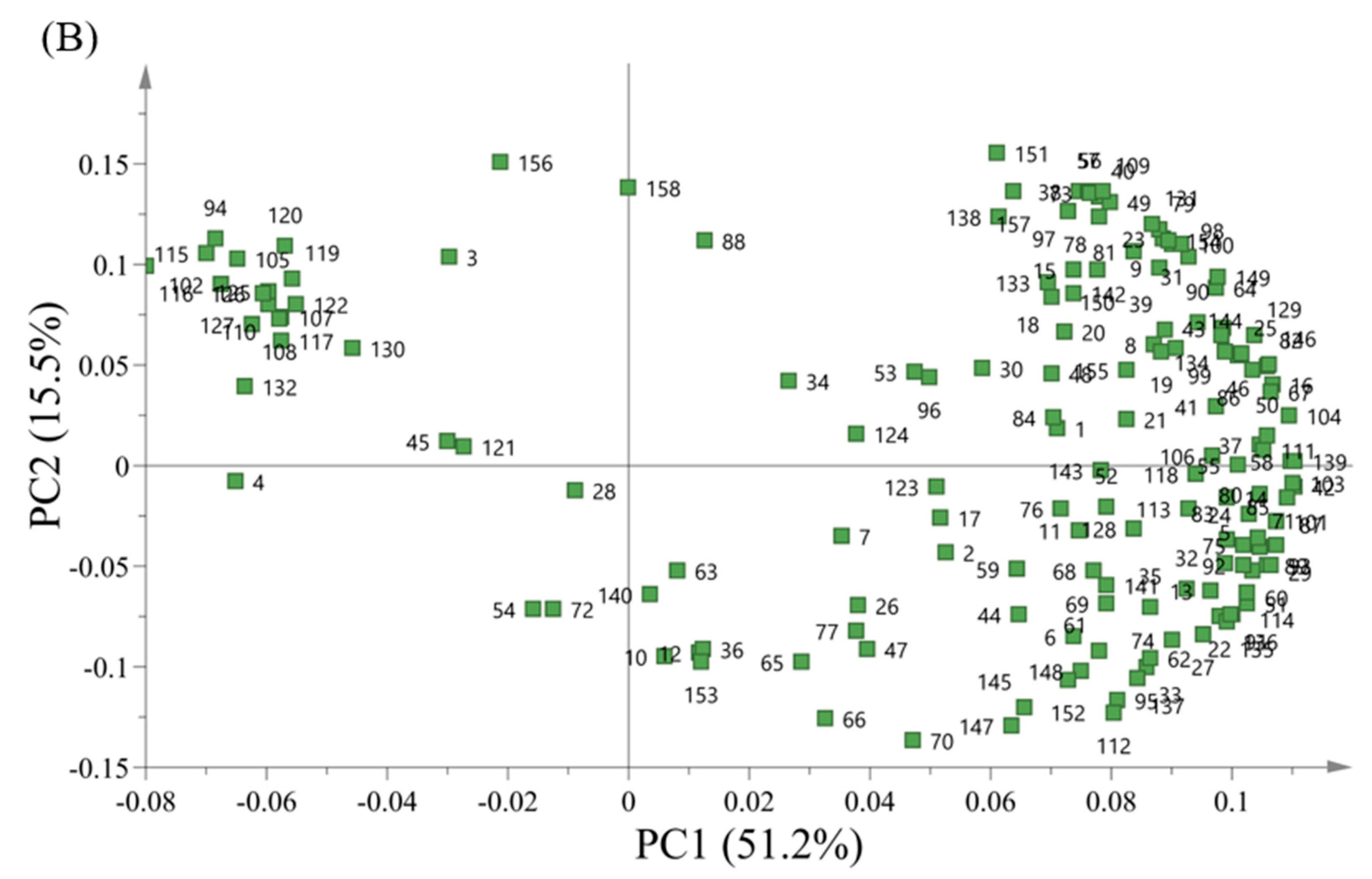
3.8. VCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| DO | Deoxygenated packaging |
| DMC | Dark muscle |
| IC-AR | Storage on ice under air |
| IC-DO | Deoxygenated packaging on ice |
| MAP | Modified atmosphere packaging |
| NGS | Next-generation sequencing |
| OMC | Dorsal portion of ordinary muscle |
| SC | Super-chilled |
| OTUs | Operational taxonomic units |
| SC-AR | SC storage under air |
| SC-DO | Deoxygenated packaging with SC storage |
| TBARS | Thiobarbituric acid reactive substances |
| TMA | Trimethylamine |
| TMAO | Trimethylamine oxide |
| TVB-N | Total volatile basic nitrogen |
| VBC | Viable bacterial count |
| VC | Volatile compound |
References
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Cheng, L.; Zhu, W.; Li, S.; Chen, X. Mechanisms underlying the deterioration of fish quality after harvest and methods of preservation. Food Control. 2022, 135, 108805. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish spoilage mechanisms and preservation techniques. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Smith, J.P.; Simpson, B.K.; Haard, N.F. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef]
- Tavares, J.; Martins, A.; Fidalgo, L.G.; Lima, V.; Amaral, R.A.; Pinto, C.A.; Saraiva, J.A. Fresh fish degradation and advances in preservation using physical emerging technologies. Foods 2021, 10, 780. [Google Scholar] [CrossRef] [PubMed]
- Ababouch, L. Assuring fish safety and quality in international fish trade. Mar. Pollut. Bull. 2006, 53, 561–568. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, X.L.; Wang, G.B.; Mo, F.Y.; Zhang, X.R. Application of super-cooled storage of aquatic products: A review. Int. J. Refrig. 2023, 154, 66–72. [Google Scholar] [CrossRef]
- Bahuaud, D.; Mørkøre, T.; Langsrud, Ø.; Sinnes, K.; Veiseth, E.; Ofstad, R.; Thomassen, M.S. Effects of −1.5 °C super-chilling on quality of Atlantic salmon (Salmo salar) pre-rigor fillets: Cathepsin activity, muscle histology, texture, and liquid leakage. Food Chem. 2008, 111, 329–339. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1747–1759. [Google Scholar] [CrossRef]
- Fan, X.; Jin, Z.; Liu, Y.; Chen, Y.; Konno, K.; Zhu, B.; Dong, X. Effects of super-chilling storage on shelf-life and quality indicators of Coregonus peled based on proteomics analysis. Food Res. Int. 2021, 143, 110229. [Google Scholar] [CrossRef]
- Kitabayashi, K.; Tanimoto, S.; Kikutani, H.; Ohkita, T.; Mabuchi, R.; Shimoda, M. Effect of nitrogen gas packaging on odor development in yellowtail Seriola quinqueradiata muscle during ice storage. Fish. Sci. 2019, 85, 247–257. [Google Scholar] [CrossRef]
- Martin, D.; Joly, C.; Dupas-Farrugia, C.; Adt, I.; Oulahal, N.; Degraeve, P. Volatilome analysis and evolution in the headspace of packed refrigerated fish. Foods 2023, 12, 2657. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Mittal, A.; Benjakul, S. Undesirable discoloration in edible fish muscle: Impact of indigenous pigments, chemical reactions, processing, and its prevention. Compr. Rev. Food Sci. Food Saf. 2022, 21, 580–603. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Wang, Y.; Hao, S.; Zhang, K.; Tian, J.; Jin, Y. Effect of changes in the structure of myoglobin on the color of meat products. Food Mater. Res. 2024, 4, e011. [Google Scholar] [CrossRef]
- Opara, U.L.; Fadiji, T.; Caleb, O.J.; Oluwole, A.O. Changes in Volatile Composition of Cape Hake Fillets under Modified Atmosphere Packaging Systems during Cold Storage. Foods 2022, 11, 1292. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Ravishankar, C.N. Oxygen scavenger packaging for seafood preservation. In Seafood Processing and Preservation; CRC Press: Boca Raton, FL, USA, 2020; Volume 57, pp. 147–155. [Google Scholar]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.J.; Stanley, R.A. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Ritter, J.C.; Budge, S.M. Key lipid oxidation products can be used to predict sensory quality of fish oils with different levels of EPA and DHA. Lipids 2012, 47, 1169–1179. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry and Fisheries, Japan. Production Statistics of the Fishery and Aquaculture Industries for 2020. Available online: https://www.e-stat.go.jp/stat-search/files?page=1&layout=datalist&toukei=00500216&tstat=000001015174&cycle=7&year=20220&month=0&tclass1=000001015175&tclass2=000001214760 (accessed on 8 August 2025).
- Ji, Y.; Ishizu, S.; Matsumoto, A.; Furuta, A.; Okada, G.; Tanimoto, S. Changes in Bacterial Flora and Quality of Yellowtail (Seriola quinqueradiata) Muscle Stored at Different Temperatures. Appl. Sci. 2025, 15, 2996. [Google Scholar] [CrossRef]
- Rehbein, H.; Oehlenschläger, J. Fishery Products: Quality, Safety and Authenticity; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Tanimoto, S.; Shimoda, M. Changes in volatile compounds of dark and ordinary yellowtail muscles (Seriola quinqueradiata) during short-term cold storage. J. Aquat. Food Prod. Technol. 2016, 25, 185–196. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kikutani, H.; Kitabayashi, K.; Ohkita, T.; Arita, R.; Nishimura, S.; Takemoto, R.; Mabuchi, R.; Shimoda, M. Qualitative changes in each part of yellowtail (Seriola quinqueradiata) flesh during cold storage. Fish. Sci. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Tanimoto, S.; Hirata, Y.; Ishizu, S.; Wang, R.; Furuta, A.; Mabuchi, R.; Okada, G. Changes in the quality and microflora of yellowtail (Seriola quinqueradiata) muscles during cold storage. Foods 2024, 13, 1086. [Google Scholar] [CrossRef]
- Mukojima, K.; Yoshii, M.; Nakashio, A.; Mabuchi, R.; Furuta, A.; Tanimoto, S. Effect of vacuum packing on the odor of yellowtail Seriola quinqueradiata flesh stored after heating. Fish. Sci. 2023, 89, 709–721. [Google Scholar] [CrossRef]
- Isonhood, J.H.; Drake, M. Aeromonas Species in Foods. J. Food Prot. 2002, 65, 575–582. [Google Scholar] [CrossRef]
- Farmer, J.J., III; Farmer, M.K.; Holmes, B. The Enterobacteriaceae. In Topley & Wilson’s Microbiology and Microbial Infections; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Gross, R.J.; Holmes, B. The Enterobacteriaceae. In Topley & Wilson’s Microbiology and Microbial Infections, 9th ed.; Parker, M.T., Duerden, B.I., Eds.; Edward Arnold: London, UK, 1990; Volume 2, pp. 601–621. [Google Scholar]
- Remenant, B.; Jaffrès, E.; Dousset, X.; Pilet, M.-F.; Zagorec, M. Bacterial spoilers of food: Behavior, fitness and functional properties. Food Microbiol. 2015, 45 Pt A, 45–53. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Yang, X.; Chen, S.; Zhao, Y.; Li, C. Changes in microbial composition and quality characteristics of yellowfin tuna under different storage temperatures. Qual. Assur. Saf. Crop. Foods 2023, 13, 988. [Google Scholar] [CrossRef]
- Li, B.; Liu, S.; Chen, X.; Su, Y.; Pan, N.; Liao, D.; Qiao, K. Dynamic changes in the microbial composition and spoilage characteristics of refrigerated large yellow croaker (Larimichthys crocea) during storage. Foods 2023, 12, 3994. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Rathod, N.B.; Nirmal, N.P.; Pagarkar, A.; Özogul, F.; Rocha, J.M. Antimicrobial impacts of microbial metabolites on the preservation of fish and fishery products: A review with current knowledge. Microorganisms 2022, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T. Studies on the trimethylamine oxide-reductase-I. Reduction of trimethylamine oxide in the dark muscle of pelagic migrating fish under aseptic condition. Nippon. Suisan Gakkaishi 1953, 19, 505–512. [Google Scholar] [CrossRef]
- Tokunaga, T. Trimethylamine oxide and its decomposition in the bloody muscle of fish-II. Formation of DMA and TMA during storage. Nippon. Suisan Gakkaishi 1970, 36, 510–515. [Google Scholar] [CrossRef][Green Version]
- Ahn, D.U.; Ajuyah, A.O.; Wolfe, F.H.; Sim, J.S. Oxygen availability affects prooxidant-catalyzed lipid oxidation of cooked turkey patties. J. Food Sci. 1993, 58, 278–282. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Xie, J. Effect of vacuum and modified atmosphere packaging on moisture state, quality, and microbial communities of grouper (Epinephelus coioides) fillets during cold storage. Food Res. Int. 2023, 173, 113340. [Google Scholar] [CrossRef] [PubMed]
- Adele, S.; Sandra, A.; Juliette, S.R.; Jean, J.J.; Katia, R.; Françoise, L. Quality assessment of ice-stored tropical yellowfin tuna (Thunnus albacares) and influence of vacuum and modified atmosphere packaging. Food Microbiol. 2016, 60, 62–72. [Google Scholar] [CrossRef]
- Gokoglu, N.; Yerlikaya, P. Effects of modified atmosphere packaging with varied CO2 and O2 concentrations on the quality and shelf life of yellowfin tuna (Thunnus albacares) fillets. Foods 2021, 10, 2904. [Google Scholar]
- Parlapani, F.F.; Haroutounian, S.A.; Nychas, G.J.; Boziaris, I.S. Microbiota succession of whole and filleted European sea bass (Dicentrarchus labrax) during storage under aerobic or modified atmosphere packaging conditions. Microorganisms 2022, 10, 548. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.H.; Kim, K.J.; Kim, H.S. Effects of vacuum packaging in freezer on oxidative spoilage, freezing quality, and storage stability of fish fillets. Food Sci. Nutr. 2021, 9, 1704–1712. [Google Scholar]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef]
- Qin, D.; Ma, C.; Lv, M.; Yu, C.P. Sphingobium estronivorans sp. nov. and Sphingobium bisphenolivorans sp. nov., isolated from a wastewater treatment plant. Int. J. Syst. Evol. Microbiol. 2020, 70, 1822–1829. [Google Scholar] [CrossRef]
- Wittich, R.M.; Busse, H.J.; Kämpfer, P.; Tiirola, M.; Wieser, M.; Macedo, A.J.; Abraham, W.R. Sphingobium aromaticiconvertens sp. nov., a xenobiotic-compound-degrading bacterium from polluted river sediment. Int. J. Syst. Evol. Microbiol. 2007, 57 Pt 2, 306–310. [Google Scholar] [CrossRef]
- Pan, Z.; Li, L.; Shen, Z.; Chen, Y.; Li, M. Characterization of the microbiota in air- or vacuum-packed crisp grass carp (Ctenopharyngodon idella C. et V.) fillets by 16S rRNA PCR-Denaturing Gradient Gel Electrophoresis and high-throughput sequencing. J. Food Prot. 2018, 81, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Liu, X.; Lei, Y.; Regenstein, J.M.; Luo, Y. Characterization of the microbial composition and quality of lightly salted grass carp (Ctenopharyngodon idellus) fillets with vacuum or modified atmosphere packaging. Int. J. Food Microbiol. 2019, 293, 87–93. [Google Scholar] [CrossRef]
- Venugopal, V. Biosensors in fish production and quality control. Biosens. Bioelectron. 2002, 17, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ariyani, F.; Barokah, G.R.; Wibowo, S. Bombay duck (Harpodon nehereus) natural formaldehyde levels and changes during frozen storage. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1137, No. 1; p. 012031. [Google Scholar]
- Wang, F.; Xu, J.; Jakovlić, I.; Wang, W.M.; Zhao, Y.H. Dietary betaine reduces liver lipid accumulation via improvement of bile acid and trimethylamine-N-oxide metabolism in blunt-snout bream. Food Funct. 2019, 10, 6675–6689. [Google Scholar] [CrossRef] [PubMed]
- Harimana, Y.; Tang, X.; Le, G.; Xing, X.; Zhang, K.; Sun, Y.; Li, Y.; Ma, S.; Karangwa, E.; Tuyishimire, M.A. Quality parameters of black carp (Mylopharyngodon piceus) raised in lotic and lentic freshwater systems. LWT 2018, 90, 45–52. [Google Scholar] [CrossRef]
- Ghosh, I.; Liu, H.; Simpson, B.K.; Zhang, Y. Endogenous Enzymes. In Handbook of Seafood and Seafood Products Analysis, 2nd ed.; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 33–50. [Google Scholar]
- Syropoulou, F.; Parlapani, F.F.; Anagnostopoulos, D.A.; Stamatiou, A.; Mallouchos, A.; Boziaris, I.S. Spoilage investigation of chill-stored meagre (Argyrosomus regius) using modern microbiological and analytical techniques. Foods 2021, 10, 3109. [Google Scholar] [CrossRef]




| Packaging Conditions | Muscle Type | Storage Time at 0 °C (Days) | Storage Time at −3 °C (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 56 | 0 | 28 | 56 | 77 | 98 | 133 | |||
| Mesophilic bacteria | Atmosphere | OMC | 2.2 | 4.0 * | 7.7 * | – | 2.2 | 3.8 | 7.1 * | 8.2 * | – | – |
| DMC | 3.0 | 3.9 * | 7.5 * | – | 3.0 | 3.8 | 6.7 * | 7.7 * | – | – | ||
| Deoxygenation | OMC | 2.2 | 2.5 | 1.9 | 5.8 * | 2.2 | 3.4 | 2.6 | – | 2.5 | 2.7 | |
| DMC | 3.0 | 2.6 | 2.4 | 5.6 * | 3.0 | 3.8 | 2.8 | – | 2.9 | 3.3 | ||
| B. thermosphacta | Atmosphere | OMC | 0.0 | 0.0 | 2.9 * | – | 0.0 | 2.2 * | 3.1 * | 5.0 * | – | – |
| DMC | 0.0 | 1.5 * | 2.8 * | – | 0.0 | 2.9 * | 3.6 * | 3.8 * | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.4 | 1.9 * | 0.0 | 0.0 | 1.7 * | 0.0 | – | 2.8 * | 2.3 * | |
| DMC | 0.0 | 0.0 | 3.0 * | 0.1 | 0.0 | 2.6 * | 0.0 | – | 2.9 * | 2.8 * | ||
| Lactic acid bacteria | Atmosphere | OMC | 0.0 | 0.0 | 1.5 * | – | 0.0 | 0.4 | 1.8 * | 3.1 * | – | – |
| DMC | 0.0 | 0.0 | 0.8 | – | 0.0 | 1.2 * | 1.7 * | 3.0 * | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | – | 0.1 | 0.6 | |
| DMC | 0.0 | 0.0 | 0.9 | 0.1 | 0.0 | 1.4 * | 0.3 | – | 0.3 | 0.9 | ||
| Enterobacteriaceae | Atmosphere | OMC | 0.0 | 2.3 * | 6.3 * | – | 0.0 | 2.1 * | 6.1 * | 7.1 * | – | – |
| DMC | 0.0 | 2.7 * | 6.6 * | – | 0.0 | 2.4 * | 6.1 * | 6.9 * | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.0 | 0.0 | 4.3 * | 0.0 | 1.4 | 0.6 | – | 0.0 | 1.1 | |
| DMC | 0.0 | 0.0 | 0.0 | 5.3 * | 0.0 | 2.5 | 0.0 | – | 0.0 | 1.5 | ||
| Aeromonas spp. | Atmosphere | OMC | 0.0 | 4.3 * | 8.2 * | – | 0.0 | 5.3 * | 7.6 * | 8.0 * | – | – |
| DMC | 0.0 | 4.3 * | 8.1 * | – | 0.0 | 5.0 * | 6.9 * | 7.9 * | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.0 | 0.0 | 5.7 * | 0.0 | 4.5 * | 1.3 | – | 0.0 | 1.8 | |
| DMC | 0.0 | 0.0 | 0.5 | 5.9 * | 0.0 | 4.2 * | 0.0 | – | 0.0 | 1.4 | ||
| Marine bacteria | Atmosphere | OMC | 0.0 | 4.0 * | 7.7 * | – | 0.0 | 4.4 * | 7.1 * | 7.7 * | – | – |
| DMC | 0.0 | 3.9 * | 7.5 * | – | 0.0 | 3.9 * | 6.7 * | 7.4 * | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.0 | 0.5 | 3.4 * | 0.0 | 3.4 * | 0.6 | – | 2.7 * | 2.1 | |
| DMC | 0.0 | 0.0 | 1.3 | 3.6 * | 0.0 | 3.7 * | 0.7 | – | 3.1 * | 2.9 * | ||
| Pseudomonas spp. | Atmosphere | OMC | 0.0 | 3.3 * | 5.9 * | – | 0.0 | 1.7 | 6.4 * | 6.8 * | – | – |
| DMC | 0.0 | 3.8 | 5.2 * | – | 0.0 | 3.8 * | 5.9 | 6.8 | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.7 | 0.8 | 1.3 | 0.0 | 3.3 * | 0.0 | – | 0.0 | 2.2 * | |
| DMC | 0.0 | 0.0 | 3.1 | 3.1 | 0.0 | 0.0 | 0.0 | – | 1.9 | 3.0 | ||
| H2S−producing bacteria | Atmosphere | OMC | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | 0.0 | 0.0 | – | – |
| DMC | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | 0.0 | 0.0 | – | – | ||
| Deoxygenation | OMC | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | |
| DMC | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | – | 0.0 | 0.0 | ||
| Packaging Conditions | Muscle Type | Storage Time at 0 °C (Days) | Storage Time at −3 °C (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 56 | 0 | 28 | 56 | 77 | 98 | 133 | |||
| Total volatile basic nitrogen (mg/100 g) | Atmosphere | OMC | 6.5 | 8.3 | 10.7 * | – | 6.5 | 8.0 | 6.9 | 13.4 * | – | – |
| DMC | 2.9 | 9.4 * | 10.3 * | – | 2.9 | 9.2 * | 10.7 * | 13.0 * | – | – | ||
| Deoxygenation | OMC | 6.5 | 10.7 * | 9.8 | 8.7 | 6.5 | 9.7 | 10.5 * | – | 10.8 * | 12.5 * | |
| DMC | 2.9 | 9.7 * | 13.3 * | 14.4 * | 2.9 | 10.4 * | 13.3 * | – | 15.2 * | 15.3 * | ||
| Trimethylamine (μg/g) | Atmosphere | OMC | 0.40 | 18.4 * | 36.7 * | – | 0.40 | 21.1 | 52.4 * | 51.4 * | – | – |
| DMC | 18.7 | 180.3 * | 146.7 * | – | 18.7 | 157.7 * | 133.0 | 107.9 | – | – | ||
| Deoxygenation | OMC | 0.40 | 25.0 | 61.2 * | 109.5 * | 0.40 | 35.4 | 74.8 | – | 170.2 * | 183.9 * | |
| DMC | 18.7 | 237.8 * | 277.8 * | 210.5 * | 18.7 | 206.8 * | 188.4 * | – | 181.2 * | 176.9 * | ||
| Thiobarbituric acid reactive substances (μmol/g) | Atmosphere | OMC | 0.004 | 0.031 * | 0.046 * | – | 0.004 | 0.026 * | 0.042 * | 0.054 * | – | – |
| DMC | 0.056 | 0.280 * | 0.321 * | – | 0.056 | 0.368 * | 0.420 * | 0.428 * | – | – | ||
| Deoxygenation | OMC | 0.004 | 0.005 | 0.005 | 0.006 | 0.004 | 0.003 | 0.005 | – | 0.003 | 0.003 | |
| DMC | 0.056 | 0.149 * | 0.176 * | 0.173 * | 0.056 | 0.045 | 0.132 * | – | 0.238 * | 0.208 * | ||
| Packaging Conditions | Storage Time at 0 °C (Days) | Storage Time at −3 °C (Days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 56 | 0 | 28 | 56 | 77 | 98 | 133 | |
| Atmosphere | 0.42 ± 0.1 | 1.20 ± 0.4 * | 1.20 ± 0.2 * | – | 0.42 ± 0.1 | 1.30 ± 0.4 * | 1.60 ± 0.3 * | 1.40 ± 0.3 * | – | – |
| Deoxygenation | 0.42 ± 0.1 | 0.70 ± 0.1 * | 0.70 ± 0.1 * | 0.70 ± 0.1 * | 0.42 ± 0.1 | 0.60 ± 0.1 * | 0.70 ± 0.1 * | – | 0.70 ± 0.1 * | 0.70 ± 0.1 * |
| Packaging Conditions | Muscle Type | Storage Time at 0°C (Days) | Storage Time at −3°C (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 56 | 0 | 28 | 56 | 77 | 98 | 133 | |||
| Spoiled odor | Atmosphere | OMC | 1.6 | 1.8 | 2.2 * | − | 1.6 | 1.9 | 2.2 * | 2.5 * | − | − |
| DMC | 1.9 | 2.5 * | 2.6 * | − | 1.9 | 2.4 | 2.4 | 2.7 * | − | − | ||
| Deoxygenation | OMC | 1.6 | 1.7 | 1.7 | 2.1 * | 1.6 | 1.6 | 1.9 | − | 2.0 | 1.9 | |
| DMC | 1.9 | 2.2 | 1.8 | 1.9 | 1.9 | 2.0 | 2.3 | − | 2.1 | 2.1 | ||
| Odor intensity | Atmosphere | OMC | 1.7 | 2.6 * | 2.9 * | − | 1.7 | 2.3 | 2.7 * | 3.5 * | − | − |
| DMC | 2.8 | 4.0 * | 3.8 * | − | 2.8 | 3.7 | 3.8 * | 4.4 * | − | − | ||
| Deoxygenation | OMC | 1.7 | 2.0 | 2.0 | 2.1 * | 1.7 | 1.9 | 2.4 | − | 2.5 * | 2.4 * | |
| DMC | 2.8 | 3.3 | 2.6 | 3.2 | 2.8 | 3.2 | 3.4 | − | 3.1 | 3.1 | ||
| Packaging Conditions | Storage Time at 0 °C (Days) | Storage Time at −3 °C (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 56 | 0 | 28 | 56 | 77 | 98 | 133 | ||
| Observed OTUs | Atmosphere | 83.0 | 53.0 | 16.3 | – | 83.0 | 31.7 | 21.0 | 14.3 | – | – |
| Deoxygenation | 83.0 | 58.3 | 12.7 | 27.0 | 83.0 | 63.0 | 57.3 | – | 43.0 | 36.7 | |
| Shannon | Atmosphere | 5.01 | 4.05 | 2.09 * | – | 5.01 | 3.90 | 1.75 * | 2.55 * | – | – |
| Deoxygenation | 5.01 | 4.47 | 2.80 * | 3.80 | 5.01 | 4.49 | 4.53 | – | 4.25 | 3.21 | |
| Chao1 | Atmosphere | 83.0 | 54.7 | 18.3 | – | 83.0 | 33.0 | 23.3 | 15.7 | – | – |
| Deoxygenation | 83.0 | 61.3 | 12.7 | 27.0 | 83.0 | 68.3 | 59.0 | – | 44.0 | 38.0 | |
| ACE | Atmosphere | 83.0 | 54.7 | 18.3 | – | 83.0 | 33.0 | 23.3 | 15.7 | – | – |
| Deoxygenation | 83.0 | 61.3 | 12.7 | 27.0 | 83.0 | 68.3 | 59.0 | – | 44.0 | 38.0 | |
| Simpson | Atmosphere | 0.94 | 0.88 | 0.59 * | – | 0.94 | 0.91 | 0.49 * | 0.73 | – | – |
| Deoxygenation | 0.94 | 0.92 | 0.77 | 0.89 | 0.94 | 0.92 | 0.91 | – | 0.91 | 0.79 | |
| Good Coverage (%) | Atmosphere | 100 | 100 | 100 | – | 100 | 100 | 100 | 100 | – | – |
| Deoxygenation | 100 | 100 | 100 | 100 | 100 | 100 | 100 | – | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Kondo, Y.; Wang, R.; Matsumoto, A.; Furuta, A.; Okada, G.; Tanimoto, S. Effects of Deoxygenated Packaging and Super-Chilled Storage on Yellowtail (Seriola quinqueradiata) Quality Deterioration. Appl. Sci. 2025, 15, 9686. https://doi.org/10.3390/app15179686
Ji Y, Kondo Y, Wang R, Matsumoto A, Furuta A, Okada G, Tanimoto S. Effects of Deoxygenated Packaging and Super-Chilled Storage on Yellowtail (Seriola quinqueradiata) Quality Deterioration. Applied Sciences. 2025; 15(17):9686. https://doi.org/10.3390/app15179686
Chicago/Turabian StyleJi, Yajing, Yu Kondo, Run Wang, Akane Matsumoto, Ayumi Furuta, Genya Okada, and Shota Tanimoto. 2025. "Effects of Deoxygenated Packaging and Super-Chilled Storage on Yellowtail (Seriola quinqueradiata) Quality Deterioration" Applied Sciences 15, no. 17: 9686. https://doi.org/10.3390/app15179686
APA StyleJi, Y., Kondo, Y., Wang, R., Matsumoto, A., Furuta, A., Okada, G., & Tanimoto, S. (2025). Effects of Deoxygenated Packaging and Super-Chilled Storage on Yellowtail (Seriola quinqueradiata) Quality Deterioration. Applied Sciences, 15(17), 9686. https://doi.org/10.3390/app15179686







