Abstract
A growing number of scientific and policy institutions recognize predominantly plant-based diets as the most sustainable dietary patterns, for both public health and the environment, and are therefore encouraging citizens to shift their eating habits. Alongside this, more people are adopting vegan or vegetarian diets in response to environmental concerns and animal welfare. In this context, the responsibility for planning nutritionally adequate diets cannot be solely individual, with household solutions, but collective actions are needed at the industrial and agricultural level. Historically, the food industry has improved the nutritional value of animal-derived foods through feed modification and supplementation. Today, similar efforts are needed to enhance the nutrient profile of plant-based foods. In addition, nutrient recommendations originally designed for mixed diets may not be directly applicable to populations following exclusively plant-based diets, given the differences in bioavailability and metabolism. This review aims to (1) identify nutrients that may be absent or present in insufficient amounts in plant-based and especially vegan diets; (2) explore potential solutions, ranging from soil management and crop biofortification to food processing, fortification, and supplementation; and (3) call for a revision of dietary reference intakes that better reflects the specific needs of populations consuming total or predominantly plant-based diets.
1. Introduction
The adoption of healthier and more sustainable diets is a planetary priority [1]. Unhealthy diets are one of the strongest predictors of morbidity and mortality, whereas the current food production system is the main driver of environmental degradation [2]. In addition, the COVID-19 pandemic has been a reminder of the many health risks associated with the livestock industry: emergence of new pathogens capable of producing similar or more dangerous pandemics, foodborne zoonoses, and antibiotic resistance [3].
Along with the mixed diet or omnivorous diet, which includes foods of animal and plant origin, there are different types of plant-based diets. The concept of ‘plant-based diet’ refers to a broad range of eating patterns that prioritize plant foods, such as fruits, vegetables, whole grains, legumes, nuts, and seeds while limiting or avoiding animal-derived products with different levels of exclusion [4,5]. There is a considerable heterogeneity in definitions of plant-based diets, ranging from traditional vegetarian patterns (lacto-vegetarian, ovo-vegetarian, lacto-ovo vegetarian, and vegan diet which excludes all animal products to semi-vegetarian or flexitarian models [5,6,7]. Also, dietary patterns initially developed in relation to favorable health outcomes, such as the Portfolio, Mediterranean, DASH, Healthy US-style, Planetary Health, and Nordic diets, have increasingly been subsumed under the PB framework due to their pronounced emphasis on plant-derived components [5,6].
In this context, the EAT-Lancet Commission on Healthy Diets From Sustainable Food Systems defined in 2019 healthy and sustainable diets as those “consisting largely of a diversity of plant-based foods”, with only “modest (and optional) amounts of animal foods”… and “limited amounts of refined grains, highly processed foods and added sugars” [2].
Many governments, health institutions, and social organizations are following the same steps and recommending a significant reduction in meat consumption in their dietary guidelines [8,9]. Although these recommendations do not necessarily mention vegetarian or vegan diets, a growing number of people are adopting these eating patterns, and even more are reducing the consumption of animal products for health or environmental reasons [10].
Among these patterns, the vegan diet, the most restrictive dietary pattern, has also been growing not only in Western countries but also in the rest of the world, being followed by people of all ages and physiological conditions [11]. In the past decades, the health and nutritional status of vegan adults and children have been the focus of extensive research. Although the vast majority of studies have highlighted the benefits of plant-based diets on longevity and prevalence of non-communicable diseases [11,12], some have also pointed out that these diets carry an increased risk of not meeting the requirements for certain micronutrients [13].
Human nutrition has never been the result of individual food choices alone. Cultural traditions, food availability and quality, education and knowledge about food, income and costs, and especially, food policy strategies, have had a significant impact on the nutritional status of individuals and communities throughout history [14]. The identification and synthesis of most vitamins and minerals during the first half of the 20th century and their use to prevent nutritional deficiencies by fortification of selected foods led to the virtual eradication of deficiency diseases in Western countries [15]. At the end of the 20th century, folic acid fortification programs proved to be a resounding success in preventing neural tube birth defects [16].
In contrast with the systematic public effort to improve the nutritional status of the general population, the same has not always been carried out with plant-based nutrition and, for example, vegans have been made to take individual responsibility for finding alternative source of nutrients [17]. As a result, citizens are receiving conflicting messages: on the one hand they are being told that they should reduce their consumption of meat and animal products for health and environmental reasons and on the other they are being told that if they adopt a vegan diet they must be aware of the risk of deficiencies and plan their diet very carefully.
Descriptive studies that show the average nutritional intake and nutritional status in vegan populations are very useful to understand the specific needs of this group and to design public policies that guarantee access to a sufficient variety of natural and fortified foods to meet their requirements at all ages. Addressing nutritional challenges in plant-based and especially vegan diets focusing on agricultural, industrial, and household solutions is becoming more important as the number of people adopting plant-based diets, including a vegan diet, is expected to continue rising.
This review aims to (1) describe the nutrients that might not be found, or not in enough quantities, in plant foods; (2) explore possible solutions that governments and institutions, as well as the food industry, could adopt; and (3) assess the need to reconsider the current Recommended Dietary Intakes (RDIs), which were originally published for populations following diets that included animal and plant foods.
2. Nutrients That Might Be Absent or in Very Small Amounts in Vegan or Plant-Based Diets
It is unanimously accepted that while vitamin B12 (cobalamin) can only be found in animal foods and needs to be supplemented in vegan and vegetarian diets, the rest of the nutrients can be obtained from plants or from sunlight (such as calciferol or vitamin D) [18,19]. Although this is technically correct, there is growing evidence that other nutrients may not be found in plant foods.
According to available studies at different times of the life cycle, exclusively plant-based diets may be lower in vitamins B2 (riboflavin), niacin (B3), B12, D, iodine, zinc, calcium, potassium, and selenium as compared with non-vegan diets [13]. This lower intake is not always associated with impaired health. Of these, the intake of vitamin B12 among vegans is significantly lower than the recommendations, and the intake of calcium in most vegans is below the recommendations [10,13,17].
Also, although less studied, retinol (a preformed form of active vitamin A found in animal products) and DHA (docosahexaenoic acid) are mainly found in seafood. Even though their precursors exist in plants, it is unknown if the amount converted is adequate. Another situation refers to those nutrients whose quantity in common plant foods may be low or very low, (especially selenium and iodine), making it difficult for vegans to meet their requirements unless specific adjustments are made. This highlights the need for appropriate dietary advice for those who choose to not consume foods of animal origin, including not only the supplementation of vitamin B12, but also of those nutrients that are repeatedly below the recommended amounts.
2.1. Omega3 Fatty Acids: Eicosapentaenoic (EPA) and Docosahexaenoic (DHA)
The n-6 fatty acids include linoleic (18:2n-6; LA) and arachidonic (20:4n-6; AA) acids, while the n-3 fatty acids include α-linolenic (18:3n-3; ALA), eicosapentaenoic (20:5n-3; EPA), and docosahexaenoic (22:6n-3; DHA) acids [20]. LA and ALA are considered essential and must be supplied by the diet, whereas EPA and DHA are not considered essential as they can be transformed from ALA. However, the formation of EPA and DHA by ALA elongation is limited and decreased by several factors, such as high dietary LA content [21]; insufficient intake of energy and some nutrients such as protein, pyridoxine, biotin, calcium, copper, magnesium, and zinc [21,22]. Therefore, attention should be paid to EPA and DHA, as plant-based diets are often lacking in these fatty acids, unless they include fish, supplements, or fortified foods [23,24,25]. DHA is especially important in the perinatal period and early childhood due to its role in the synthesis of cell membranes, phospholipids, retinal photoreceptors (vision), and brain gray matter [26,27].
The only n-3 fatty acid present in useful amounts in plant foods is ALA. Its main sources are flax seeds, hemp seeds, chia seeds, walnuts and their oils, soy products, and some algae [28]. The only plant sources of DHA and EPA are certain algae [28,29]. Although some experts believe that well-planned vegan diets can satisfy the requirements of n-3 fatty acids [19], more studies that measure plasma values of ALA, EPA, and DHA in adults, children, and adolescents are needed, since the current data are very scarce [30,31,32]. Keeping an optimal omega-6/omega-3 ratio in the diet is important for optimal EPA and DHA conversion, but it can be difficult to obtain enough ALA without also increasing the amount of LA in the diet, unless specific foods high in ALA are included on a daily basis [30]. Excessive LA (found in sunflower and other seed oils), as well as trans fats (margarine) and tropical oils rich in saturated fats (coconut, palm, and palm kernel oils) can interfere in the conversion of ALA into EPA and DHA [28,30,33].
The European Food Safety Authority (EFSA) Panel proposes an Adequate Intake for ALA of 0.5 total energy% for both adults and children based on the lowest estimated mean intakes of the various population groups from a number of European countries where overt ALA deficiency symptoms are not present [34]. This means for an energy intake value between 1000 and 2500 kcal/day approximately an intake of 0.6 to 1.4 g of ALA is required. This is easily achieved if the diet includes flax seeds, hemp seeds, chia seeds, and walnuts [19,28]. Some authors consider that people following plant-based diets should aim for 2.2–4.4 g/day (or 1.1 g/day/1000 Kcals), depending on the amount of LA in the diet [30].
Regarding EPA and DHA, the EFSA Panel proposes an Adequate Intake of 100 mg DHA for infants 6–24 months, 250 mg for adults, and 350–450 mg for pregnant and lactating women [34]. However, there are not enough data about the consequences of not taking DHA and EPA for long periods of time and studies on healthy adults and children show conflicting or inconsistent results [35,36]. Lower DHA levels have been found in breast milk from un-supplemented vegetarian and vegan mothers compared to mothers who eat seafood [25,37] and again, although the consequences of this are still unknown, until more data are available, it seems prudent to recommend DHA supplementation from microalgae oil during pregnancy and breastfeeding.
2.2. Vitamin A: β-Carotene and Retinol
Retinol fulfills multiple functions in vision, cell growth and differentiation, embryogenesis, the maintenance of epithelial barriers, and immunity [38]. Preformed vitamin A exists only in animal products. However, there are about 50 carotenoids that the body can convert into vitamin A; the most common one is beta-carotene. The biological value of substances with vitamin A activity is expressed as retinol equivalent (RE). The EFSA Panel has proposed the following conversion factors: 1 μg of RE is equivalent to 1 μg of retinol, 6 μg of β-carotene, and 12 μg of another provitamin A [39] based on available evidence.
Preformed vitamin A is efficiently absorbed (70–90%), and its absorption increases when taken with foods containing fat, as it is a fat-soluble vitamin. However, β-carotene absorption appears to be highly variable (5–65%) and dependent on factors such as diet, genetic characteristics, and health status [40,41], with the small intestine being the primary tissue where dietary provitamin A carotenoids are converted to retinol. Therefore, vitamin A status is expressed in terms of total body retinol storage or, alternatively, as hepatic concentration of the vitamin [40].
Blood retinol concentration is used to assess vitamin A status, despite its lack of sensitivity and specificity. A blood vitamin A concentration below 0.70 μmol/L (200 μg/L) indicates insufficient intake and levels above 1.05 μmol/L (300 μg/L) indicate adequate vitamin A status [38]. To prevent clinical signs of deficiency and provide adequate stores, a concentration of at least 20 μg retinol/g liver in adults is assumed to maintain adequate plasma retinol concentration.
The EFSA Panel considers that these values can be used as a target value for establishing the average requirement (AR) for vitamin A for all age groups. There is some evidence that the catabolic rate of retinol may be higher in children than in adults, but the data are limited, so EFSA used the adult catabolic rate value and corrected it based on a growth factor. In Europe, the recommended dietary intake is set at 250 μg of RE/day between 7 and 36 months of age and at 650 μg of RE/day between 15 and 17 years of age and adults [39].
Although plant-based diets can be rich in beta-carotene and therefore in retinol precursors, little is known about the vitamin A status of vegans, including beta-carotene intake. In fact, lower vitamin A intakes have been reported in vegan adults [42,43]. Although two other research studies in adults [44] and young children [31] found no differences in total vitamin A intake in retinol activity equivalents (RAEs) between vegans and non-vegans, blood biomarkers of vitamin A showed markedly low levels in both adults and children following a vegan diet, with retinol-binding protein (RBP) in all six children in the vegan diet group falling below the cut-off level for insufficiency [31]
It is important to take into account, especially when testing children, that RBP is a negative acute-phase reactant (decreases in serum when there is an acute infection or inflammation) [45]. Normal levels of vitamin A are required for bone health. Provitamin A carotenoids promote bone formation and inhibit bone resorption. However, excessively high levels of preformed vitamin A (but not carotene) may inhibit bone mineralization. The deleterious effect of hypervitaminosis A in bone mineral density is more pronounced if there is concomitant vitamin D deficiency [46].
The vitamin A status in plant-based diets deserves more attention. This could be assessed by direct measurements of serum retinol and dark adaptation tests. It is not known how genetic variability affects the conversion of carotenoids into vitamin A and whether recommendations should vary according to people’s genetic types [47]. It is also unknown how many provitamin A carotenoids are required for an adequate retinol synthesis in people following plant-based diets. Until more data are available, including a good range and amount of carotene-rich foods in the diet (sweet potatoes, carrots, bright orange winter squash or pumpkin, dark green leafy vegetables, sweet red peppers) and eating them with fat-containing food (avocado, nuts, olive oil) may lead to greater absorption and conversion and should be recommended [47,48,49,50].
2.3. Iodine
Iodine is an essential mineral required for the synthesis of thyroid hormones. In the perinatal period, optimal levels of thyroid hormones are critical for normal growth and neurological development [51].
Iodine deficiency in the fetus and infant is the most common preventable cause of brain damage worldwide [52]. Similarly, excess iodine can also lead to thyroid dysfunction, but evidence for excess iodine in healthy conditions is more limited than that for deficiency [53]. Iodine is highly absorbed, with over 90% of ingested iodine, since the thyroid is the main storage site for iodine in the body and the kidney is the main route of excretion. In healthy conditions, urinary iodine accounts for over 90% of dietary intake, making it a good indicator of body status [54]. A urinary iodine concentration ≥ 100 µg/L is accepted as indicating sufficient iodine intake and preventing goiter and hypothyroidism. This corresponds to an iodine intake of 70 µg/day in infants aged 7–11 months, 90–130 µg/day in children depending on age, 150 µg/day in adults, and 200 µg/day in pregnant and lactating women [55]. Iodine is found primarily in seafood (fish, shellfish, and seaweed). Cow’s milk and eggs can be good sources if animal feed is supplemented; iodine in milk can also be obtained by disinfecting teats with iodine-containing substances [55]. Because iodine content in plant foods and water varies widely, iodized salt or supplements are accepted as the safest way for people consuming plant-based diets to meet their daily needs [19]. However, there are several problems associated with the use of iodized salt. The degree of iodization of salt varies between countries and even between salt brands, despite regulations. Most studies on nutritional intakes do not consider the contribution of iodized salt (both household and processed foods) to total iodine intake [56]. Similarly, the World Health Organization (WHO) and the International Council for the Control of Iodine Deficiency Disorders (ICCIDD) consider that a country may have an adequate iodine supply when more than 90% of families consume iodized salt [56,57,58]. In vegan adults, iodine intakes have been observed to be lower than those of non-vegans [43,59,60,61,62,63,64] and often lower than the RNI (150 μg/d). On the other hand, several studies [62,63,64] have found urinary and serum iodine concentrations in vegans close to or below the minimum recommended levels. However, some vegans had excessive iodine intakes due to high seaweed consumption [65].
To our knowledge, few studies on urinary iodine in vegan children were carried out [66]. In infants, iodized salt is not advised before 12 months of age and after 12 months, the recommended iodine intake can be achieved with 2–5 g/day of salt, depending on its content which differs in each country [67]. However, the use of iodized salt in infants and young children cannot be considered as the optimal form of supplying iodine. Vegans and plant-based consumers living in countries where no iodized salt is available or who avoid the use of salt are also at higher risk of iodine deficiency. It would probably be more beneficial in the long term to fortify products that are routinely consumed by a large percentage of plant-based consumers, such as plant-based milks.
Sea vegetables are a good source of iodine; however, their iodine content is highly variable (low in nori, moderate in wakame and dulse, and very high in arame and kombu); therefore, general recommendations about this food group are not possible. Another difficulty is the lack of information about iodine content per portion in food containing sea vegetables. Some species might have high levels of heavy metals [68,69], which further restrict their consumption.
Iodine status is still a public health problem. Several measures are urgent: to ensure an adequate iodine intake in the population, especially in the most vulnerable vegan groups (babies and children, women of reproductive age, pregnant and lactating women); to control effectively the iodization of salt or other foods; to obtain a better knowledge of the nutritional indicators of iodine in the population; and to promote proper labeling of foods containing seaweeds and other iodine-rich foods, so that they show the amount of iodine provided per serving of food.
2.4. Selenium
Selenium is a free radical scavenger mineral with antioxidant and anticarcinogenic properties. It also plays an important role in thyroid function, the immune and reproductive systems, as well as mental health [70]. Thus, insufficient selenium intake has been associated with thyroid autoimmune disease, poorer immune response against viruses, reproductive difficulties, cognitive impairment and dementia, type 2 diabetes, and some cancers [71]. The majority of the world’s population has suboptimal selenium intake where the recommended daily intakes are not reached [72,73]. Blood concentrations in healthy adults from 69 countries also indicate that nutritional selenium deficiency is highly prevalent in 21 countries and moderately prevalent in 16 countries (using the reference value of 70 μg Se/L in serum or plasma [72]. In the diet, this oligoelement is supplied mainly in the form of L-selenomethionine and L-selenocysteine, and also in inorganic compounds, such as selenate and selenite, although in smaller amounts. All forms of selenium appear to be well absorbed from the diet [74]. A total of 25 selenoproteins with diverse functions have been identified. Selenoprotein P (SEPP1) plays a central role in the delivery of selenium to tissues and is involved in the regulation of its metabolism in the body. It is considered the most informative biomarker of selenium function due to its role in selenium transport and metabolism and its response to different forms of selenium intake. Intervention studies with different levels of selenium intake showed that plasma SEPP1 concentration stabilizes in response to increasing selenium doses. This stabilization of plasma SEPP1 concentration is considered indicative of adequate selenium delivery to all tissues and reflects saturation of functional selenium stores, ensuring that selenium requirements are met. An Adequate Intake (AI) of 70 µg/day has been established for adult men and women. For infants 7–11 months of age, an AI of 15 µg/day was obtained by extrapolating upwards from the estimated selenium intake of exclusively breastfed infants and accounting for differences in reference body weights. For children and adolescents, selenium AIs were extrapolated from the adult AI by isometric scaling and applying a growth factor [74].
The selenium content of plant foods depends mainly on the selenium content of the soil and its chemical characteristics. Some plant foods, such as Brazil nuts, garlic, onions, leeks, broccoli, and cabbage, absorb more selenium from the soil and are known as “selenium accumulators”. The amount of selenium from animal foods is easier to control, as it depends mostly on the diet of the animals, which makes the intake of selenium from animal sources more constant [74]. Therefore, consumers of fully or partially plant-based diets are more subject to lower selenium intakes. Among them, vegans are the most likely to have lower selenium intakes [64,75,76]. Kristensen et al. [43] found that vegans had the lowest selenium intake, which was close to the WHO RNI. Because of their role in thyroid function along with iodine, some vegans, especially women, may be more vulnerable to thyroid disorders if their iodine and selenium intake is inadequate [77].
Although the selenium deficit may be exacerbated in vegan diets, it is an issue that must be addressed globally. Planet selenium sources are limited and should be used efficiently to increase the selenium concentration in food. Direct addition of selenium compounds to food (process fortification) can be undertaken by the food industry. Increased human intake may be achieved by the use of selenium fertilizers, increased consumption of plants that naturally accumulate more selenium, sprouting seeds in selenium-enriched soil, plant breeding for enhanced selenium accumulation, plant production in the most selenium-rich areas, food fortification and supplementation of individuals [78]. There is less wastage if selenium is added late in the production chain rather than early [78].
2.5. Other Nutrients
Iron and zinc have been traditionally reported as more difficult to obtain from plant foods because of the presence of phytate and other inhibitors in plants. Several studies have reported that although intakes among plant-based diets are adequate, serum levels are usually lower. This is not the same scenario as with the nutrients discussed above, because iron and zinc are abundant in plant foods. However, the current RDI for these nutrients might not be optimal for people following plant-based diets. We will analyze this in more detail in the following sections.
3. Optimizing Micronutrient Content in Plant Foods
Ensuring a supply of high-quality, nutrient-dense plant foods is vital if we are to promote adherence to the EAT-Lancet diet and support the health and nutritional status of a population increasingly following plant-based diets.
The food system is the sum of actors and interactions along the food chain—from input supply and production of crops, to transportation, processing, retailing, wholesaling, and preparation of foods to consumption and disposal. Food systems also include the enabling policy environments and cultural norms around food [79].
The agricultural system has traditionally been focused on increasing grain yield and crop productivity [80], and subsequently allocating a high percentage of these crops to feed livestock. One of the consequences of this strategy has been the loss of organic matter and nutrients in soil and a decrease in the quality of plant foods intended for human consumption [81]. Although populations from low- and middle-income countries have traditionally suffered from this type of micronutrient malnutrition (known as ‘hidden hunger’) [80], it is likely that this problem will become more prevalent in high-income countries as the population adopts a more plant-based diet, unless significant changes in the food production system take place.
Adopting a more sustainable approach to agricultural production (such as Conservation Agriculture) to prevent further soil erosion and rehabilitate agricultural land will automatically increase nutrient content in soil and nutrient retention by plants [81]. In addition, biofortification of crops which increases or improves the nutritional value of crops is a cost-effective way to improve the nutritional quality of plant foods. This can be achieved through different processes, including conventional breeding, agronomic approaches, genetic engineering, or mutational breeding [82,83].
These approaches seek to increase the levels of specific nutrients such as iron, zinc, and vitamin A and selenium in commonly consumed foods [84,85]. Traditional plant breeding involves selecting and crossing plants with higher amounts of nutrients of interest to obtain new varieties rich in these nutrients, for example, identifying and crossing varieties rich in iron, zinc, or vitamin A, especially in legumes and cereals. Considering that some minerals have lower bioavailability in plant-based diets, the possibility of enriching fresh produce, such as many vegetables, by adopting specific agronomic approaches, should be considered. Agronomic biofortification of vegetables, with the aim of increasing the content of important minerals in edible portions, such as calcium, magnesium, iodine, zinc, selenium, iron, copper, and silicon, can be carried out, and studies have reported on it. To date, attention has focused on the biofortification of selenium and iodine, while in the case of the other mineral elements, aspects related to plant typology, genotypes, chemical form, and application protocols are far from well-defined. Although agronomic fortification is considered an easy-to-implement technique, the approach is complex considering the various interactions that occur at the crop level, as well as the bioavailability of the various minerals for the consumer. Considering this, only a few studies have broadly examined both the definition of biofortification protocols and the quantification of the bioavailable fraction of the element [86].
On the other hand, agronomic practices include the application of mineral fertilizers, such as zinc and iron fertilizers, either to the soil or through foliar sprays; soil management, optimizing soil conditions to improve nutrient availability and, consequently, crop uptake and accumulation. Finally, microbial inoculation, using beneficial microorganisms to improve nutrient availability and uptake [84,87].
Finally, through genetic engineering, gene editing can be performed using techniques to modify genes involved in the absorption, transport or synthesis or bioavailability of nutrients in the plant or the introduction of genes from other species to improve these functions [84].
Biofortification strategies should be environmentally sustainable and contribute to food security by allowing not only more nutrient-rich foods but also by developing studies to study the bioavailability of these nutrients in the human organism.
Food industry can play a key role at enriching plant foods. Food fortification (the addition of micronutrients to a food that does not contain those compounds naturally), food enrichment (the intentional increase in some micronutrients already present in the food) [83], and food restoration (the addition of essential nutrients to a food after these have been lost during the course of Good Manufacturing Process (GMP), or during normal storage and handling procedures, in the same amounts that were present in origin) can improve the contribution of micronutrients in plant-based diets. According to WHO/FAO, there are three main types of food fortification based on application and scope: mass-, target-, and market-driven fortifications [88,89]. Mass fortification is mainly used in some low- and middle-income countries, where nutritional deficiencies are widely spread, and fortification becomes mandatory and instigated by governments. In this case, the standards are usually made compulsory through specific regulations. In high-income countries, mass food fortification is not common and, in many cases, food products fortification is voluntary for food system agents. Nowadays only some countries such as the United Kingdom include the mandatory fortification of basic foods such as wheat flour with micronutrients like calcium, iron, niacin, and thiamin [90].
In these countries, addressing potential nutritional deficiencies in plant-based diets should be supported through the combination of the other two fortification systems proposed by FAO: targeted and market-driven. Targeted fortification should be an enrichment strategy designed specifically for people following plant-based diets, based on scientific criteria and with governmental support. The success of this strategy lies in choosing those products that are most frequently consumed by vegans or people following predominantly plant-based diets. One example could be plant-based milks. Plant milks are an excellent vehicle to provide highly absorbable calcium, vitamin D, B12, and iodine. Fortified plant milks are especially valuable for children aged 1–3 years. Plant milks targeted for young children should also include vitamin A, vitamin B2, and DHA, and should provide a minimum amount of calories and protein.
Market-driven fortification should be carried out by the food industry. The market and the variety of vegan products have increased in recent years. In the European Union, REGULATION (EC) No 1925/2006 on the addition of vitamins, minerals, and certain other substances to food allows the addition of vitamins and minerals as shown in Table 1 in bioavailable form to food when (i) there is a deficiency in the specific population or population groups that can be clinically demonstrated or suggested by estimated low intake levels; or (ii) there is a potential to improve the nutritional status of the population or specific population groups and/or to correct possible vitamin or mineral deficiencies due to changes in eating habits [91]. As has been exposed throughout this review, the current intake and nutritional status of certain nutrients in the vegan population would justify both premises. Furthermore, enrichment by food manufacturers of the products commonly consumed by this population would give them added value. Manufacturers should consider the recommended intakes of each nutrient before designing and formulating their products.

Table 1.
Relevant nutrients that can be added as fortifiers in plant foods and their formulation allowed by current legislation in the European Union [86].
In addition to fortification, certain industrial food-processing practices, such as canning and extrusion cooking, malting, milling, and hydrothermal treatments have the potential to reduce dietary phytate in cereals and therefore increase the bioavailability of iron, zinc, magnesium, and calcium [92,93], as mentioned in Figure 1.
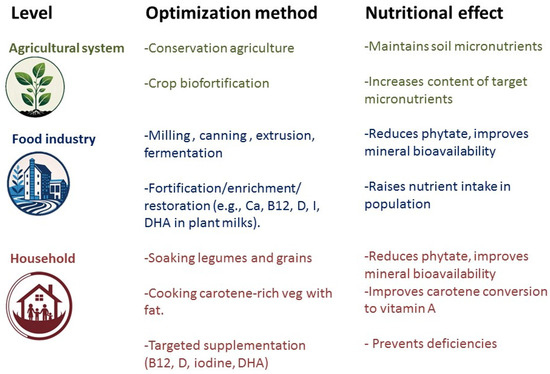
Figure 1.
Optimizing micronutrient content in plant foods at different levels of the food system.
Also, the way foods are prepared and cooked at home can have a significant impact in the absorption of nutrients. Soaking cereals and legumes in water (followed by decanting the water) before cooking them can reduce their phytate content [92]. Cooking (heating) carotene-rich vegetables and combining them with a source of fat will improve conversion to vitamin A [49,50]. Other food combinations that enhance nutrient absorption are iron plus vitamin C and other organic acids and calcium plus protein [88].
Finally, and at least until the food chain can guarantee a sufficient supply of all nutrients, people who follow vegan or plant-based diets should take a regular supplement of vitamin B12, and in most cases, of vitamin D too (Figure 1).
Table 2, summarizes, for each nutrient mentioned above, the aspects to consider in terms of its main source, bioavailability, and possible solutions to increase its amount in plants and plant-based foods.

Table 2.
Main plant source, bioavailability, and possible solutions to increase the amount of nutrients in plant-based foods.
Besides these points, the plant-based food industry faces the dual challenge of meeting consumer demand for convenient, tasty products while addressing nutritional concerns associated with plant-based diets. In this way, new emerging meat alternatives with innovations in taste, texture, and variety, closely aligned with familiar culinary experiences, can further increase acceptance. The latest innovations focus on emerging meat alternatives. The three major technological approaches driving innovation in sustainable protein production are cultivated meat, fermentation-derived proteins, and 3D-printed protein structures. The cultivated meat (cell-based) is produced by growing animal cells in bioreactors, yielding real muscle and fat tissue without slaughter. Key challenges remain in scaling up, reducing costs, and improving texture and consumer acceptance [94]. The fermentation-derived proteins [95] are used with the microorganisms to produce protein biomass or specific functional proteins (e.g., dairy, egg, or heme proteins via precision fermentation). This method is highly scalable, efficient, and precise, though regulatory frameworks and consumer perception are still evolving. The 3D-printed protein structures [96] employ additive manufacturing to replicate the fibrous structure of whole-cut meat using plant-based or hybrid “inks.” Industrial-scale operations now exist, but limitations include slow printing speed, high costs, and weaker sensory qualities compared to conventional plant-based alternatives. Overall, these three approaches complement each other in creating novel, sustainable meat alternatives, though each faces distinct technological, economic, and cultural hurdles before reaching mainstream adoption.
Finally, another point on which more studies are also needed is the increased exposure to pesticides with increased adherence to plant-based diets. The results are contradictory, insofar as there are studies that pesticide exposure increases as plant-based food intake increases, such as acetamiprid, azadirachtin, cypermethrin, pyrethrins, and spinose [95]. However, other studies show that the consumption of organic foods greatly decreases exposure to these pesticides and that many of the consumers who adopt vegetarian diets are also those with a higher consumption of organic foods whose regular consumption decreases exposure to pesticides [97].
4. Are Current Dietary Reference Intakes Valid for Plant-Based Diets?
Dietary Reference Intakes (DRIs) are a set of several related concepts of reference values that reflect scientific knowledge about the nutrient requirements of healthy populations [98]. These values are issued by different public health administrations, depending on the country (Table 3), and provide the scientific basis on which nutritional recommendations are built, being the references used in dietary assessment and planning, at the population and individual level. Likewise, they are used in the food industry to establish reference values in food labeling and food fortification [99].

Table 3.
Definitions of the reference values of nutrient intake and the names used by different institutions.
In all countries, recommended reference values are established for populations that follow mixed diets that include foods of animal and plant origin [99]. It is quoted that “if RDAs are achieved through diets composed of a variety of foods derived from different food groups rather than by supplementation or fortification, such diets are likely to be adequate in all other nutrients for which RDAs cannot currently be established” [100]. In addition, “normal” or “mixed” diets are based on the usual daily intake of healthy people and have been calculated to contain the average nutrient intake of healthy populations, with the implication that both bioavailability and requirement studies are based on a diet with foods from all groups [99,101].
When recommended reference values are calculated for each nutrient, its chemistry, functions, physiology and metabolism, its interaction with other nutrients, bioavailability, and absorption rate are considered [102]. For many nutrients, digestion, absorption, or both, from plant foods, are different when compared to animal foods. For example, the absorption of heme iron is higher and independent from other components of the diet, whereas the absorption of non-heme iron is usually lower and affected (positively and negatively) by other dietary components. The RDA for people following mixed diets account for this, but in plant-based diets, nonheme iron is the only source of iron. Some experts have proposed that recommended intakes in people following plant-based diets should be higher to allow for the decreased absorption [18]. Also, carotenoids are the main or only source of vitamin A in plant-based diets and the efficiency with which they are converted into vitamin A should be considered when establishing the RDI for retinol equivalents. Similarly, the pattern of essential amino acids provided by plant-based diets is not equivalent to that of mixed diets and the total protein requirements may not be the same. Table 3 shows the dietary sources of ALA and carotenes precursors, as well as iron and zinc, and proposed recommendations based on the available scientific literature.
Food patterns that include mainly plant foods, as well as the culinary practices, result in specific food matrix effects regarding inhibitory/promoting effects (therefore, bioavailability and bioconversion) and this should be considered when establishing nutrient requirements. The relative importance of such factors varies from nutrient to nutrient and new studies of food consumption and nutritional status in populations following these diets should be considered in order to modify the RDA or PRI values if necessary. The EFSA has recently conducted this regarding the PRI for zinc, correcting the requirements for the level of phytic acid in the diet [99], but there is a lack of equivalent data for other nutrients. It is a priority to investigate in a structured way how the current intake and absorption levels for each nutrient determine the nutritional status of populations that follow plant-based diets, in order to update the DRIs/DRVs accordingly (Table 4).

Table 4.
Proposed values for some nutrients’ intake for plant-based diets.
In addition to reconsidering the RDIs, another important step is to develop specific dietary guidelines for vegan or plant-based diets that show the ideal distribution of the plant food groups that ensures optimal macro- and micronutrient intakes. Although an increasing number of national and regional food-based dietary guidelines in the world recommend limitation or reduction in meat consumption and almost half already include both animal and plant sources of protein in the same group, stating or implying that animal foods can be replaced with plant protein-rich foods, all of them still include animal foods [98]. This creates confusion among vegan or plant-based consumers and even among health professionals, and is not the best way to promote good plant-based nutritional education.
The main limitations of this work are that it does not focus specifically on any particular aspect of plant-based diets, and that it presents some ideas and points that need further research. The authors intend to draw attention to the fact that there are points at the systemic, community, and food chain levels that are not consistent with the current recommendations to reduce animal food consumption. More studies are needed on the bioavailability of some nutrients in plant foods to support new recommendations for nutrient intakes in plant-based diets, as recommendations for mixed diets cannot be transferred to plant-based diets. Finally, more technological feasibility studies are needed for the introduction into the food chain of plant foods with higher nutritional value, either with fortification or with food production itself.
5. Conclusions
A shift towards more plant-based diets would have many benefits in multiple areas and should therefore be a priority for states and societies. Currently, people who try to reduce or eliminate animal products from their diet face numerous obstacles, which is not only is discriminatory and therefore ethically unacceptable, but is also counterproductive. In order to resolve this situation, a collective effort would be needed on three main levels:
Governments and public health institutions should publish dietary guidelines specific for plant-based eating patterns, designed so they meet all nutrient requirements. These RDI should be established from studies on nutritional status in vegan children, adolescents, and adult women and men, as well as in pregnant and lactating women. Governments should ensure that populations following plant-based diets have access to a good range of natural and fortified foods.
Medicine, nutrition, and other health-related degrees should include plant nutrition in their curricula. Vegans and other people following plant-based diets should receive appropriate and up-to-date nutritional counseling that respects their beliefs and preferences, without judging or discrimination. In settings where foods are not fortified, appropriate supplements should be prescribed.
Research should focus on identifying those nutrients whose requirements may be difficult for vegans to meet and on finding food sources that provide these nutrients and are easy to accept, affordable, and sustainable. Studies that only compare nutritional intakes in groups with different eating patterns with the intention to show that a vegan diet is deficient are not useful and do not help to improve the health and nutritional status of vegans. Furthermore, this comparison is not authentic, since it does not take into account that animal products have been fortified throughout the food chain.
Author Contributions
Conceptualization, I.M.-L. and M.M.-B.; performance of the analyses of the literature, I.M.-L., M.M.-B., M.M.-P. and S.M.-P.; writing, I.M.-L., M.M.-B., M.M.-P. and S.M.-P.; review and editing, I.M.-L. and M.M.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the Government of Aragón-Spain (grant Group AESA A06-20R).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO. Sustainable Healthy Diets: Guiding Principles. Available online: https://www.who.int/publications/i/item/9789241516648 (accessed on 15 May 2025).
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Rzymski, P.; Kulus, M.; Jankowski, M.; Dompe, C.; Bryl, R.; Petitte, J.N.; Kempisty, B.; Mozdziak, P. COVID-19 Pandemic Is a Call to Search for Alternative Protein Sources as Food and Feed: A Review of Possibilities. Nutrients 2021, 5, 150. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Rosenfeld, D.L.; Moreira, A.V.B.; Zandonadi, R.P. Plant-based and vegetarian diets: An overview and definition of these dietary patterns. Eur. J. Nutr. 2023, 62, 1109–1121. [Google Scholar] [CrossRef]
- Kent, G.; Kehoe, L.; Flynn, A.; Walton, J. Plant-based diets: A review of the definitions and nutritional role in the adult diet. Proc. Nutr. Soc. 2022, 81, 62–74. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a Healthy Diet: Evidence for The Role of Contemporary Dietary Patterns in Health and Disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Wang, T.; Masedunskas, A.; Willett, W.C.; Fontana, L. Vegetarian and vegan diets: Benefits and drawbacks. Eur. Heart J. 2023, 44, 3423–3439. [Google Scholar] [CrossRef] [PubMed]
- Menos Carne, Más Vida. Spanish Ministry of Consumer Affairs. Available online: https://www.youtube.com/watch?v=OddlzKikgeA (accessed on 15 May 2025).
- Seffen, A.E.; Dohle, S. What motivates German consumers to reduce their meat consumption? Identifying relevant beliefs. Appetite 2023, 187, 106593. [Google Scholar] [CrossRef]
- Borusiak, B.; Szymkowiak, A.; Kucharska, B.; Gálová, J.; Mravcová, A. Predictors of intention to reduce meat consumption due to environmental reasons–Results from Poland and Slovakia. Meat Sci. 2022, 184, 108674. [Google Scholar] [CrossRef] [PubMed]
- Selinger, E.; Neuenschwander, M.; Koller, A.; Gojda, J.; Kühn, T.; Schwingshackl, L.; Barbaresko, J.; Schlesinger, S. Evidence of a vegan diet for health benefits and risks—An umbrella review of meta-analyses of observational and clinical studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 9926–9936. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Rosenberg, I.; Uauy, R. History of modern nutrition science—Implications for current research, dietary guidelines, and food policy. BMJ 2018, 361, k2392. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Angell, S.Y.; Lang, T.; Rivera, J.A. Role of government policy in nutrition—Barriers to and opportunities for healthier eating. BMJ 2018, 361, k2426. [Google Scholar] [CrossRef]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Head Zauche, L.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Raj, S.; Guest, N.S.; Landry, M.J.; Mangels, A.R.; Pawlak, R.; Rozga, M. Vegetarian Dietary Patterns for Adults: A Position Paper of the Academy of Nutrition and Dietetics. J. Acad. Nutr. Diet. 2025, 125, 831–846.e2. [Google Scholar] [CrossRef]
- Spector, A.; Kim, H.Y. Discovery of Essential Fatty Acids. J. Lipid Res. 2014, 56, 11–21. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Horrobin, D.F. Nutritional and medical importance of gammalinolenic acid. Prog. Lipid Res. 1992, 31, 163–194. [Google Scholar] [CrossRef]
- Sarter, B.; Kelsey, K.S.; Schwartz, T.A.; Harris, W.S. Blood docosahexaenoic acid and eicosapentaenoic acid in vegans: Associations with age and gender and effects of an algal-derived omega-3 fatty acid supplement. Clin. Nutr. 2015, 34, 212–218. [Google Scholar] [CrossRef]
- Pinto, A.M.; Sanders, T.A.; Kendall, A.C.; Nicolaou, A.; Gray, R.; Al-Khatib, H.; Hall, W.L. A comparison of heart rate variability, n-3 PUFA status and lipid mediator profile in age- and BMI-matched middle-aged vegans and omnivores. Br. J. Nutr. 2017, 117, 669–685. [Google Scholar] [CrossRef]
- Perrin, M.T.; Pawlak, R.; Dean, L.L.; Christis, A.; Friend, L. A cross-sectional study of fatty acids and brain-derived neurotropic factor (BDNF) in human milk from lactating women following vegan vegetarian and omnivore diets. Eur. J. Clin. Nutr. 2019, 58, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, F.R.; Valenzuela, M.C.; Hernandez-Rodas, E.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fatty Acids 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Birch, E.E.; Carlson, S.E.; Hoffman, D.R.; Fitzgerald-Gustafson, K.M.; Fu, V.L.N.; Drover, J.R.; Castañeda, Y.S.; Minns, L.; Wheaton, D.K.; Mundy, D. The DIAMOND (DHA Intake and Measurement of neural development) Study: A double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am. J. Clin. Nutr. 2010, 4, 848–859. [Google Scholar] [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640S–646S. [Google Scholar] [CrossRef]
- Gebauer, S.K.; Psota, T.L.; Harris, W.S.; Kris-Etherton, P.M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am. J. Clin. Nutr. 2006, 83, 1526S–1535S. [Google Scholar] [CrossRef] [PubMed]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; San Pablo, G. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 4, 2365. [Google Scholar] [CrossRef]
- Hovinen, T.; Korkalo, L.; Freese, R.; Skaffari, E.; Isohanni, P.; Niemi, M.; Nevalainen, J.; Gylling, H.; Suomalainen, A. Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol. Med. 2021, 5, e13492. [Google Scholar] [CrossRef]
- Sanders, T.A. DHA status of vegetarians. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.E.; Wilson, M.; Hellon, T.G.; Davies, I.G. Bioavailability and conversion of plant-based sources of omega-3 fatty acids–a scoping review to update supplementation options for vegetarians and vegans. Crit. Rev. Food Sci. Nutr. 2022, 62, 4982–4997. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 30, 11. [Google Scholar] [CrossRef]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review. Evid. Rep. Technol. Assess. 2016, 224, 1–826. [Google Scholar] [CrossRef]
- Karcz, K.; Królak-Olejnik, B. Vegan or vegetarian diet and breast milk composition–a systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1081–1098. [Google Scholar] [CrossRef]
- Vidailhet, M.; Rieu, D.; Feillet, F.; Bocquet, A.; Chouraqui, J.P.; Darmaun, D.; Dupont, C.; Frelut, M.L.; Girardet, J.P.; Hankard, R.; et al. Vitamin A in pediatrics: An update from the Nutrition Committee of the French Society of Pediatrics. Arch. Pediatr. 2017, 24, 288–297. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on Dietary Reference Values for vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- Strobel, M.; Tinz, J.; Biesalski, H.K. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur. J. Nutr. 2007, 46, I1–I20. [Google Scholar] [CrossRef]
- Borel, P.; Desmarchelier, C. Genetic Variations Associated with Vitamin A Status and Vitamin A Bioavailability. Nutrients 2017, 8, 246. [Google Scholar] [CrossRef]
- Li, D.; Sinclair, A.J.; Mann, N.J.; Turner, A.; Ball, M.J. Selected micronutrient intake and status in men with differing meat intakes, vegetarians and vegans. Asia Pac. J. Clin. Nutr. 2000, 9, 18–23. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gøbel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 30, 115. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 31, 575–582. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; Russell, R.M.; Stephensen, C.B.; Gannon, B.M.; Craft, N.E.; Haskell, M.J.; Lietz, G.; Schulze, K.; Raiten, D.J. Biomarkers of nutrition for development (BOND)—Vitamin A review. J. Nutr. 2016, 146, 1816S–1848S. [Google Scholar] [CrossRef]
- Yee, M.M.F.; Chin, K.Y.; Ima-Nirwana, S.; Wong, S.K. Vitamin A and Bone Health: A Review on Current Evidence. Molecules 2021, 21, 1757. [Google Scholar] [CrossRef]
- Chen, G.D.; Zhu, Y.Y.; Cao, Y.; Liu, J.; Shi, W.Q.; Liu, Z.M.; Chen, Y.M. Association of dietary consumption and serum levels of vitamin A and β-carotene with bone mineral density in Chinese adults. Bone 2015, 79, 110–115. [Google Scholar] [CrossRef]
- Kopec, R.E.; Cooperstone, J.L.; Schweiggert, R.M.; Young, G.S.; Harrison, E.H.; Francis, D.M.; Clinton, S.K.; Schwartz, S.J. Avocado Consumption Enhances Human Postprandial Provitamin A. Absorption and Conversion from a Novel High-β-Carotene Tomato Sauce and from Carrots. J. Nutr. 2014, 144, 1158–1166. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef] [PubMed]
- White, W.S.; Zhou, Y.; Crane, A.; Dixon, P.; Quadt, F.; Flendrig, L.M. Modeling the dose effects of soybean oil in salad dressing on carotenoid and fat-soluble vitamin bioavailability in salad vegetables. Am. J. Clin. Nutr. 2017, 106, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011, 22, 645–652. [Google Scholar] [CrossRef]
- Kapil, U. Health consequences of iodine deficiency. Sultan Qaboos Univ. Med. J. 2007, 7, 267–272. [Google Scholar] [CrossRef]
- Pearce, E.N.; Lazarus, J.H.; Moreno-Reyes, R.; Zimmermann, M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016, 104, 918s–923s. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.N.; Caldwell, K.L. Urinary iodine, thyroid function, and thyroglobulin as biomarkers of iodine status. Am. J. Clin. Nutr. 2016, 104, 898–901. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- Skeaff, A.S. Iodine Nutrition in Pregnancy. In Comprehensive Handbook of Iodine; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- WHO; ICCIDD; UNICEF. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide for Programme Managers. 2007. Available online: https://iris.who.int/bitstream/handle/10665/43781/9789241595827_eng.pdf (accessed on 21 July 2025).
- Vila, L.; Lucas, A.; Donnay, S.; De la Vieja, A.; Wengrovicz, S.; Santiago, P.; Bandrés, O.; Velasco, I.; Garcia-Fuentes, E.; Ares, S.; et al. Iodine nutrition status in Spain Needs for the future. Endocrinol. Diabetes Nutr. 2020, 67, 61–69. [Google Scholar] [CrossRef]
- Brantsæter, A.L.; Knutsen, H.K.; Johansen, N.C.; Nyheim, K.A.; Erlund, I.; Meltzer, H.M.; Henjum, S. Inadequate Iodine Intake in Population Groups Defined by Age, Life Stage and Vegetarian Dietary Practice in a Norwegian Convenience Sample. Nutrients 2018, 17, 230. [Google Scholar] [CrossRef]
- Lightowler, H.; Davies, G. Assessment of iodine intake in vegans: Weighed dietary record vs duplicate portion technique. Eur. J. Clin. Nutr. 2002, 56, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Groufh-Jacobsen, S.; Hess, S.Y.; Aakre, I.; Folven-Gjengedal, E.L.; Blandhoel-Pettersen, K.; Henjum, S. Vegans, Vegetarians and Pescatarians Are at Risk of Iodine Deficiency in Norway. Nutrients 2020, 20, 3555. [Google Scholar] [CrossRef] [PubMed]
- Krajčovičová-Kudláčková, M.; Bučková, K.; Klimeš, I.; Šeboková, E. Iodine Deficiency in Vegetarians and Vegans. Ann. Nutr. Metab. 2003, 47, 183–185. [Google Scholar] [CrossRef]
- Alles, B.; Baudry, J.; Mejean, C.; Touvier, M.; Peneau, S.; Hercberg, S.; Kesse-Guyot, E. Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the nutriNet-santé study. Nutrients 2017, 9, 1023. [Google Scholar] [CrossRef]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Eveleigh, E.R.; Coneyworth, L.J.; Avery, A.; Welham, S.J.M. Vegans, Vegetarians, and Omnivores: How Does Dietary Choice Influence Iodine Intake? A Systematic Review. Nutrients 2020, 29, 1606. [Google Scholar] [CrossRef]
- Světnička, M.; Heniková, M.; Selinger, E.; Ouřadová, A.; Potočková, J.; Kuhn, T.; Gojda, J.; El-Lababidi, E. Prevalence of iodine deficiency among vegan compared to vegetarian and omnivore children in the Czech Republic: Cross-sectional study. Eur. J. Clin. Nutr. 2023, 77, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Zava, T.T.; Zava, D.T. Assessment of Japanese iodine intake based on seaweed consumption in Japan: A literature-based analysis. Thyroid. Res. 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Bouga, M.; Combet, E. Emergence of Seaweed and Seaweed-Containing Foods in the UK: Focus on Labeling, Iodine Content, Toxicity and Nutrition. Foods 2015, 15, 240–253. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium−Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Rayman, M.P. The use of high-Se yeast to raise Se status: How does it measure up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- Schupbach, R.; Wegmuller, R.; Berguerand, C.; Bui, M.; Herter-Aeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Am. J. Clin. Nutr. 2017, 56, 283–293. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef]
- Fallon, N.; Dillon, S.A. Low Intakes of Iodine and Selenium Amongst Vegan and Vegetarian Women Highlight a Potential Nutritional Vulnerability. Front. Nutr. 2020, 20, 72. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar] [CrossRef]
- International Food Policy Research Institute. Available online: https://www.ifpri.org/topic/food-systems (accessed on 3 June 2025).
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 14, 12. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Hotz, C. Biofortification. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 175–181. [Google Scholar]
- Białowąs, W.; Blicharska, E.; Drabik, K. Biofortification of Plant- and Animal-Based Foods in Limiting the Problem of Microelement Deficiencies-A Narrative Review. Nutrients 2024, 16, 1481. [Google Scholar] [CrossRef] [PubMed]
- Medina-Lozano, I.; Díaz, A. Applications of Genomic Tools in Plant Breeding: Crop Biofortification. Int. J. Mol. Sci. 2022, 23, 3086. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Qu, J.; Pu, Y.; Rao, S.; Xu, F.; Wu, C. Selenium Biofortification of Crop Food by Beneficial Microorganisms. J. Fungi 2020, 6, 59. [Google Scholar] [CrossRef]
- Avnee, S.-S.; Chaudhary, D.R.; Jhorar, P.; Rana, R.S. Biofortification: An approach to eradicate micronutrient deficiency. Front. Nutr. 2023, 10, 1233070. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Dary, O.; Mora, J.O. Food Fortification: Technological Aspects. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 306–314. [Google Scholar]
- FAO; WHO. Codex Alimentarius, 2nd ed.; FAO: Rome, Italy, 1994; Volume 4. [Google Scholar]
- Global Fortification Data Exchange. Available online: https://fortificationdata.org/ (accessed on 3 June 2025).
- European Council. Regulation (EC) No 1925/2006 of the european parliament and of the council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Off. J. Eur. Union 2006, L 404, 26–38. [Google Scholar]
- Gibson, R.S.; Raboy, V.; King, J.C. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018, 76, 793–804. [Google Scholar] [CrossRef]
- Teucher, B.; Olivares, M.; Cori, H. Enhancers of iron absorption: Ascorbic acid and other organic acids. Int. J. Vitam. Nutr. Res. 2004, 74, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Kerstetter, J.E.; O’Brien, K.O.; Caseria, D.M.; Wall, D.E.; Insogna, K.L. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J. Clin. Endocrinol. Metab. 2005, 90, 26–31. [Google Scholar] [CrossRef]
- Powell, D.J.; Li, D.; Smith, B.; Chen, W.N. Cultivated meat microbiological safety considerations and practices. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70077. [Google Scholar] [CrossRef]
- Liu, Y.; Aimutis, W.R.; Drake, M. Dairy, Plant, and Novel Proteins: Scientific and Technological Aspects. Foods 2024, 13, 1010. [Google Scholar] [CrossRef]
- Dey, S.; Hettiarachchy, N.; Bisly, A.A.; Luthra, K.; Atungulu, G.G.; Ubeyitogullari, A.; Mozzoni, L.A. Physical and textural properties of functional edible protein films from soybean using an innovative 3D printing technology. J. Food Sci. 2022, 87, 4808–4819. [Google Scholar] [CrossRef]
- Rebouillat, P.; Vidal, R.; Cravedi, J.P.; Taupier-Letage, B.; Debrauwer, L.; Gamet-Payrastre, L.; Touvier, M.; Hercberg, S.; Lairon, D.; Baudry, J.; et al. Estimated dietary pesticide exposure from plant-based foods using NMF-derived profiles in a large sample of French adults. Eur. J. Nutr. 2021, 60, 1475–1488. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary Reference Values. Available online: https://www.efsa.europa.eu/en/topics/topic/dietary-reference-values (accessed on 13 May 2025).
- European Food Safety Authority (EFSA). Dietary Reference Values for nutrients. Summary Report. EFSA Support. Publ. 2017, 14, e15121. Available online: https://www.efsa.europa.eu/en/supporting/pub/e15121 (accessed on 14 May 2025). [CrossRef]
- National Research Council (US). Subcommittee on the tenth edition of the recommended dietary allowances. In Recommended Dietary Allowances, 10th ed.; National Academies Press: Washington, DC, USA, 1989. Available online: https://www.ncbi.nlm.nih.gov/books/NBK234926/ (accessed on 14 May 2025).
- Subcommittee on Interpretation and Uses of Dietary Reference Intakes and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes: Applications in Dietary Planning; National Academies Press: Washington, DC, USA, 2003; Available online: http://www.nap.edu/catalog/10609.html (accessed on 14 May 2025).
- SCF (Scientific Committee on Food). Nutrient and energy intakes for the European Community. In Reports of the Scientific Committee for Food, 31st Series. Food–Science and Technique; European Commission: Luxembourg, 1993; Volume 248, p. 3. [Google Scholar]
- U.S. Department of Agriculture. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 2 February 2022).
- Lane, K.; Derbyshire, E.; Li, W.; Brennan, C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: A review of the literature. Crit. Rev. Food. Sci. Nutr. 2014, 54, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Armah, S.M.; Carriquiry, A.; Sullivan, D.; Cook, J.D.; Reddy, M.B. A complete diet-based algorithm for predicting nonheme iron absorption in adults. J. Nutr. 2013, 143, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R.; Roughead, Z.K. Nonheme-iron absorption, fecal ferritin excretion, and blood indexes of iron status in women consuming controlled lactoovovegetarian diets for 8 week. Am. J. Clin. Nutr. 1999, 69, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).