The Role of Gut Microbiota in Food Allergies and the Potential Role of Probiotics for Their Treatment
Abstract
1. Introduction
2. Search Strategy
2.1. Mechanisms of Action of Resident Gut Immune Cells
2.2. Role of Short-Chain Fatty Acids in Gut Immune Regulation
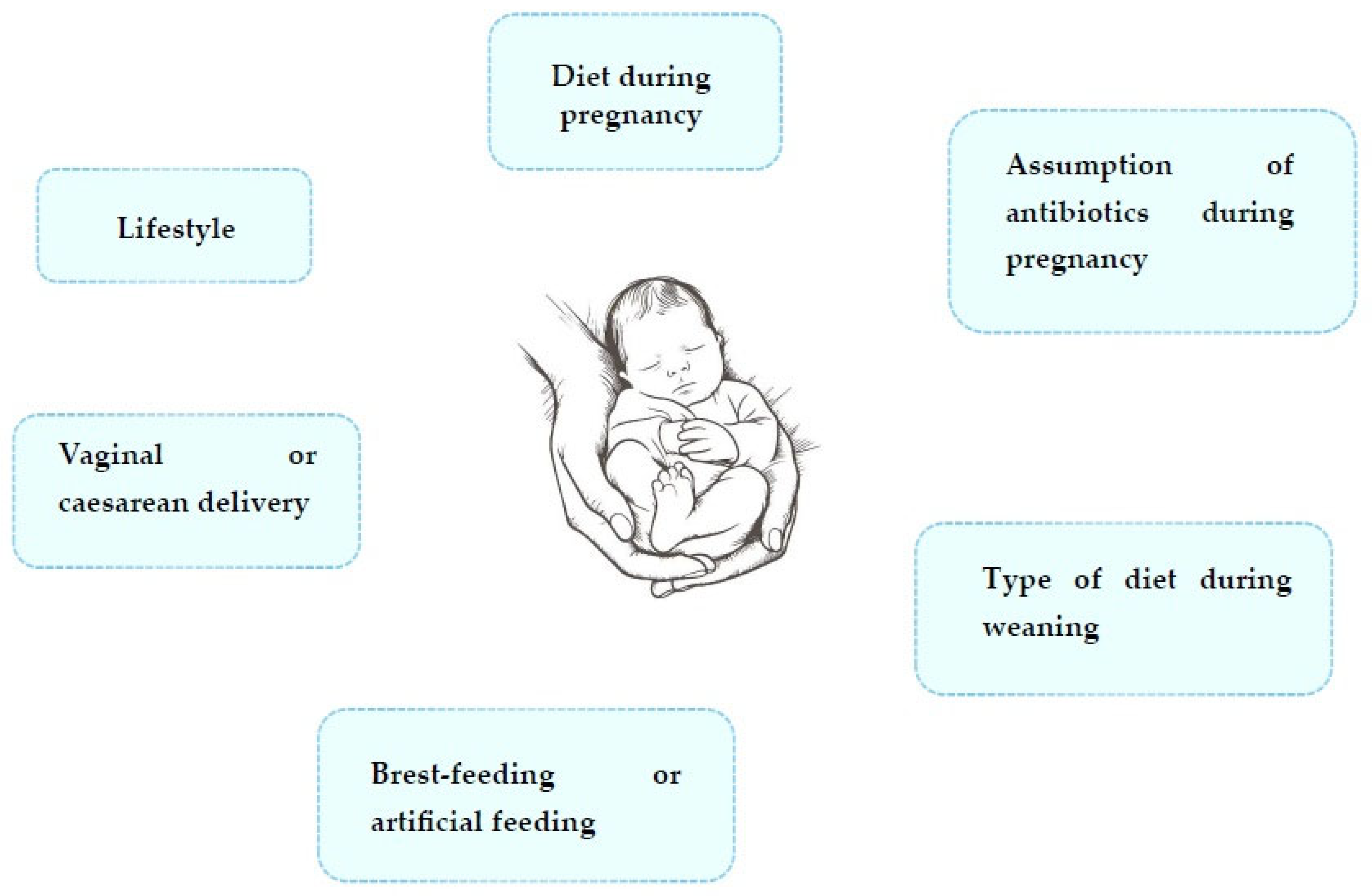
2.3. Relationship Between the Development of Food Allergies and Changes in Gut Microbiota in Early Life
2.4. Interventional Effects of Probiotics and Prebiotics
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| AGE | Advanced glycation end product |
| AhR | Aryl hydrocarbon receptor |
| AMPs | Antimicrobial peptides |
| cDCs | Conventional DCs |
| CMA | Cow milk allergy |
| CS | Caesarean section |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| DCs | Dendritic cells |
| EHCF | Extensively hydrolyzed casein formula |
| FAs | Food allergies |
| HMO | Human milk oligosaccharide |
| IECs | Intestinal epithelial cells |
| IL | Interleukin |
| LGG | L. rhamnosus GG |
| LPS | Lipopolysaccharides |
| M cells | Microfold cells |
| MCs | Mast cells |
| MHC | Major histocompatibility complex |
| mTECs | Medullary thymic epithelial cells |
| pDCs | Plasmacytoid DCs |
| PDL-1 | Programmed cell death protein 1 |
| PPs | Peyer Patches |
| PRR | Pattern recognition receptor |
| pTreg | Peripheral T regulatory |
| SCFAs | Short-chain fatty acids |
| sIgA | Secretory IgA |
| TCR | T cell receptor |
| TGF-β | Tumor growth factor-β |
| Th | T helper cells |
| TLR | Toll-like receptor |
| Treg | Regulatory T cell |
| tTreg | Thymic-derived Treg cell |
| UPF | Ultra-processed food |
References
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and Burden of Food Allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef]
- Gupta, R.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W. Food allergies around the world. Front. Nutr. 2024, 11, 1373110. [Google Scholar] [CrossRef]
- Kılıç, M.; Beyazıt, E.; Önalan, E.E.; Kaymaz, T.; Taşkın, E. Evaluation of toll-like receptors 2 and 4 polymorphism and intestinal microbiota in children with food allergies. Turk. J. Pediatr. 2023, 65, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. The interplay between the gut immune system and microbiota in health and disease: Nutraceutical intervention for restoring intestinal homeostasis. Curr. Pharm. Des. 2013, 19, 1329–1342. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides: Phylogenic Sources and Biological Activities. First of Two Parts. Curr. Pharm. Des. 2018, 24, 1043–1053. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Charitos, I.A.; Scacco, S.; Cotoia, A.; Castellaneta, F.; Castellana, G.; Pasqualotto, F.; Venneri, M.; Ferrulli, A.; Aliani, M.; Santacroce, L.; et al. Intestinal Microbiota Dysbiosis Role and Bacterial Translocation as a Factor for Septic Risk. Int. J. Mol. Sci. 2025, 26, 2028. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Bottalico, L.; Charitos, I.A.; Castellaneta, F.; Gaxhja, E.; Topi, S.; Palmirotta, R.; Jirillo, E. Exploitation of Natural By-Products for the Promotion of Healthy Outcomes in Humans: Special Focus on Antioxidant and Anti-Inflammatory Mechanisms and Modulation of the Gut Microbiota. Antioxidants 2024, 13, 796. [Google Scholar] [CrossRef]
- Costa, J.; Villa, C.; Verhoeckx, K.; Cirkovic-Velickovic, T.; Schrama, D.; Roncada, P.; Rodrigues, P.M.; Piras, C.; Martín-Pedraza, L.; Monaci, L.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens? Clin. Rev. Allergy Immunol. 2022, 62, 1–36. [Google Scholar] [CrossRef]
- Anvari, S.; Miller, J.; Yeh, C.Y.; Davis, C.M. IgE-Mediated Food Allergy. Clin. Rev. Allergy Immunol. 2019, 57, 244–260. [Google Scholar] [CrossRef]
- Leonard, S.A. Non-IgE-mediated Adverse Food Reactions. Curr. Allergy Asthma Rep. 2017, 17, 84. [Google Scholar] [CrossRef]
- Niess, J.H.; Kaymak, T. Eosinophilic Esophagitis Pathogenesis: All Clear? Inflamm. Intest. Dis. 2025, 10, 135–150. [Google Scholar] [CrossRef]
- Charitos, I.A.; Castellaneta, F.; Santacroce, L.; Bottalico, L. Historical Anecdotes and Breakthroughs of Histamine: From Discovery to Date. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 801–814. [Google Scholar] [CrossRef]
- León, B. Understanding the development of Th2 cell-driven allergic airway disease in early life. Front. Allergy 2023, 3, 1080153. [Google Scholar] [CrossRef] [PubMed]
- Worbs, T.; Hammerschmidt, S.I.; Förster, R. Dendritic cell migration in health and disease. Nat. Rev. Immunol. 2017, 17, 30–48. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Khosravi, A.; Zadeh, L.J.; Parizad, E.G. Role of IL-25 in Immunity. J. Clin. Diagn. Res. 2015, 9, OE01-4. [Google Scholar] [CrossRef]
- Berni Canani, R.; Caminati, M.; Carucci, L.; Eguiluz-Gracia, I. Skin, gut, and lung barrier: Physiological interface and target of intervention for preventing and treating allergic diseases. Allergy 2024, 79, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. New Perspectives in Food Allergy. Int. J. Mol. Sci. 2020, 21, 1474. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Jirillo, E. Mast Cells as a Double-Edged Sword in Immunity: Their Function in Health and Disease. First of Two Parts. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 654–669. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, C.; Zou, M.; Galvez, E.; Beckstette, M.; Ebel, M.; Strowig, T.; Huehn, J.; Pezoldt, J. The microbiota is dispensable for the early stages of peripheral regulatory T cell induction within mesenteric lymph nodes. Cell. Mol. Immunol. 2021, 18, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Cabeza-Cabrerizo, M.; Cardoso, A.; Minutti, C.M.; Pereira da Costa, M.; Reis e Sousa, C. Dendritic Cells Revisited. Annu. Rev. Immunol. 2021, 39, 131–166. [Google Scholar] [CrossRef]
- Tai, J.; Kwak, J.; Han, M.; Kim, T.H. Different Roles of Dendritic Cells for Chronic Rhinosinusitis Treatment According to Phenotype. Int. J. Mol. Sci. 2022, 23, 8032. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, I.; Kato, T.; Hemmi, H.; Fukuda-Ohta, Y.; Wakaki-Nishiyama, N.; Yamamoto, A.; Kaisho, T. Conventional Type 1 Dendritic Cells in Intestinal Immune Homeostasis. Front. Immunol. 2022, 13, 857954. [Google Scholar] [CrossRef]
- Carroll, S.L.; Pasare, C.; Barton, G.M. Control of adaptive immunity by pattern recognition receptors. Immunity 2024, 57, 632–648. [Google Scholar] [CrossRef]
- Rawat, K.; Tewari, A.; Li, X.; Mara, A.B.; King, W.T.; Gibbings, S.L.; Nnam, C.F.; Kolling, F.W.; Lambrecht, B.N.; Jakubzick, C.V. CCL5-producing migratory dendritic cells guide CCR5+ monocytes into the draining lymph nodes. J. Exp. Med. 2023, 220, e20222129. [Google Scholar] [CrossRef]
- Ellenbogen, Y.; Jiménez-Saiz, R.; Spill, P.; Chu, D.K.; Waserman, S.; Jordana, M. The Initiation of Th2 Immunity Towards Food Allergens. Int. J. Mol. Sci. 2018, 19, 1447. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Szajewska, H.; Lack, G. Food allergy and the gut. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 241–257. [Google Scholar] [CrossRef]
- Georgiev, P.; Charbonnier, L.M.; Chatila, T.A. Regulatory T Cells: The Many Faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Arroyo Hornero, R.; Hamad, I.; Côrte-Real, B.; Kleinewietfeld, M. The Impact of Dietary Components on Regulatory T Cells and Disease. Front. Immunol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Kondo, K.; Takada, K.; Takahama, Y. Antigen processing and presentation in the thymus: Implications for T cell repertoire selection. Curr. Opin. Immunol. 2017, 46, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Jirillo, E. The Tolerant Immune System: Biological Significance and Clinical Implications of T Cell Tolerance. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jiang, Y.; Xia, S. Regulation of thymic T regulatory cell differentiation by TECs in health and disease. Scand. J. Immunol. 2021, 94, e13094. [Google Scholar] [CrossRef] [PubMed]
- Santosh Nirmala, S.; Kayani, K.; Gliwiński, M.; Hu, Y.; Iwaszkiewicz-Grześ, D.; Piotrowska-Mieczkowska, M.; Sakowska, J.; Tomaszewicz, M.; Marín Morales, J.M.; Lakshmi, K.; et al. Beyond FOXP3: A 20-year journey unravelling human regulatory T-cell heterogeneity. Front. Immunol. 2024, 14, 1321228. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wan, Y.Y. Intricacies of TGF-beta signaling in Treg and Th17 cell biology. Cell. Mol. Immunol. 2023, 20, 1002–1022. [Google Scholar] [CrossRef]
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117. [Google Scholar] [CrossRef]
- Allen, F.; Tong, A.A.; Huang, A.Y. Unique Transcompartmental Bridge: Antigen-Presenting Cells Sampling across Endothelial and Mucosal Barriers. Front. Immunol. 2016, 7, 231. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Miyamoto, K.; Chida, A.; Okuzawa, A.T.; Yoshimatsu, Y.; Kudo, Y.; Sujino, T. Localization and movement of Tregs in gastrointestinal tract: A systematic review. Inflamm. Regen. 2022, 42, 47. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef] [PubMed]
- Losol, P.; Wolska, M.; Wypych, T.P.; Yao, L.; O’Mahony, L.; Sokolowska, M. A cross talk between microbial metabolites and host immunity: Its relevance for allergic diseases. Clin. Transl. Allergy 2024, 14, e12339. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell. Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Luu, M.; Visekruna, A. Short-chain fatty acids: Bacterial messengers modulating the immunometabolism of T cells. Eur. J. Immunol. 2019, 49, 842–848. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Berin, M.C. Food allergy and the microbiome: Current understandings and future directions. J. Allergy Clin. Immunol. 2019, 144, 1468–1477. [Google Scholar] [CrossRef]
- Narushima, S.; Sugiura, Y.; Oshima, K.; Atarashi, K.; Hattori, M.; Suematsu, M.; Honda, K. Characterization of the 17 strains of regulatory T cell-inducing human-derived Clostridia. Gut Microbes 2014, 5, 333–339. [Google Scholar] [CrossRef]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Kellermann, L.; Jensen, K.B.; Bergenheim, F.; Gubatan, J.; Chou, N.D.; Moss, A.; Nielsen, O.H. Mucosal vitamin D signaling in inflammatory bowel disease. Autoimmun. Rev. 2020, 19, 102672. [Google Scholar] [CrossRef]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial barrier hypothesis: Effect of the external exposome on the microbiome and epithelial barriers in allergic disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Wang, T.M.; Wang, R.X.; Colgan, S.P. Physiologic hypoxia in the intestinal mucosa: A central role for short-chain fatty acids. Am. J. Physiol. Cell. Physiol. 2024, 327, C1087–C1093. [Google Scholar] [CrossRef]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids-A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell. Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Seo, J.; Yeun, J.; Choi, H.; Kim, Y.I.; Chang, S.Y. The role of mucosal barriers in human gut health. Arch. Pharm. Res. 2021, 44, 325–341. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Kim, K.S.; Hong, S.W.; Han, D.; Yi, J.; Jung, J.; Yang, B.G.; Lee, J.Y.; Lee, M.; Surh, C.D. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 2016, 351, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, Y.M.; Kim, B.; Tae, I.H.; Kim, N.E.; Pranata, M.; Kim, T.; Won, S.; Kang, N.J.; Lee, Y.K.; et al. Disordered development of gut microbiome interferes with the establishment of the gut ecosystem during early childhood with atopic dermatitis. Gut Microbes 2022, 14, 2068366. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Ververis, K.; Karagiannis, T.C. Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy 2011, 2011, 869647. [Google Scholar] [CrossRef]
- Rajput, M.; Momin, T.; Singh, A.; Banerjee, S.; Villasenor, A.; Sheldon, J.; Paudel, P.; Rajput, R. Determining the association between gut microbiota and its metabolites with higher intestinal Immunoglobulin A response. Vet. Anim. Sci. 2022, 19, 100279. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.H.; Ellis, J.A.; Saffery, R.; Allen, K.J. The role of genetics and environment in the rise of childhood food allergy. Clin. Exp. Allergy 2012, 42, 20–29. [Google Scholar] [CrossRef]
- Renz, H.; Holt, P.G.; Inouye, M.; Logan, A.C.; Prescott, S.L.; Sly, P.D. An exposome perspective: Early-life events and immune development in a changing world. J. Allergy Clin. Immunol. 2017, 140, 24–40. [Google Scholar] [CrossRef]
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Montoya-Williams, D.; Lemas, D.J.; Spiryda, L.; Patel, K.; Carney, O.O.; Neu, J.; Carson, T.L. The neonatal microbiome and its partial role in mediating the association between birth by cesarean section and adverse pediatric outcomes. Neonatology 2018, 114, 103–111. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Ye, Y.; Wang, F.; Chen, F.; Zheng, C. The Role of Microbiota in Infant Health: From Early Life to Adulthood. Front. Immunol. 2021, 12, 708472. [Google Scholar] [CrossRef]
- Pan, K.; Zhang, C.; Tian, J. The Effects of Different Modes of Delivery on the Structure and Predicted Function of Intestinal Microbiota in Neonates and Early Infants. Pol. J. Microbiol. 2021, 70, 45–55. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Björkstén, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef]
- Papathoma, E.; Triga, M.; Fouzas, S.; Dimitriou, G. Cesarean Section Delivery and Development of Food Allergy and Atopic Dermatitis in Early Childhood. Pediatr. Allergy Immunol. 2016, 27, 419–424. [Google Scholar] [CrossRef]
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of lactation stage, gestational age and mode of delivery on breast milk microbiota. J. Perinatol. 2014, 34, 599–605. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Antimicrobial Peptides in Human Disease: Therapeutic Approaches. Second of Two Parts. Curr. Pharm. Des. 2018, 24, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the gut microbiota by breastfeeding: The gateway to allergy prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Ongena, R.; Dierick, M.; Vanrompay, D.; Cox, E.; Devriendt, B. Lactoferrin impairs pathogen virulence through its proteolytic activity. Front. Vet. Sci. 2024, 11, 1428156. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.K.; McGuire, M.A. Got bacteria? The astounding, yet not-so-surprising, microbiome of human milk. Curr. Opin. Biotechnol. 2017, 44, 63–68. [Google Scholar] [CrossRef]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, B.; Hu, Y.; Zhao, Y. New insights into gut-bacteria-derived indole and its derivatives in intestinal and liver diseases. Front. Pharmacol. 2021, 12, 769501. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.; Sung, G.Y. Effects of indole-3-lactic acid, a metabolite of tryptophan, on IL-4 and IL-13-induced human skin-equivalent atopic dermatitis models. Int. J. Mol. Sci. 2022, 23, 13520. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl hydrocarbon receptor activation modulates intestinal epithelial barrier function by maintaining tight junction integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez Longo, M.N.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Kirjavainen, P.; Eerola, E.; Kero, P.; Salminen, S.; Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was not developing. J. Allergy Clin. Immunol. 2001, 107, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, T.R.; Jakobsson, H.E.; Andersson, A.F.; Björkstén, B.; Engstrand, L.; Jenmalm, M.C. Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 2012, 129, 434–440. [Google Scholar] [CrossRef]
- Fieten, K.B.; Totté, J.E.E.; Levin, E.; Reyman, M.; Meijer, Y.; Knulst, A.; Schuren, F.; Pasmans, S.G.M.A. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. Int. Arch. Allergy Immunol. 2018, 175, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Brugman, S.; Perdijk, O.; van Neerven, R.J.; Savelkoul, H.F. Mucosal immune development in early life: Setting the stage. Arch. Immunol. Ther. Exp. 2015, 63, 251–268. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic immune system development in newborn children. Cell 2018, 174, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Rigo-Adrover, M.D.M.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Preclinical immunomodulation by the probiotic Bifidobacterium breve M-16V in early life. PLoS ONE 2016, 11, e0166082. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J.; et al. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial signature in IgE-mediated food allergies. Genome Med. 2020, 12, 92. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Caffarelli, C.; Di Mauro, D.; Mastrorilli, C.; Bottau, P.; Cipriani, F.; Ricci, G. Solid food introduction and the development of food allergies. Nutrients 2018, 10, 1790. [Google Scholar] [CrossRef] [PubMed]
- Koukou, Z.; Papadopoulou, E.; Panteris, E.; Papadopoulou, S.; Skordou, A.; Karamaliki, M.; Diamanti, E. The effect of breastfeeding on food allergies in newborns and infants. Children 2023, 10, 1046. [Google Scholar] [CrossRef]
- Scarpone, R.; Kimkool, P.; Ierodiakonou, D.; Leonardi-Bee, J.; Garcia-Larsen, V.; Perkin, M.R.; Boyle, R.J. Timing of allergenic food introduction and risk of immunoglobulin E-mediated food allergy: A systematic review and meta-analysis. JAMA Pediatr. 2023, 177, 489–497. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Skjerven, H.O.; Lie, A.; Vettukattil, R.; Rehbinder, E.M.; LeBlanc, M.; Asarnoj, A.; Carlsen, K.H.; Despriee, Å.W.; Färdig, M.; Gerdin, S.W.; et al. Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet. 2022, 399, 2398–23411. [Google Scholar] [CrossRef]
- Tsuang, A.J.; Nowak-Węgrzyn, A.H. Increased food diversity in the first year of life is inversely associated with allergic diseases. Pediatrics 2014, 134, S139–S140. [Google Scholar] [CrossRef]
- Du Toit, G.; Foong, R.X.; Lack, G. Corrigendum to “Prevention of food allergy-Early dietary interventions” [Allergol Int 65 (2016) 370-377]. Allergol. Int. 2017, 66, 159. [Google Scholar] [CrossRef]
- Augustine, T.; Kumar, M.; Al Khodor, S.; van Panhuys, N. Microbial dysbiosis tunes the immune response towards allergic disease outcomes. Clin. Rev. Allergy Immunol. 2023, 65, 43–71. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; van Neerven, R.J.J. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 36273641. [Google Scholar] [CrossRef]
- Catanzaro, J.R.; Strauss, J.D.; Bielecka, A.; Porto, A.F.; Lobo, F.M.; Urban, A.; Schofield, W.B.; Palm, N.W. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci. Rep. 2019, 9, 13574. [Google Scholar] [CrossRef]
- Kukkonen, K.; Kuitunen, M.; Haahtela, T.; Korpela, R.; Poussa, T.; Savilahti, E. High intestinal IgA associates with reduced risk of IgE-associated allergic diseases. Pediatr. Allergy Immunol. 2010, 21, 67–73. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Stephen-Victor, E.; Gerber, G.K.; Noval Rivas, M.; Wang, S.; Harb, H.; Wang, L.; Li, N.; Crestani, E.; Spielman, S.; et al. Microbiota therapy acts via a regulatory T cell MyD88/RORγt pathway to suppress food allergy. Nat. Med. 2019, 25, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Ballini, A.; Boccellino, M.; Scacco, S.; Lovero, R.; Charitos, I.A.; Santacroce, L. The Intestinal Microbiota May Be a Potential Theranostic Tool for Personalized Medicine. J. Pers. Med. 2022, 12, 523. [Google Scholar] [CrossRef]
- Jones, A.L.; Curran-Everett, D.; Leung, D.Y.M. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J. Allergy Clin. Immunol. 2016, 137, 1247–1248.e3. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Guttman-Yassky, E. Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. J. Allergy Clin. Immunol. 2014, 134, 769–779. [Google Scholar] [CrossRef]
- Tham, E.; Chia, M.; Riggioni, C.; Nagarajan, N.; Common, J.E.A.; Kong, H.H. The skin microbiome in pediatric atopic dermatitis and food allergy. Allergy. 2024, 79, 1470–1484. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Hu, C.; Liu, Y.; Wang, X.; Zhang, J.; Wang, F.; Zhu, M. Short-chain fatty acids as a target for prevention against food allergy by regulatory T cells. JGH Open 2019, 3, 190–195. [Google Scholar] [CrossRef]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.; Hanekamp, D.; Veldhoen, M.; et al. Diet-derived short chain fatty acids stimulate intestinal epithelial cells to induce mucosal tolerogenic dendritic cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef]
- Wu, W.; Sun, M.; Chen, F.; Cao, A.T.; Liu, H.; Zhao, Y.; Huang, X.; Xiao, Y.; Yao, S.; Zhao, Q.; et al. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol. 2017, 10, 946–956. [Google Scholar] [CrossRef]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- de Taeye, S.W.; Rispens, T.; Vidarsson, G. The ligands for human IgG and their effector functions. Antibodies 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.M.; Rantanen, V.; Lin, J.S.; Karinen, S.; Saarinen, K.M.; Goldis, M.; Mäkelä, M.J.; Hautaniemi, S.; Savilahti, E.; Sampson, H.A. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J. Allergy Clin. Immunol. 2010, 125, 1315–1321.e9. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; James, L.K.; Bahnson, H.T.; Shamji, M.H.; Couto-Francisco, N.C.; Islam, S.; Houghton, S.; Clark, A.T.; Stephens, A.; Turcanu, V.; et al. IgG4 inhibits peanut-induced basophil and mast cell activation in peanut-tolerant children sensitized to peanut major allergens. J. Allergy Clin. Immunol. 2015, 135, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Brichacek, A.L.; Florkowski, M.; Abiona, E.; Frank, K.M. Ultra-Processed Foods: A Narrative Review of the Impact on the Human Gut Microbiome and Variations in Classification Methods. Nutrients 2024, 16, 1738. [Google Scholar] [CrossRef]
- Davis, K.E.; Prasad, C.; Vijayagopal, P.; Juma, S.; Adams-Huet, B.; Imrhan, V. Contribution of dietary advanced glycation end products (AGE) to circulating AGE: Role of dietary fat. Br. J. Nutr. 2015, 114, 1797–1806. [Google Scholar] [CrossRef]
- Sterenczak, K.A.; Nolte, I.; Murua, E.H. RAGE splicing variants in mammals. Methods Mol. Biol. 2013, 963, 265–276. [Google Scholar] [CrossRef]
- Forde, B.; Yao, L.; Shaha, R.; Murphy, S.; Lunjani, N.; O’Mahony, L. Immunomodulation by foods and microbes: Unravelling the molecular tango. Allergy 2022, 77, 3513–3526. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, A.; Martínez, M.; Babio, N.; Konstanti, P.; Tinahones, F.J.; Vioque, J.; Corella, D.; Fitó, M.; Vidal, J.; Moreno-Indias, I.; et al. Association between ultra-processed food consumption and gut microbiota in senior subjects with overweight/obesity and metabolic syndrome. Front. Nutr. 2022, 9, 976547. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martinez, J.A.; Riezu-Boj, J.I. Gut microbiota differences according to ultra-processed food consumption in a Spanish population. Nutrients 2021, 13, 2710. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Jang, H.H.; Kim, G.; Zouiouich, S.; Cho, S.Y.; Kim, H.J.; Kim, J.; Choe, J.S.; Gunter, M.J.; Ferrari, P.; et al. Taxonomic composition and diversity of the gut microbiota in relation to habitual dietary intake in Korean adults. Nutrients 2021, 13, 366. [Google Scholar] [CrossRef]
- Akagawa, S.; Kaneko, K. Gut microbiota and allergic diseases in children. Allergol. Int. 2022, 71, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J.; et al. Reduced Genetic Potential for Butyrate Fermentation in the Gut Microbiome of Infants Who Develop Allergic Sensitization. J. Allergy Clin. Immunol. 2019, 44, 1638–1647.e3. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High Levels of Butyrate and Propionate in Early Life Are Associated with Protection Against Atopy. Allergy 2019, 74, 799–809. [Google Scholar] [CrossRef]
- Berdi, M.; de Lauzon-Guillain, B.; Forhan, A.; Castelli, F.A.; Fenaille, F.; Charles, M.A.; Heude, B.; Junot, C.; Adel-Patient, K.; EDEN Mother-Child Cohort Study Group. Immune Components of Early Breastmilk: Association with Maternal Factors and with Reported Food Allergy in Childhood. Pediatr. Allergy Immunol. 2019, 30, 107–116. [Google Scholar] [CrossRef]
- Savage, J.; Sicherer, S.; Wood, R. The Natural History of Food Allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203, quiz 204. [Google Scholar] [CrossRef]
- Leonard, S.A.; Sampson, H.A.; Sicherer, S.H.; Noone, S.; Moshier, E.L.; Godbold, J.; Nowak-Wegrzyn, A. Dietary baked egg accelerates resolution of egg allergy in children. J. Allergy Clin. Immunol. 2012, 130, 473–480.e471. [Google Scholar] [CrossRef]
- Adam, T.; Divaret-Chauveau, A.; Roduit, C.; Adel-Patient, K.; Deschildre, A.; Raherison, C.; Charles, M.A.; Nicklaus, S.; de Lauzon-Guillain, B. Complementary feeding practices are related to the risk of food allergy in the ELFE cohort. Allergy 2023, 78, 2456–2466. [Google Scholar] [CrossRef]
- Hua, X.; Song, L.; Yu, G.; Vogtmann, E.; Goedert, J.J.; Abnet, C.C.; Landi, M.T.; Shi, J. MicrobiomeGWAS: A Tool for identi-fying Host Genetic Variants Associated with Microbiome Composition. Genes 2022, 13, 1224. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Matsumoto, M.; Aranami, A.; Ishige, A.; Watanabe, K.; Benno, Y. LKM512 yogurt consumption improves the intestinal environment and induces the T-helper type 1 cytokine in adult patients with intractable atopic dermatitis. Clin. Exp. Allergy 2007, 37, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Cela, L.; Brindisi, G.; Gravina, A.; Pastore, F.; Semeraro, A.; Bringheli, I.; Marchetti, L.; Morelli, R.; Cinicola, B.; Capponi, M.; et al. Molecular Mechanism and Clinical Effects of Probiotics in the Management of Cow’s Milk Protein Allergy. Int. J. Mol. Sci. 2023, 24, 9781. [Google Scholar] [CrossRef]
- Ahanchian, H.; Nouri, Z.; Jafari, S.A.; Moghiman, T.; Amirian, M.H.; Ezzati, A.; Kianifar, H.R. Synbiotics in Children with Cow’s Milk Allergy: A Randomized Controlled Trial. Iran. J. Pediatr. 2014, 24, 29–34. [Google Scholar]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: A randomized trial. J. Allergy Clin. Immunol. 2012, 129, 580–582.e5. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D.; Althera Study Group. Treating cow’s milk protein allergy: A double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr. 2013, 102, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Sharma, G.; Im, S.H. Probiotics as a Potential Immunomodulating Pharmabiotics in Allergic Diseases: Current Status and Future Prospects. Allergy Asthma Immunol. Res. 2018, 10, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Mahallei, M.; Pormohammad, A.; Varshochi, M.; Ganbarov, K.; Zeinalzadeh, E.; Yousefi, B.; Bastami, M.; Tanomand, A.; Mahmood, S.S.; et al. Microbial balance in the intestinal microbiota and its association with diabetes, obesity and allergic disease. Microb. Pathog. 2019, 127, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Morgano, G.P.; Zhang, Y.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Gandhi, S.; et al. World Allergy Organization-McMaster University guidelines for allergic disease prevention (GLAD-P): Prebiotics. World Allergy Organ J. 2016, 9, 10. [Google Scholar] [CrossRef]
- Santos, S.C.D.; Konstantyner, T.; Cocco, R.R. Effects of probiotics in the treatment of food hypersensitivity in children: A systematic review. Allergol. Immunopathol. 2020, 48, 95–104. [Google Scholar] [CrossRef]
- Qamer, S.; Deshmukh, M.; Patole, S. Probiotics for cow’s milk protein allergy: A systematic review of randomized controlled trials. Eur. J. Pediatr. 2019, 178, 1139–1149. [Google Scholar] [CrossRef]
- Canani, R.B.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: A prospective multicenter study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar] [CrossRef]
- Cukrowska, B.; Ceregra, A.; Maciorkowska, E.; Surowska, B.; Zegadło-Mylik, M.A.; Konopka, E.; Trojanowska, I.; Zakrzewska, M.; Bierła, J.B.; Zakrzewski, M.; et al. The Effectiveness of Probiotic Lactobacillus rhamnosus and Lactobacillus casei Strains in Children with Atopic Dermatitis and Cow’s Milk Protein Allergy: A Multicenter, Randomized, Double Blind, Placebo Controlled Study. Nutrients 2021, 13, 1169. [Google Scholar] [CrossRef]
- Sorensen, K.; Cawood, A.L.; Cooke, L.H.; Acosta-Mena, D.; Stratton, R.J. The Use of an Amino Acid Formula Containing Synbiotics in Infants with Cow’s Milk Protein Allergy-Effect on Clinical Outcomes. Nutrients 2021, 13, 2205. [Google Scholar] [CrossRef]
- Chatchatee, P.; Nowak-Wegrzyn, A.; Lange, L.; Benjaponpitak, S.; Chong, K.W.; Sangsupawanich, P.; van Ampting, M.T.J.; Oude Nijhuis, M.M.; Harthoorn, L.F.; Langford, J.E.; et al. Tolerance development in cow’s milk-allergic infants receiving amino acid-based formula: A randomized controlled trial. J. Allergy Clin. Immunol. 2022, 149, 650–658.e5. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Węgrzyn, A.; Chatchatee, P. Mechanisms of Tolerance Induction. Ann. Nutr. Metab. 2017, 70, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Aureli, P.; Capurso, L.; Castellazzi, A.M.; Clerici, M.; Giovannini, M.; Morelli, L.; Poli, A.; Pregliasco, F.; Salvini, F.; Zuccotti, G.V. Probiotics and health: An evidence-based review. Pharmacol. Res. 2011, 63, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics and prebiotics-Progress and challenges. Int. Dairy J. 2008, 18, 969–975. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925. [Google Scholar] [CrossRef]
- Baba, N.; Samson, S.; Bourdet-Sicard, R.; Rubio, M.; Sarfati, M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. J. Leukoc. Biol. 2008, 84, 468–476. [Google Scholar] [CrossRef]

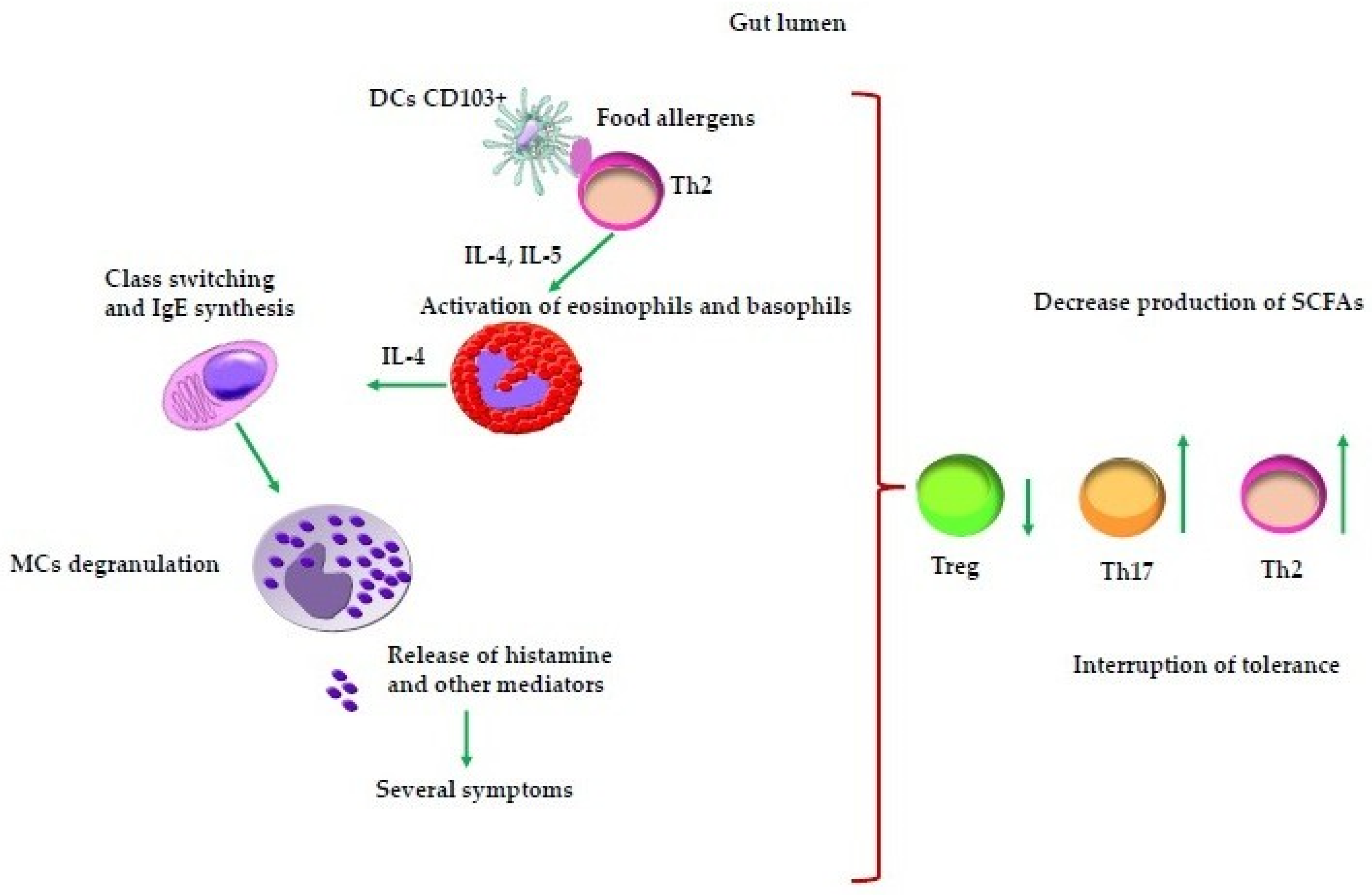
| Cell Type/Subset | Phenotypic Markers | Main Functions | Role in FAs | Role in Immune Tolerance |
|---|---|---|---|---|
| DCs | cDCs: CD11c+, MHC-II, pDC subsets; | Antigen capture, processing, and presentation; PRR sensing; IL secretion | Present allergens to naive CD3+CD4+ T cells; Th2 polarization via IL-4, IL-5, IL-13; Promote IgE class switching in B cells | ----- |
| Tolerogenic DCs | CD103+ PDL-1, MHC-II | Antigen capture, processing, TGF-β and IL-10 secretion | Induce Treg differentiation | In the absence of inflammation, promote peripheral tolerance |
| Naïve CD4+ T lymphocytes | CD3+, CD4+ | Differentiate into several subsets depending on IL milieu | IL-4/IL-13 influence, differentiation into Th2 amplifying allergic inflammation | Under TGF-β/retinoic acid, differentiate into pTreg, maintaining tolerance |
| Th2 lymphocytes | CD3+CD4+, GATA-3+ | Secrete IL-4, IL-5, IL-13 | IL-4: IgE class switching; IL-5: Eosinophil/basophil recruitment; IL-13: Mucus production, barrier alteration | ----- |
| B lymphocytes/Plasma cells | CD3−CD19, CD3−CD20+/CD138+ | Antibody production | Production of allergen-specific IgE that binds FcεRI on MCs/basophils | Under IL-10/TGF-β influence, produce IgG4 (blocking antibodies) |
| MCs | FcεRI+, c-kit+ | Degranulation: histamine, Leukotrienes, Prostaglandins release | Type I hypersensitivity reaction: vasodilation, smooth muscle contraction, mucus secretion | Regulated activation prevents inappropriate inflammation |
| Basophils | FcεRI+, IL-5Rα+ | Circulating effector cells, IL producers | Amplify Th2 inflammation; release mediators on IgE crosslinking | Minimal or null role in immune tolerance |
| Eosinophils | CCR3+, IL-5Rα+ | Cytotoxic granule release, IL secretion | Tissue damage and amplification of inflammation in FAs | ----- |
| Treg cells | CD3+CD4+ CD25+FoxP3+ | Immunosuppression via IL-10, TGF-β, CTLA-4 | Deficiency/dysfunction: Loss of tolerance; FA development | Maintain tolerance to food antigens, commensals, and self-antigens |
| Goblet cells and CX3CR1+ macrophages | MUC2+/CX3CR1+ | Antigen sampling and transfer to DCs | When the barrier is impaired, increased allergen translocation can occur | Facilitate tolerogenic Ag presentation to DCs |
| Gut microbiota | ----- | Modulate immune development and barrier function | Dysbiosis leads to Th2 skewing and FA risk | Eubiosis supports Treg induction and mucosal barrier integrity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magrone, T.; Magrone, M.; Notaristefano, R.; Gaxhja, E.; Rezaeinezhad, N.; Topi, S.; Santacroce, L.; Colella, M. The Role of Gut Microbiota in Food Allergies and the Potential Role of Probiotics for Their Treatment. Appl. Sci. 2025, 15, 9590. https://doi.org/10.3390/app15179590
Magrone T, Magrone M, Notaristefano R, Gaxhja E, Rezaeinezhad N, Topi S, Santacroce L, Colella M. The Role of Gut Microbiota in Food Allergies and the Potential Role of Probiotics for Their Treatment. Applied Sciences. 2025; 15(17):9590. https://doi.org/10.3390/app15179590
Chicago/Turabian StyleMagrone, Thea, Manrico Magrone, Rossana Notaristefano, Elona Gaxhja, Najmeh Rezaeinezhad, Skender Topi, Luigi Santacroce, and Marica Colella. 2025. "The Role of Gut Microbiota in Food Allergies and the Potential Role of Probiotics for Their Treatment" Applied Sciences 15, no. 17: 9590. https://doi.org/10.3390/app15179590
APA StyleMagrone, T., Magrone, M., Notaristefano, R., Gaxhja, E., Rezaeinezhad, N., Topi, S., Santacroce, L., & Colella, M. (2025). The Role of Gut Microbiota in Food Allergies and the Potential Role of Probiotics for Their Treatment. Applied Sciences, 15(17), 9590. https://doi.org/10.3390/app15179590








