The Use of Phase Change Materials for Thermal Management of Metal Hydride Reaction
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of CPCMs
- -
- m1 is the mass of CPCM
- -
- m0 is the mass of 3D matrix material
2.3. Characterization
2.3.1. Thermal Properties of CPCMs
2.3.2. Thermal Performance Testing Experimental Design and Setup
- -
- k is the rate constant, S−1
- -
- [x] the concentration of LaNi5
- -
- t is the reaction time, S
3. Results and Discussion
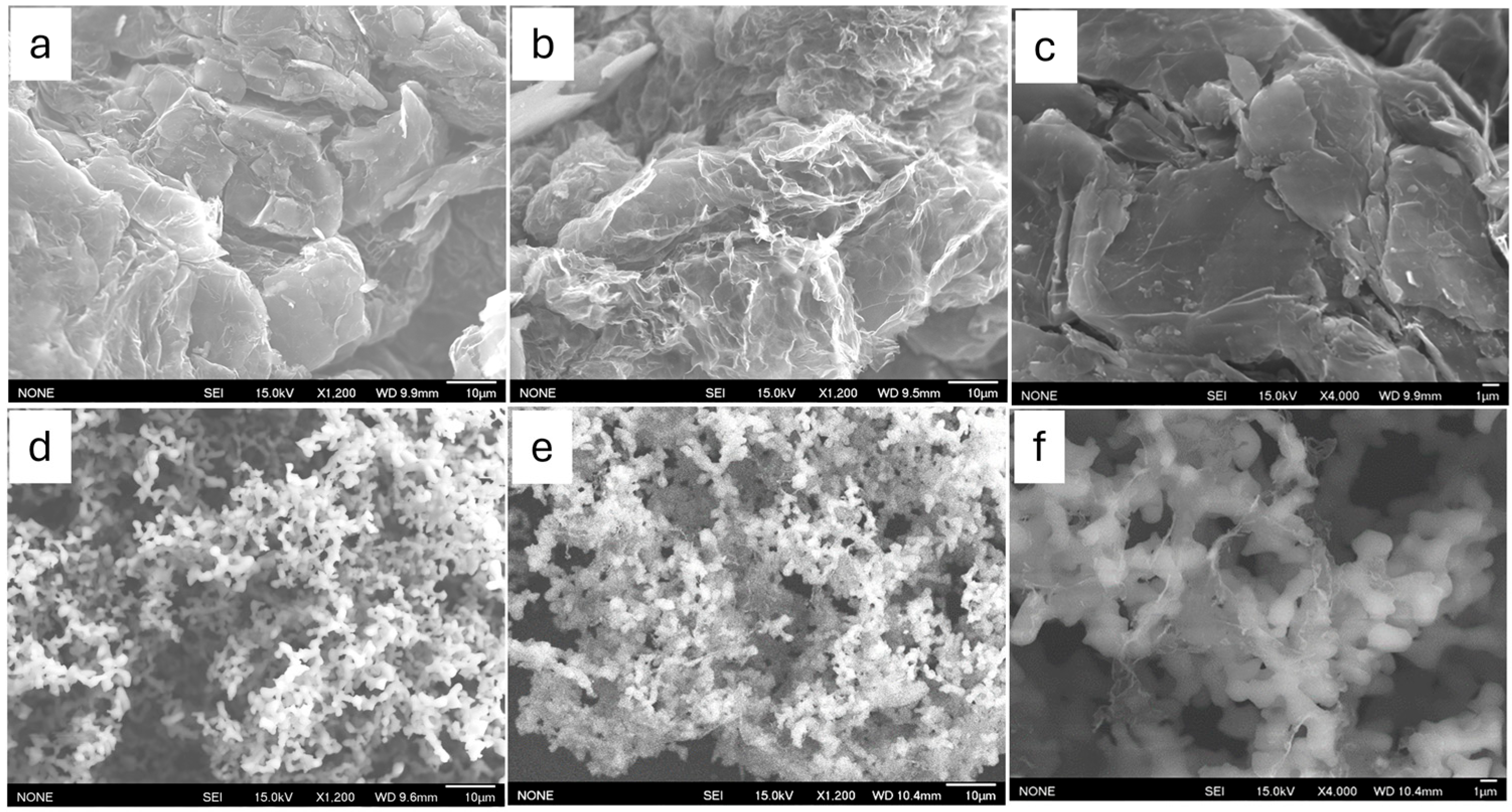
3.1. Structure Characterization
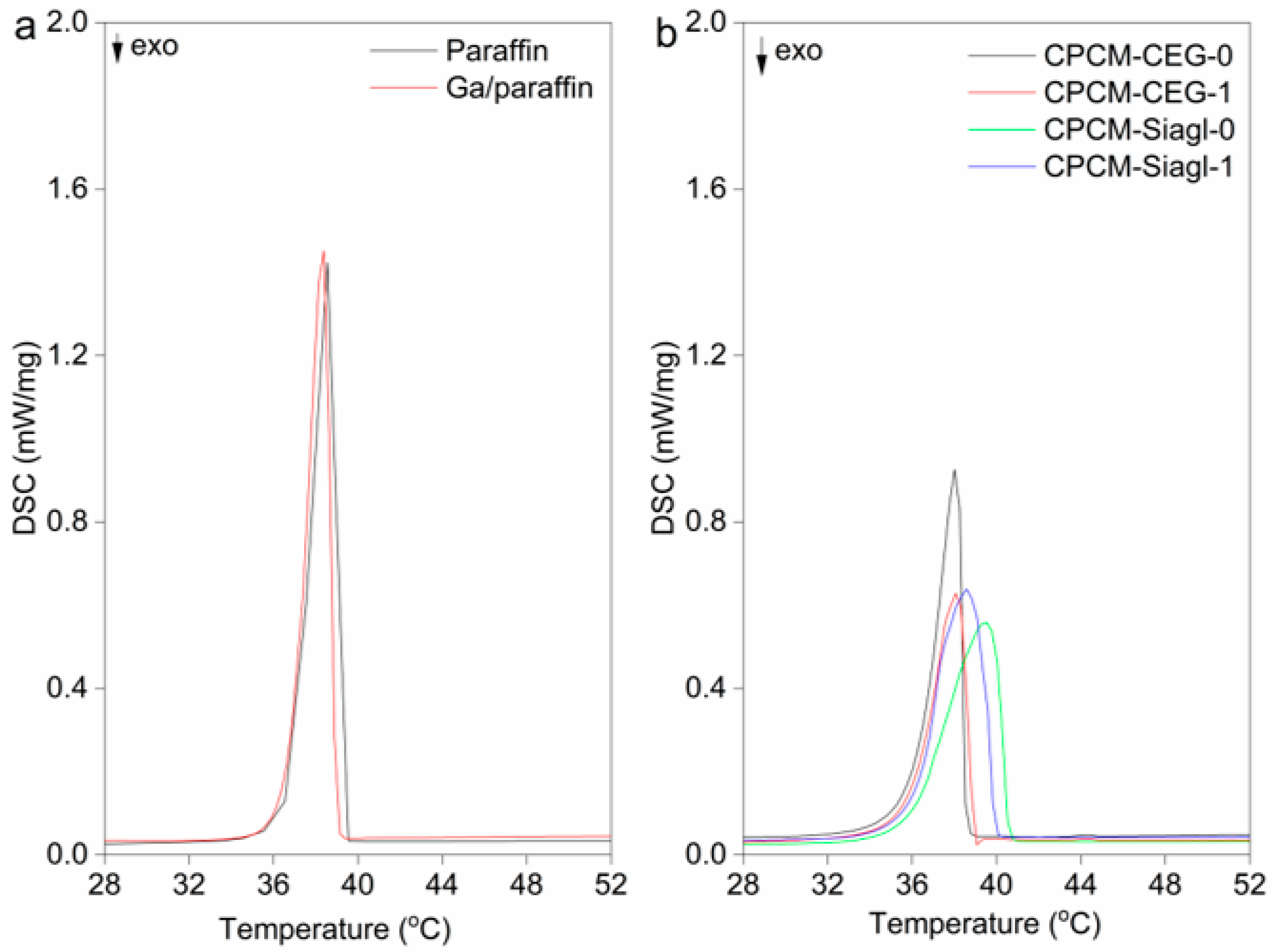
3.2. Thermal Properties
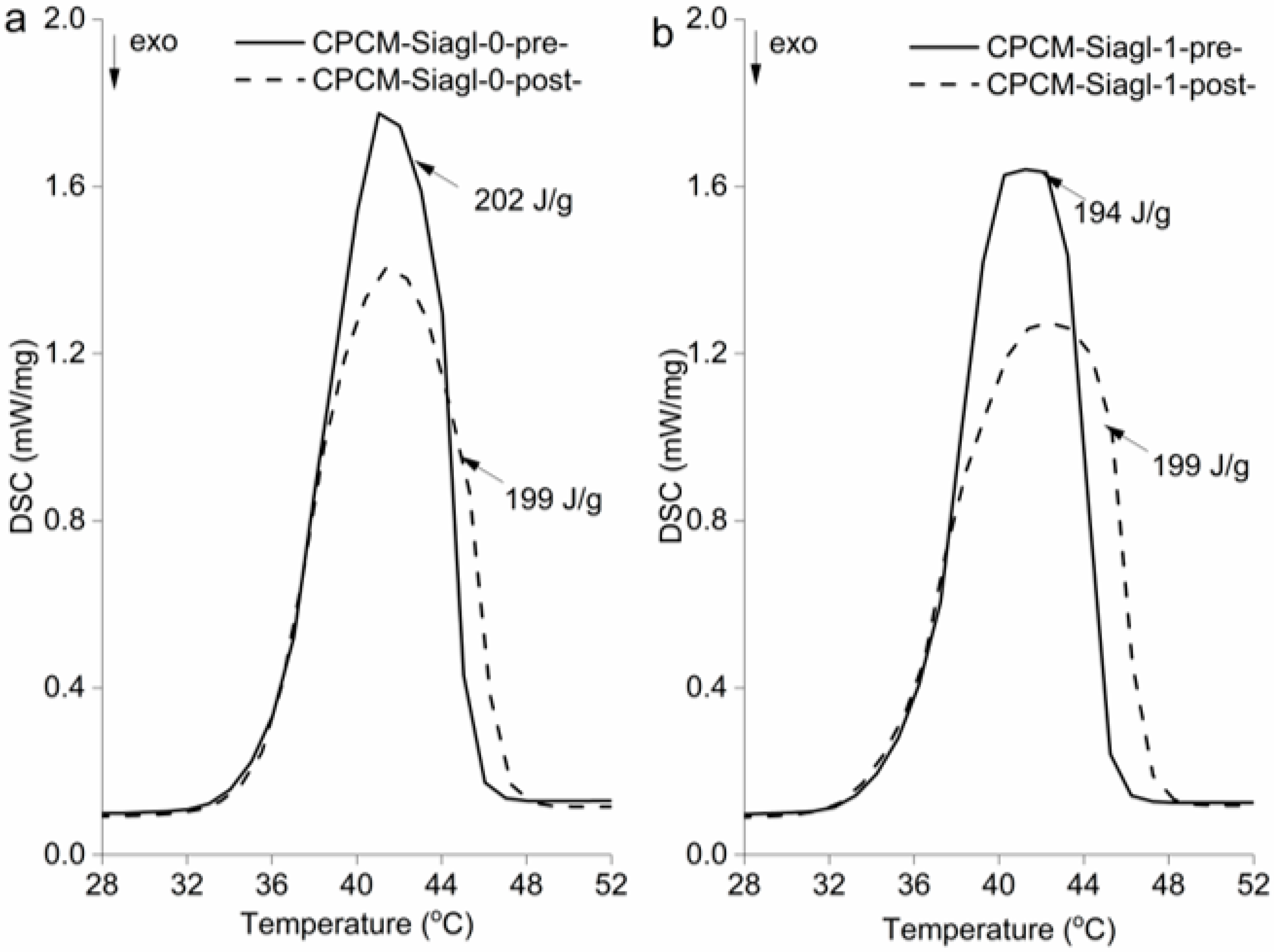
3.3. CPCMs Thermal Cycling Stability
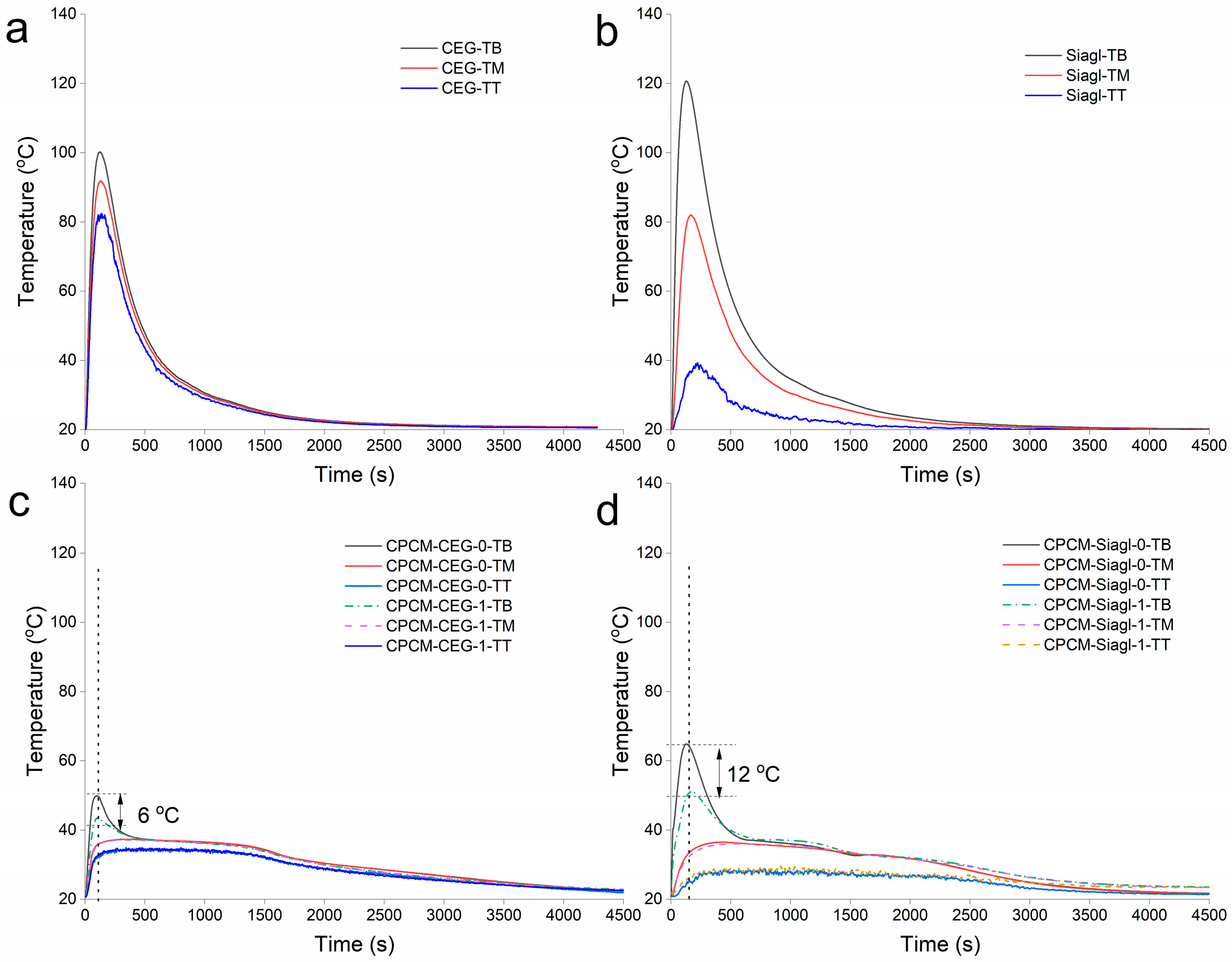
3.4. Thermal Response of CPCMs to Surface Temperature Increase
3.5. Simulated Behaviour of CPCMs in Response to Heating from Hydrogen Absorption in LaNi5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCM | Phase Change Material |
| CPCM | Composite Phase Change Material |
| MH | Metal Hydride |
| CEG | Compressed Expanded Graphite |
| Siagl | Silica aerogel |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| DSC | Differential Scanning Calorimetry |
References
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef] [PubMed]
- Kubilay Karayel, G.; Javani, N.; Dincer, I. A comprehensive assessment of energy storage options for green hydrogen. Energy Convers. Manag. 2023, 291, 117311. [Google Scholar] [CrossRef]
- Mulky, L.; Srivastava, S.; Lakshmi, T.; Sandadi, E.R.; Gour, S.; Thomas, N.A.; Shanmuga Priya, S.; Sudhakar, K. An overview of hydrogen storage technologies–Key challenges and opportunities. Mater. Chem. Phys. 2024, 325, 129710. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Liu, L.; Ilyushechkin, A.; Liang, D.; Cousins, A.; Tian, W.; Chen, C.; Yin, J.; Schoeman, L. Metal Hydride Composite Structures for Improved Heat Transfer and Stability for Hydrogen Storage and Compression Applications. Inorganics 2023, 11, 181. [Google Scholar] [CrossRef]
- Tao, J.; Luan, J.; Liu, Y.; Qu, D.; Yan, Z.; Ke, X. Technology development and application prospects of organic-based phase change materials: An overview. Renew. Sustain. Energy Rev. 2022, 159, 112175. [Google Scholar] [CrossRef]
- Jouhara, H.; Żabnieńska-Góra, A.; Khordehgah, N.; Ahmad, D.; Lipinski, T. Latent thermal energy storage technologies and applications: A review. Int. J. Thermofluids 2020, 5, 100039. [Google Scholar] [CrossRef]
- Zhu, J.; Lin, X.; Lv, L.; Li, M.; Luo, Q.; Kudiiarov, V.N.; Liu, W.; Leng, H.; Han, X.; Ma, Z. The relationship between thermal management methods and hydrogen storage performance of the metal hydride tank. J. Mater. Sci. Technol. 2024, 203, 66–77. [Google Scholar] [CrossRef]
- Raju, M.; Kumar, S. Optimization of heat exchanger designs in metal hydride based hydrogen storage systems. Int. J. Hydrogen Energy 2012, 37, 2767–2778. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, K.; Wei, Q.; Ma, L.; Ye, W.; Li, H.; Zhou, B.; Yu, Z.; Lin, C.T.; Luo, J.; et al. Thermal conductivity enhancement of phase change materials with 3D porous diamond foam for thermal energy storage. Appl. Energy 2019, 233, 208–219. [Google Scholar] [CrossRef]
- Wang, X.; Repaka, D.V.M.; Suwardi, A.; Zhu, Q.; Wu, J.; Xu, J. Thermal and Electrical Properties of Liquid Metal Gallium During Phase Transition. Trans. Tianjin Univ. 2023, 29, 209–215. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Zhu, Z.; Wang, Q.; Chen, Y.; Ma, T. Experimental study on the heat transfer performance of a gallium heat sink. Energy Convers. Manag. 2020, 213, 112853. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Zsembinszki, G.; Martín, M. Evaluation of volume change in phase change materials during their phase transition. J. Energy Storage 2020, 28, 101206. [Google Scholar] [CrossRef]
- Rahman, A.; Dickinson, M.; Farid, M. Microindentation of microencapsulated phase change materials. Adv. Mater. Res. 2011, 275, 85–88. [Google Scholar] [CrossRef]
- Vérez, D.; Borri, E.; Crespo, A.; Mselle, B.D.; de Gracia, Á.; Zsembinszki, G.; Cabeza, L.F. Experimental Study on Two PCM Macro-Encapsulation Designs in a Thermal Energy Storage Tank. Appl. Sci. 2021, 11, 6171. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials–A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Wang, X.; Yang, R.; Lin, K. Influence of additives on thermal conductivity of shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2006, 90, 1692–1702. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Farid, M.M. A novel graphite-PCM composite sphere with enhanced thermo-physical properties. Appl. Therm. Eng. 2018, 142, 401–409. [Google Scholar] [CrossRef]
- Mills, A.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal conductivity enhancement of phase change materials using a graphite matrix. Appl. Therm. Eng. 2006, 26, 1652–1661. [Google Scholar] [CrossRef]
- Xiong, F.; Zhou, J.; Jin, Y.; Zhang, Z.; Qin, M.; Han, H.; Shen, Z.; Han, S.; Geng, X.; Jia, K.; et al. Thermal shock protection with scalable heat-absorbing aerogels. Nat. Commun. 2024, 15, 7125. [Google Scholar] [CrossRef]
- Xie, L.; Wu, X.; Wang, G.; Shulga, Y.M.; Liu, Q.; Li, M.; Li, Z. Encapsulation of Paraffin Phase-Change Materials within Monolithic MTMS-Based Silica Aerogels. Gels 2023, 9, 317. [Google Scholar] [CrossRef]
- Yao, Y.; Cui, Y.; Deng, Z. Phase change composites of octadecane and gallium with expanded graphite as a carrier. RSC Adv. 2022, 12, 17217–17227. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Luo, Q.; Yang, X.; Yang, Y.; Tan, J.; Dong, Z.; Dang, J.; Li, J.; Chen, Y.; et al. Thermodynamics and kinetics of hydriding and dehydriding reactions in Mg-based hydrogen storage materials. J. Magnes. Alloys 2021, 9, 1922–1941. [Google Scholar] [CrossRef]
- Li, C.; Liu, Q.; Zhang, G.; Lin, L.; Ostrikov, K.K. Rapid synthesis of MTES-derived silica aerogel monoliths in Cetyltrimethylammonium bromide/water solvent system by ambient pressure drying. Powder Technol. 2023, 418, 118314. [Google Scholar] [CrossRef]
- Kumar, S.; Kojima, Y.; Dey, G.K. Thermodynamics and kinetics of hydrogen absorption–desorption of highly crystalline LaNi5. J. Therm. Anal. Calorim. 2018, 134, 889–894. [Google Scholar] [CrossRef]
- Muthukumar, P.; Satheesh, A.; Linder, M.; Mertz, R.; Groll, M. Studies on hydriding kinetics of some La-based metal hydride alloys. Int. J. Hydrogen Energy 2009, 34, 7253–7262. [Google Scholar] [CrossRef]
- Hubbard, W.N.; Rawlins, P.L.; Connick, P.A.; Stedwell, R. The standard enthalpy of formation of LaNi, The enthalpies of hydriding of LaNi5−xAlx. J. Chem. Thermodyn. 1983, 15, 785–798. [Google Scholar] [CrossRef]
- Albright, G.; Farid, M.; Al-Hallaj, S. Development of a model for compensating the influence of temperature gradients within the sample on DSC-results on phase change materials. J. Therm. Anal. Calorim. 2010, 101, 1155–1160. [Google Scholar] [CrossRef]
- Ghanbari, E.; Picken, S.J.; van Esch, J.H. Analysis of differential scanning calorimetry (DSC): Determining the transition temperatures, and enthalpy and heat capacity changes in multicomponent systems by analytical model fitting. J. Therm. Anal. Calorim. 2023, 148, 12393–12409. [Google Scholar] [CrossRef]






| Parameters | Paraffin (PCM38) | Gallium |
|---|---|---|
| Melting temperature (°C) | 36–38 | 29.8 |
| Latent heat (kJ/kg) | 226 | 80 |
| Volumetric latent heat (MJ/m3) | 188 | 472 |
| Liquid density (kg/L) | ~0.77 | 6.095 |
| Solid density (kg/L) | 0.83 | 5.904 |
| Specific heat (kJ/(kg·K)) | 2 | 0.4 |
| Volume expansion coefficient (%) | 7–8% | 3.1 |
| Thermal conductivity (W/(m·K)) | 0.24 | 29.28 |
| Type | S-L | S-L |
| Sample ID | Matrix | PCM | Temp. (°C) | Time | PCM Loading Mass % |
|---|---|---|---|---|---|
| CPCM-CEG-0 | CEG | paraffin | 40 | 18 h | 74–77 |
| CPCM-CEG-1 | CEG | Ga/paraffin | 40 | 18 h | 74–77 |
| CPCM-Siagl-0 | aerogel | paraffin | 40 | 10 min | 87–89 |
| CPCM1-Siagl-1 | aerogel | Ga/paraffin | 40 | 10 min | 87–89 |
| Name | Onset (°C) | End Set (°C) | M.P. (°C) | ΔH (J/g) | PCM Loading Mass % | Theoretical Latent Heat (J/g) 1 |
|---|---|---|---|---|---|---|
| Paraffin | 37.1 | 39.2 | 38.7 | 227 | - | - |
| Ga/Paraffin | 37 | 39 | 38.3 | 230 | - | - |
| CPCM-CEG-0 | 36.3 | 38.4 | 38.1 | 170 | 76.3 | 173 |
| CPCM-CEG-1 | 36.2 | 38.8 | 38.3 | 175 | 76.2 | 175 |
| CPCM-Siagl-0 | 36.2 | 40.6 | 39.5 | 197 | 88.1 | 199 |
| CPCM-Siagl-1 | 36.3 | 39.9 | 38.6 | 195 | 87.7 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; McCurdy, M.; Farid, M. The Use of Phase Change Materials for Thermal Management of Metal Hydride Reaction. Appl. Sci. 2025, 15, 9657. https://doi.org/10.3390/app15179657
Xu Y, McCurdy M, Farid M. The Use of Phase Change Materials for Thermal Management of Metal Hydride Reaction. Applied Sciences. 2025; 15(17):9657. https://doi.org/10.3390/app15179657
Chicago/Turabian StyleXu, Ying, Murray McCurdy, and Mohammed Farid. 2025. "The Use of Phase Change Materials for Thermal Management of Metal Hydride Reaction" Applied Sciences 15, no. 17: 9657. https://doi.org/10.3390/app15179657
APA StyleXu, Y., McCurdy, M., & Farid, M. (2025). The Use of Phase Change Materials for Thermal Management of Metal Hydride Reaction. Applied Sciences, 15(17), 9657. https://doi.org/10.3390/app15179657






