Genome-Wide In Silico Analysis Expanding the Potential Allergen Repertoire of Mango (Mangifera indica L.)
Abstract
1. Introduction
2. Materials and Methods
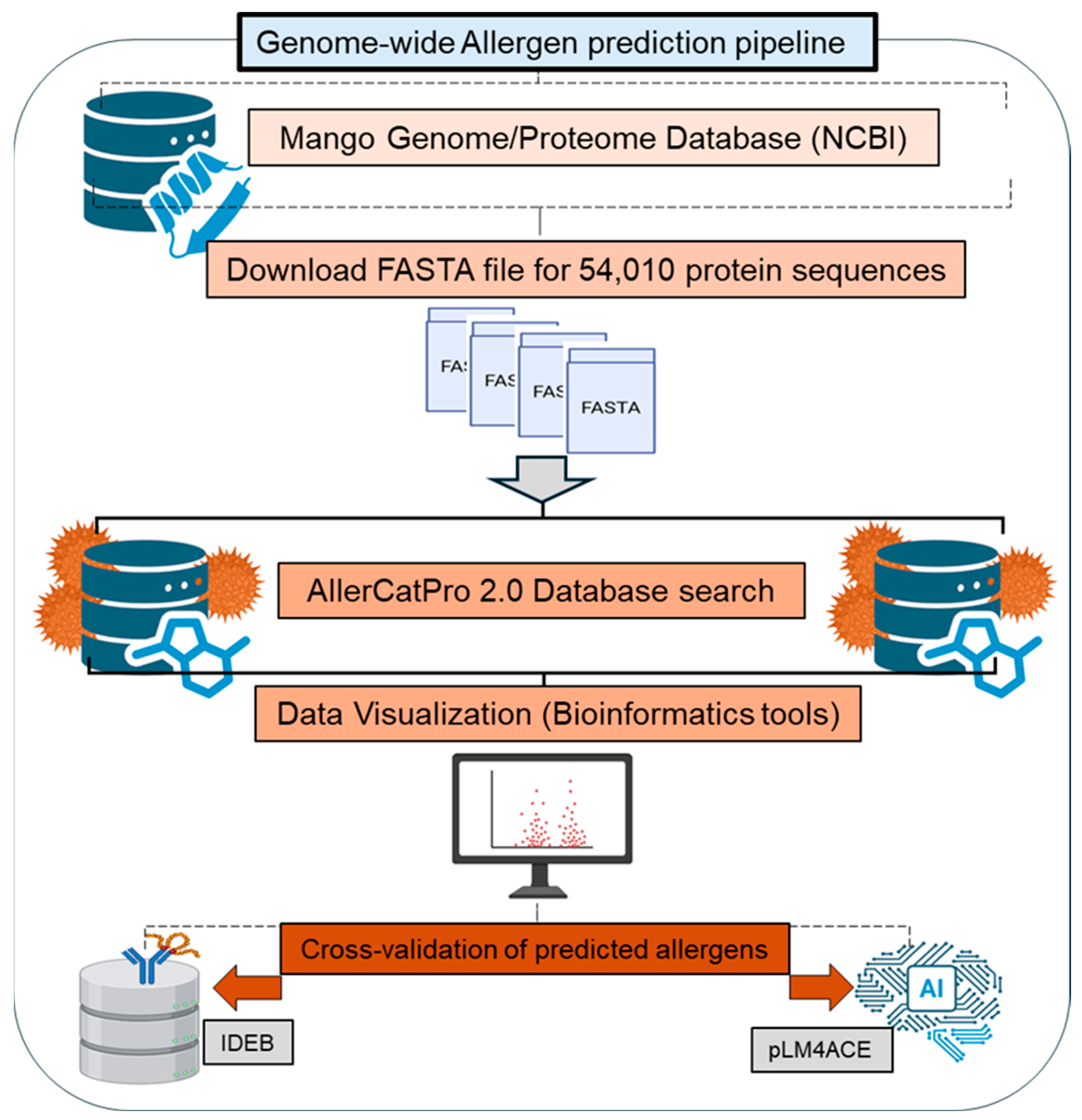
2.1. Collection of Mango Protein Sequences
2.2. In Silico Platform for Identification of Potential Mango Allergens
2.3. Bioinformatics Tools for Data Processing
3. Results and Discussion
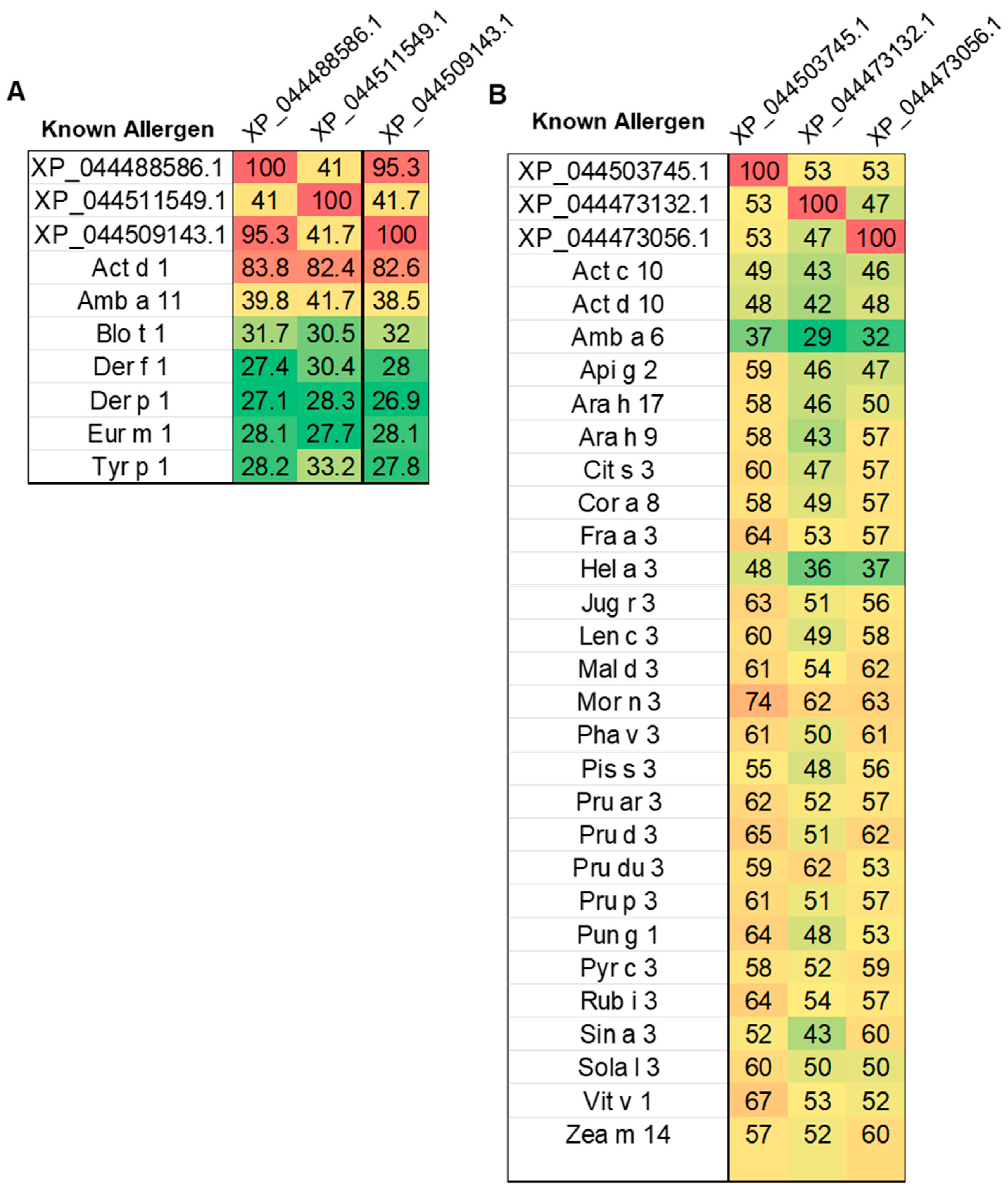
3.1. Identification of Known Mango Allergens
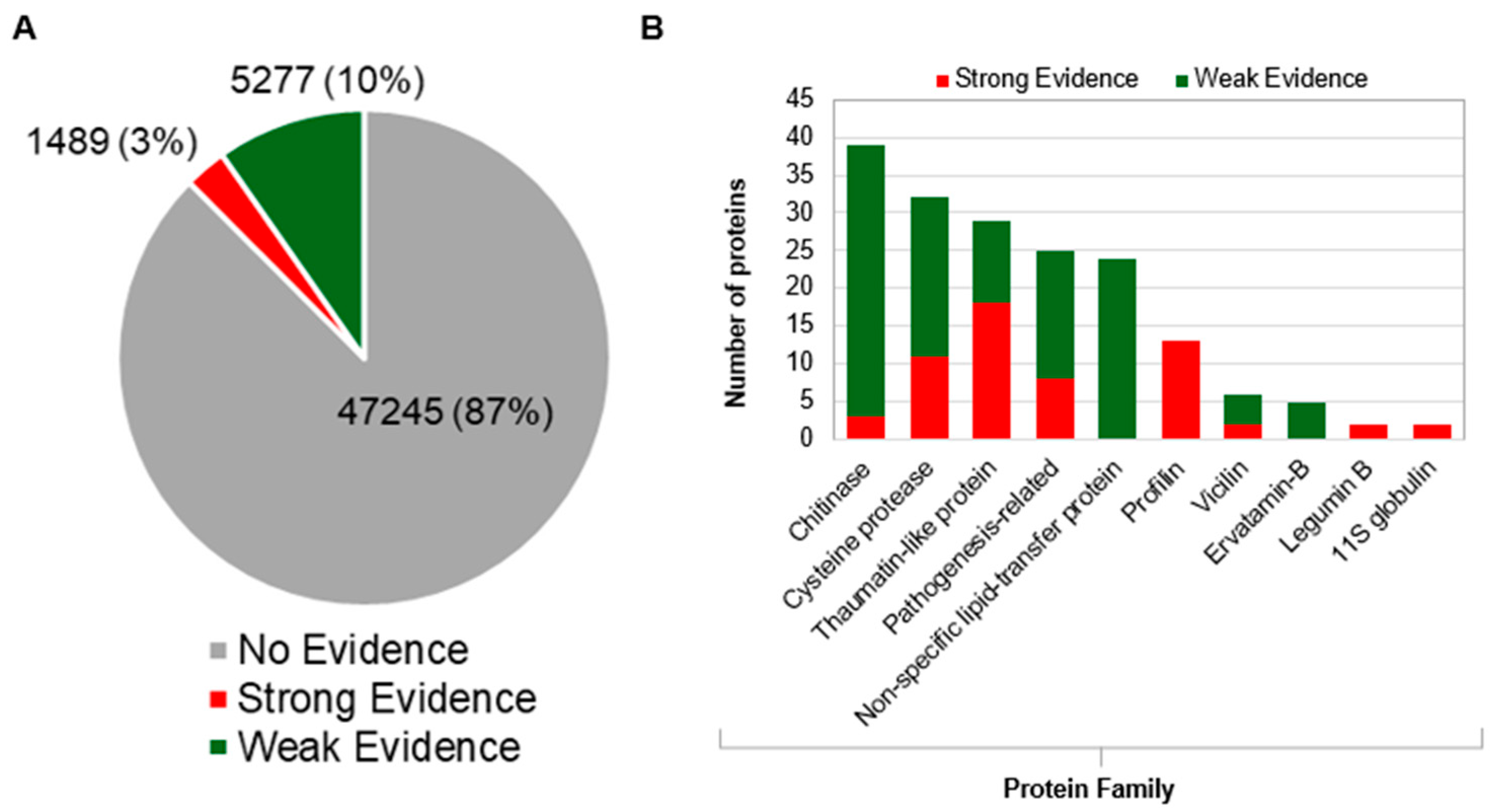
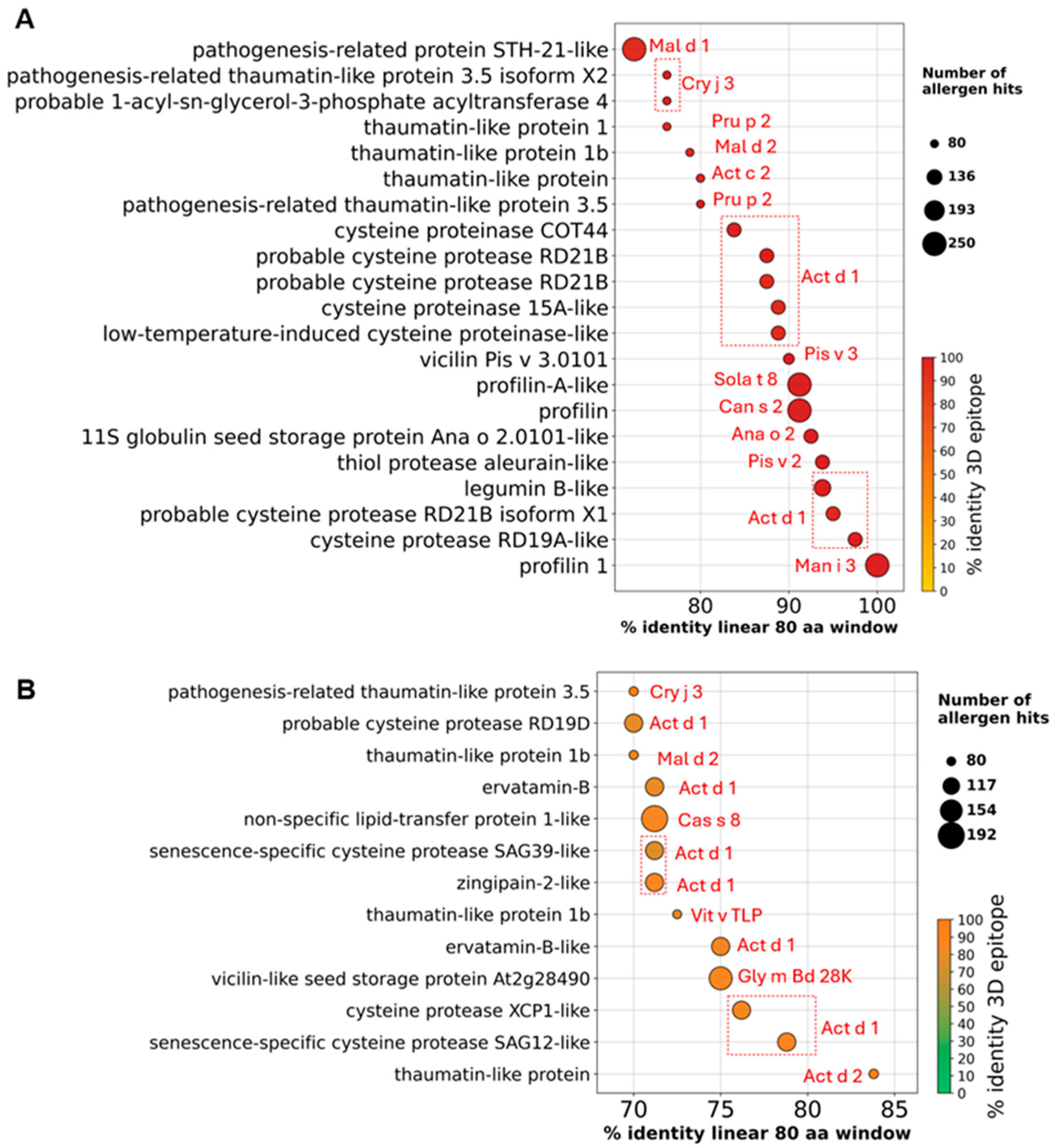
3.2. Potential Allergens in Mango Genome
3.3. Identification of High-Confidence Allergen Protein Families in Mango
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTP | Lipid Transfer Protein |
| PR | Pathogenesis-Related |
| GPI | Glycosylphosphatidylinositol |
| EFSA | European Food Safety Authority |
| IEDB | Immune Epitope Database |
| pLM4AIg | Protein Language Model-Based Predictors for Allergenic Proteins and Peptides |
| CATAS | Chinese Academy of Tropical Agricultural Sciences |
References
- Liu, W.; Yang, Q.; Wang, Z.; Wang, J.; Min, F.; Yuan, J.; Tong, P.; Li, X.; Wu, Y.; Gao, J.; et al. Quantitative food allergen risk assessment: Evolving concepts, modern approaches, and industry implications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70132. [Google Scholar] [CrossRef] [PubMed]
- Präger, L.; Simon, J.C.; Treudler, R. Food allergy—New risks through vegan diet? Overview of new allergen sources and current data on the potential risk of anaphylaxis. J. Der Dtsch. Dermatol. Ges. 2023, 21, 1308–1313. [Google Scholar] [CrossRef]
- Shaheen, N.; Hossen, M.d.S.; Akhter, K.T.; Halima, O.; Hasan, M.d.K.; Wahab, A.; Gamagedara, S.; Bhargava, K.; Holmes, T.; Najar, F.Z.; et al. Comparative Seed Proteome Profile Reveals No Alternation of Major Allergens in High-Yielding Mung Bean Cultivars. J. Agric. Food Chem. 2024, 72, 13957–13965. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cong, Y. Recent advances in the study of epitopes, allergens and immunologic cross-reactivity of edible mango. Food Sci. Hum. Wellness 2024, 13, 1186–1194. [Google Scholar] [CrossRef]
- Maurer-Stroh, S.; Krutz, N.L.; Kern, P.S.; Gunalan, V.; Nguyen, M.N.; Limviphuvadh, V.; Eisenhaber, F.; Gerberick, G.F. AllerCatPro-prediction of protein allergenicity potential from the protein sequence. Bioinformatics 2019, 35, 3020–3027. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms (GMO Panel). Scientific Opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed. EFSA J. 2010, 8, 1700. [Google Scholar] [CrossRef]
- Krikeerati, T.; Rodsaward, P.; Nawiboonwong, J.; Pinyopornpanish, K.; Phusawang, S.; Sompornrattanaphan, M. Revisiting Fruit Allergy: Prevalence across the Globe, Diagnosis, and Current Management. Foods 2023, 12, 4083. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Lopata, A.L. Editorial: Spotlight on allergy research in Asia. Front. Allergy 2024, 5, 1371795. [Google Scholar] [CrossRef]
- García-Villegas, A.; Fernández-Ochoa, Á.; Rojas-García, A.; Alañón, M.E.; Arráez-Román, D.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. The Potential of Mangifera indica L. Peel Extract to Be Revalued in Cosmetic Applications. Antioxidants 2023, 12, 1892. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Simnitt, S.; Wechsler, S.J.; Wakefield, H. Fruit and Tree Nuts Outlook: September 2023|Economic Research Service. Available online: https://www.ers.usda.gov/publications/pub-details?pubid=107539 (accessed on 24 June 2025).
- Berghea, E.C.; Craiu, M.; Ali, S.; Corcea, S.L.; Bumbacea, R.S. Contact Allergy Induced by Mango (Mangifera indica): A Relevant Topic? Medicina 2021, 57, 1240. [Google Scholar] [CrossRef] [PubMed]
- Ukleja-Sokołowska, N.; Gawrońska-Ukleja, E.; Lis, K.; Żbikowska-Gotz, M.; Sokołowski, Ł.; Bartuzi, Z. Anaphylactic reaction in patient allergic to mango. Allergy Asthma Clin. Immunol. 2018, 14, 78. [Google Scholar] [CrossRef]
- Paschke, A.; Kinder, H.; Zunker, K.; Wigotzki, M.; Weßbecher, R.; Vieluf, D.; Steinhart, H. Characterization of Allergens in Mango Fruit and Ripening Dependence of the Allergenic Potency. Food Agric Immunol. 2001, 13, 51–61. [Google Scholar] [CrossRef]
- Cardona, E.E.G.; Heathcote, K.; Teran, L.M.; Righetti, P.G.; Boschetti, E.; D’Amato, A. Novel low-abundance allergens from mango via combinatorial peptide libraries treatment: A proteomics study. Food Chem. 2018, 269, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Garino, C.; Coïsson, J.D.; Arlorio, M. In silico allergenicity prediction of several lipid transfer proteins. Comput. Biol. Chem. 2016, 60, 32–42. [Google Scholar] [CrossRef]
- Kulkarni, A.; Ananthanarayan, L.; Raman, K. Identification of putative and potential cross-reactive chickpea (Cicer arietinum) allergens through an in silico approach. Comput. Biol. Chem. 2013, 47, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Halima, O.; Najar, F.Z.; Wahab, A.; Gamagedara, S.; Chowdhury, A.I.; Foster, S.B.; Shaheen, N.; Ahsan, N. Lentil allergens identification and quantification: An update from omics perspective. Food Chem. 2022, 4, 100109. [Google Scholar] [CrossRef]
- Bastiaan-Net, S.; Pina-Pérez, M.C.; Dekkers, B.J.W.; Westphal, A.H.; America, A.H.P.; Ariëns, R.M.C.; de Jong, N.W.; Wichers, H.J.; Mes, J.J. Identification and in silico bioinformatics analysis of PR10 proteins in cashew nut. Protein Sci. 2020, 29, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Jamakhani, M.; Lele, S.S.; Rekadwad, B. In silico assessment data of allergenicity and cross-reactivity of NP24 epitopes from Solanum lycopersicum (Tomato) fruit. Data Brief 2018, 21, 660–674. [Google Scholar] [CrossRef]
- Zhou, W.; Bias, K.; Lenczewski-Jowers, D.; Henderson, J.; Cupp, V.; Ananga, A.; Ochieng, J.W.; Tsolova, V. Analysis of Protein Sequence Identity, Binding Sites, and 3D Structures Identifies Eight Pollen Species and Ten Fruit Species with High Risk of Cross-Reactive Allergies. Genes 2022, 13, 1464. [Google Scholar] [CrossRef]
- Hernández-Lao, T.; Rodríguez-Pérez, R.; Labella-Ortega, M.; Muñoz Triviño, M.; Pedrosa, M.; Rey, M.-D.; Jorrín-Novo, J.V.; Castillejo-Sánchez, M.Á. Proteomic identification of allergenic proteins in holm oak (Quercus ilex) seeds. Food Chem. 2025, 464 Pt 1, 141667. [Google Scholar] [CrossRef] [PubMed]
- Savić, A.; Mitić, D.; Smiljanić, K.; Radosavljević, J.; Stanić-Vučinić, D.; Ćirković Veličković, T. Ensemble-based in silico allergenicity prediction of black tiger shrimp Penaeus monodon. In Proceedings of the XVIII International Italian Proteomics Association Annual Meeting in partnership with the Hellenic Proteomics Society and Serbian Proteomics Association, Roma, Italy, 27–29 November 2024; p. 78. [Google Scholar]
- Nguyen, M.N.; Krutz, N.L.; Limviphuvadh, V.; Lopata, A.L.; Gerberick, G.F.; Maurer-Stroh, S. AllerCatPro 2.0: A web server for predicting protein allergenicity potential. Nucleic Acids Res. 2022, 50, W36–W43. [Google Scholar] [CrossRef]
- Krutz, N.L.; Kimber, I.; Winget, J.; Nguyen, M.N.; Limviphuvadh, V.; Maurer-Stroh, S.; Mahony, C.; Gerberick, G.F. Application of AllerCatPro 2.0 for protein safety assessments of consumer products. Front. Allergy 2023, 4, 1209495. [Google Scholar] [CrossRef] [PubMed]
- Kolaskar, A.S.; Tongaonkar, P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990, 276, 172–174. [Google Scholar] [CrossRef]
- Du, Z.; Xu, Y.; Liu, C.; Li, Y. pLM4Alg: Protein Language Model-Based Predictors for Allergenic Proteins and Peptides. J. Agric. Food Chem. 2024, 72, 752–760. [Google Scholar] [CrossRef]
- Giangrieco, I.; Ciardiello, M.A.; Tamburrini, M.; Tuppo, L.; Rafaiani, C.; Mari, A.; Alessandri, C. Comparative Analysis of the Immune Response and the Clinical Allergic Reaction to Papain-like Cysteine Proteases from Fig, Kiwifruit, Papaya, Pineapple and Mites in an Italian Population. Foods 2023, 12, 2852. [Google Scholar] [CrossRef]
- Suzuki, M.; Itoh, M.; Ohta, N.; Nakamura, Y.; Moriyama, A.; Matsumoto, T.; Ohashi, T.; Murakami, S. Blocking of protease allergens with inhibitors reduces allergic responses in allergic rhinitis and other allergic diseases. Acta Oto-Laryngol. 2006, 126, 746–751. [Google Scholar] [CrossRef]
- Szewińska, J.; Simińska, J.; Bielawski, W. The roles of cysteine proteases and phytocystatins in development and germination of cereal seeds. J. Plant Physiol. 2016, 207, 10–21. [Google Scholar] [CrossRef]
- Sharma, A.; Vashisht, S.; Mishra, R.; Gaur, S.N.; Prasad, N.; Lavasa, S.; Batra, J.K.; Arora, N. Molecular and immunological characterization of cysteine protease from Phaseolus vulgaris and evolutionary cross-reactivity. J. Food Biochem. 2022, 46, e14232. [Google Scholar] [CrossRef]
- Soh, W.T.; Zhang, J.; Hollenberg, M.D.; Vliagoftis, H.; Rothenberg, M.E.; Sokol, C.L.; Robinson, C.; Jacquet, A. Protease allergens as initiators–regulators of allergic inflammation. Allergy 2023, 78, 1148–1168. [Google Scholar] [CrossRef]
- Anagnostou, A. Lipid transfer protein allergy: An emerging allergy and a diagnostic challenge. Ann. Allergy Asthma Immunol. 2023, 130, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.-B.S.; Hall, S.; Dragsted, L.O. Identification of European allergy patterns to the allergen families PR-10, LTP, and profilin from Rosaceae fruits. Clin. Rev. Allergy Immunol. 2011, 41, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Mafra, I. Rosaceae food allergy: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7423–7460. [Google Scholar] [CrossRef] [PubMed]
- Skypala, I.J.; Asero, R.; Barber, D.; Cecchi, L.; Diaz Perales, A.; Hoffmann-Sommergruber, K.; Pastorello, E.A.; Swoboda, I.; Bartra, J.; Ebo, D.G.; et al. Non-specific lipid-transfer proteins: Allergen structure and function, cross-reactivity, sensitization, and epidemiology. Clin. Transl. Allergy 2021, 11, e12010. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Zarif, A.; Huynh, A.N.; Yang, Z.; Ahsan, N. Genome-Wide In Silico Analysis Expanding the Potential Allergen Repertoire of Mango (Mangifera indica L.). Appl. Sci. 2025, 15, 8375. https://doi.org/10.3390/app15158375
Singh A, Zarif A, Huynh AN, Yang Z, Ahsan N. Genome-Wide In Silico Analysis Expanding the Potential Allergen Repertoire of Mango (Mangifera indica L.). Applied Sciences. 2025; 15(15):8375. https://doi.org/10.3390/app15158375
Chicago/Turabian StyleSingh, Amit, Aayan Zarif, Annelise N Huynh, Zhibo Yang, and Nagib Ahsan. 2025. "Genome-Wide In Silico Analysis Expanding the Potential Allergen Repertoire of Mango (Mangifera indica L.)" Applied Sciences 15, no. 15: 8375. https://doi.org/10.3390/app15158375
APA StyleSingh, A., Zarif, A., Huynh, A. N., Yang, Z., & Ahsan, N. (2025). Genome-Wide In Silico Analysis Expanding the Potential Allergen Repertoire of Mango (Mangifera indica L.). Applied Sciences, 15(15), 8375. https://doi.org/10.3390/app15158375






