Kinetics and Morphological Characteristics of CO2 Hydrate Formation Within Sandstone Fractures
Abstract
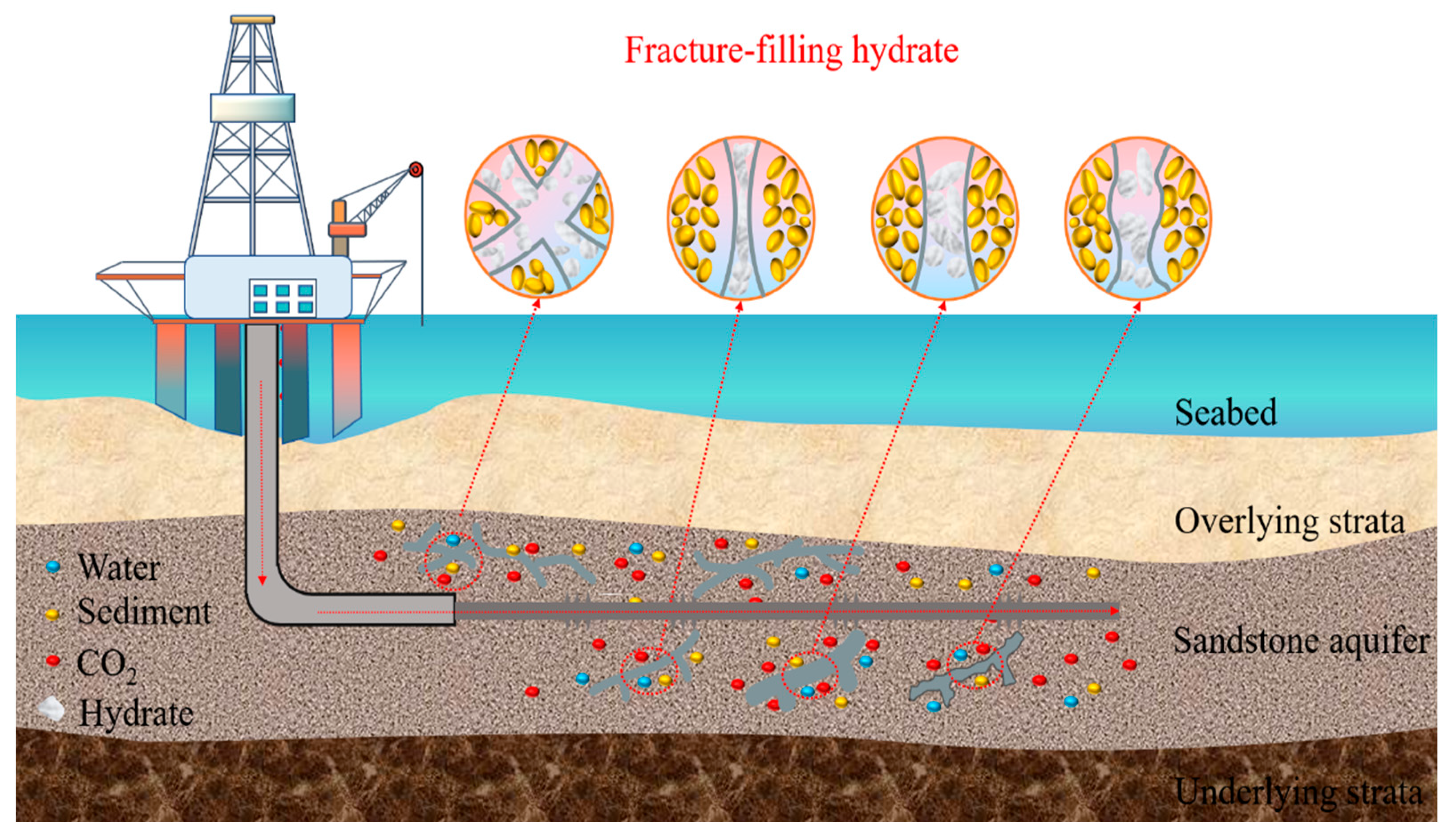
1. Introduction
2. Methodology
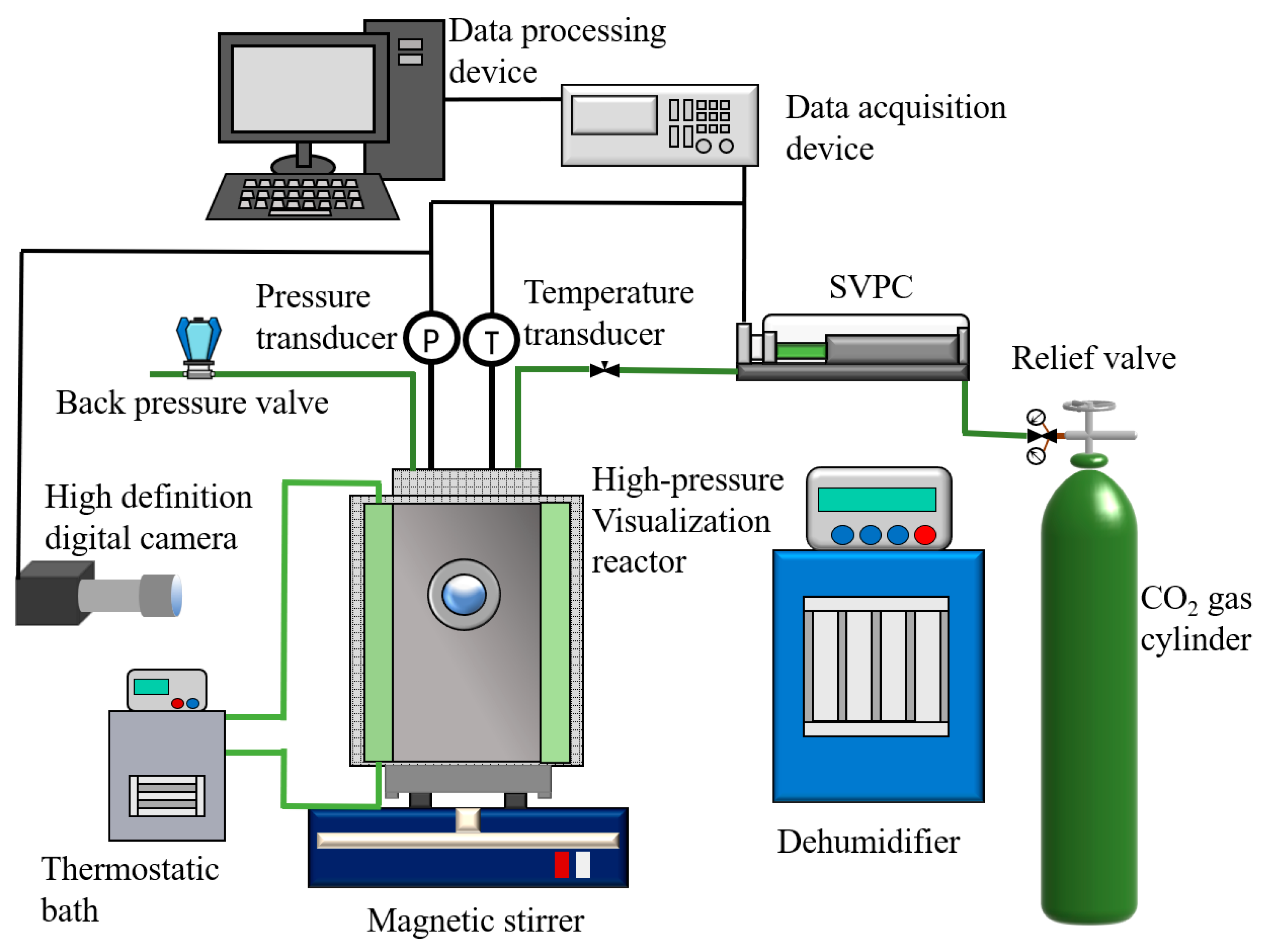
2.1. Experimental Apparatuses and Materials
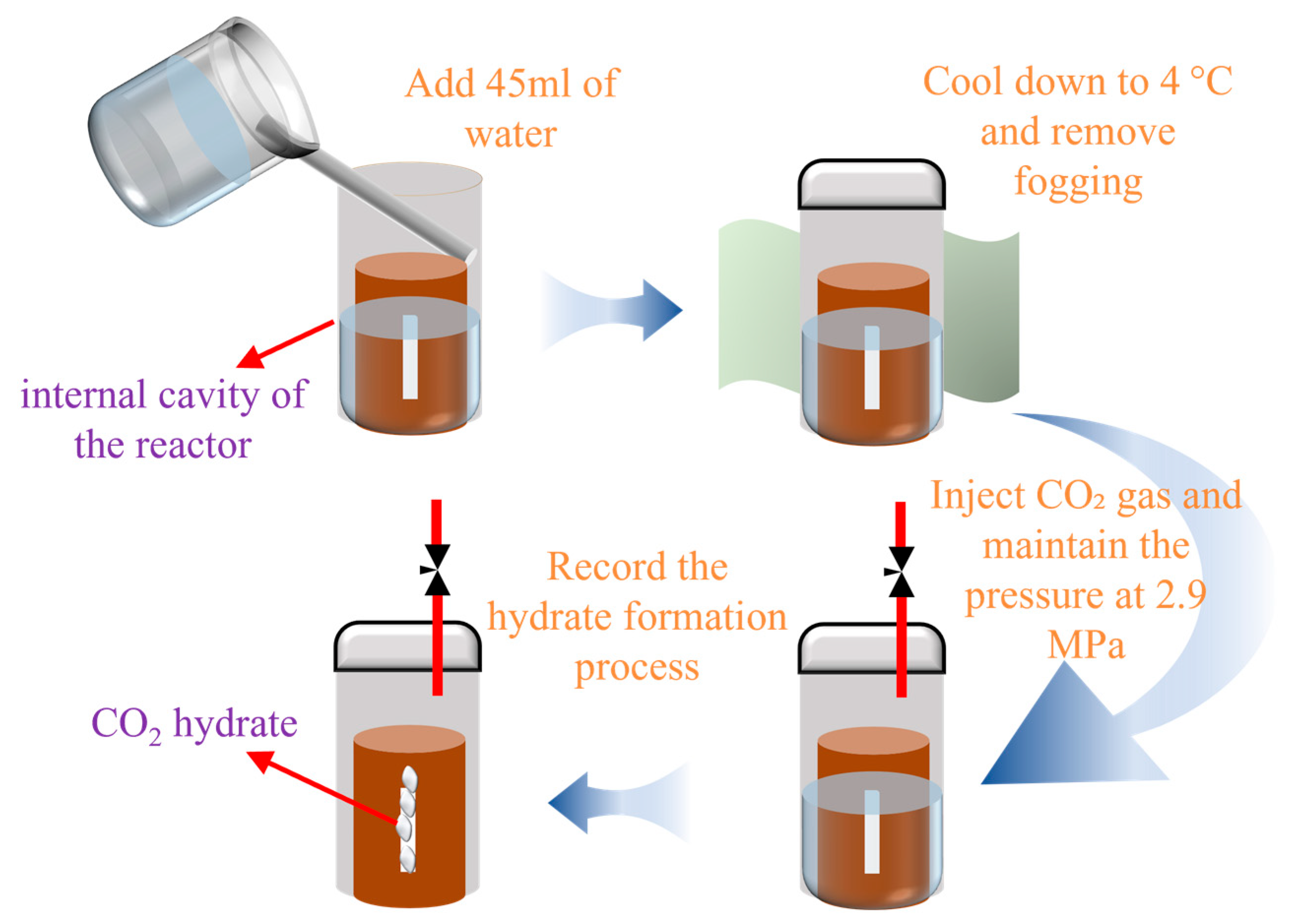
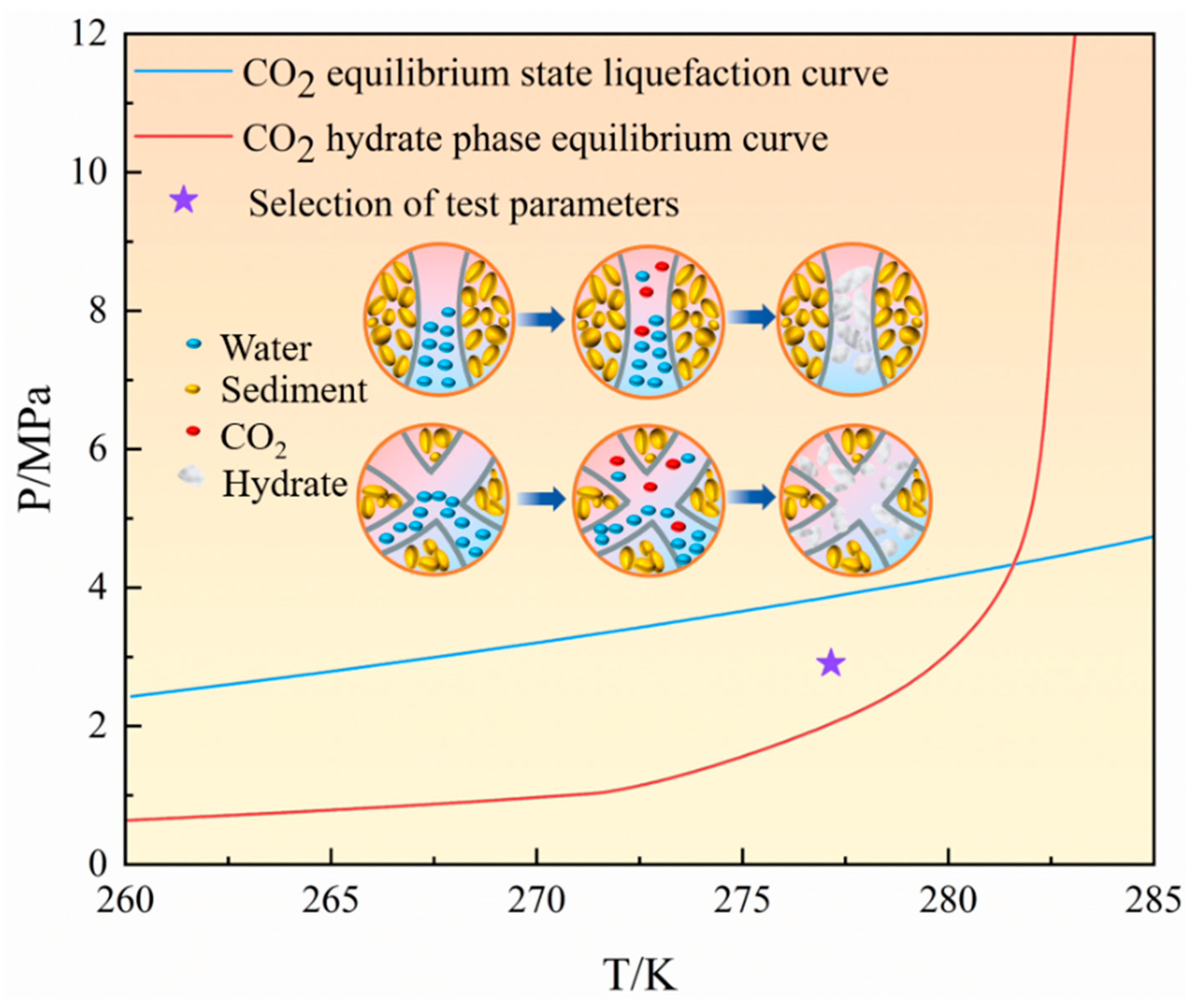
2.2. Experimental Procedures and Schemes
3. Results and Discussion
3.1. Kinetics and Morphology of CO2 Hydrate Formation Within Fractures
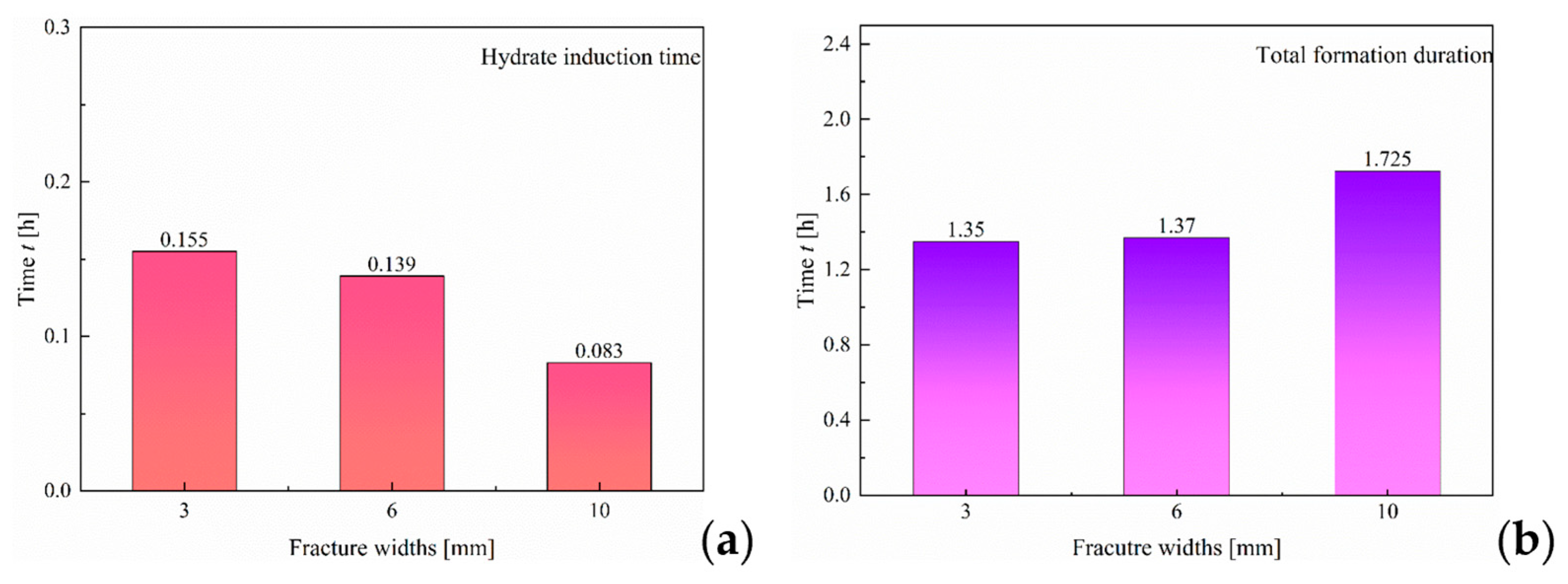
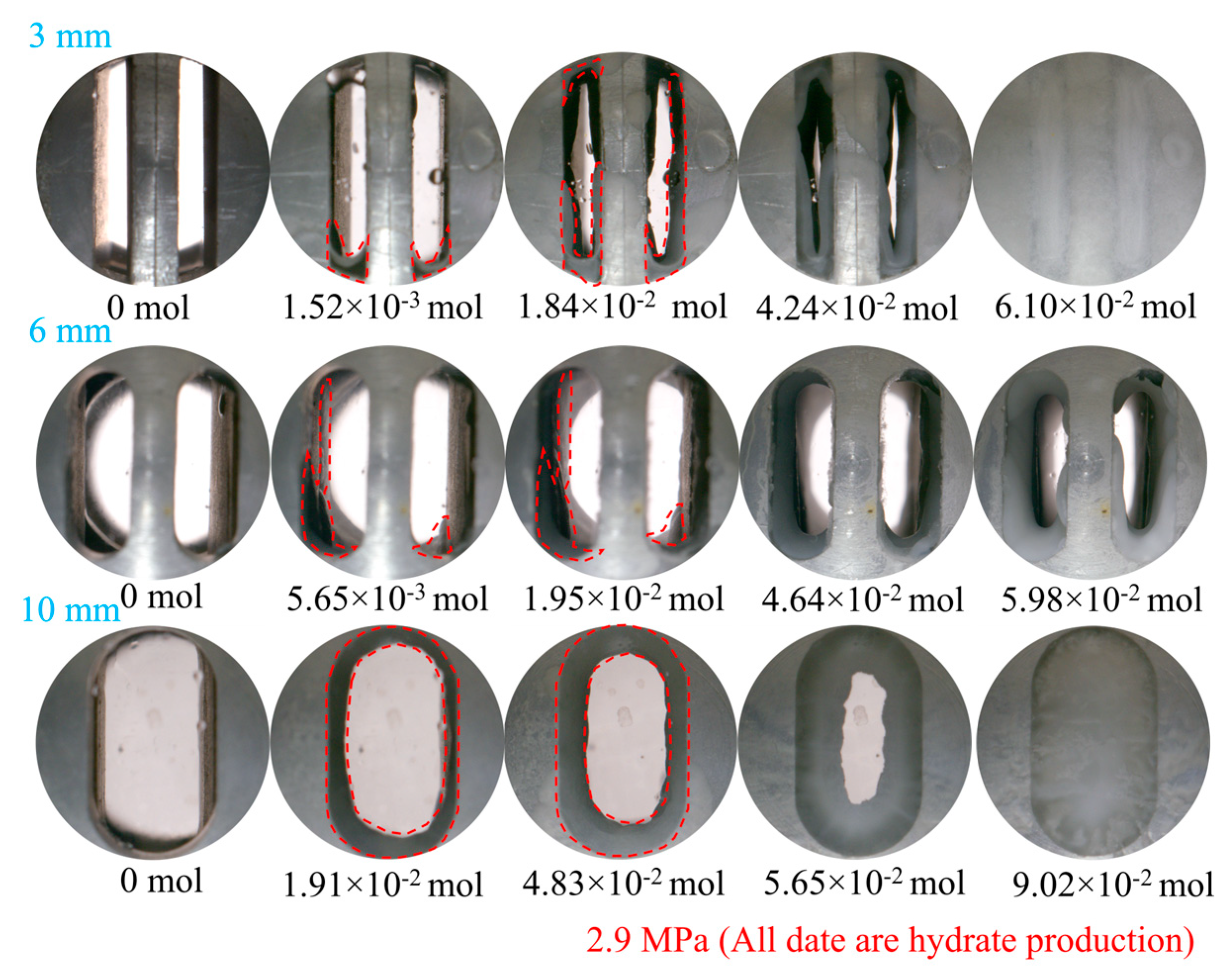
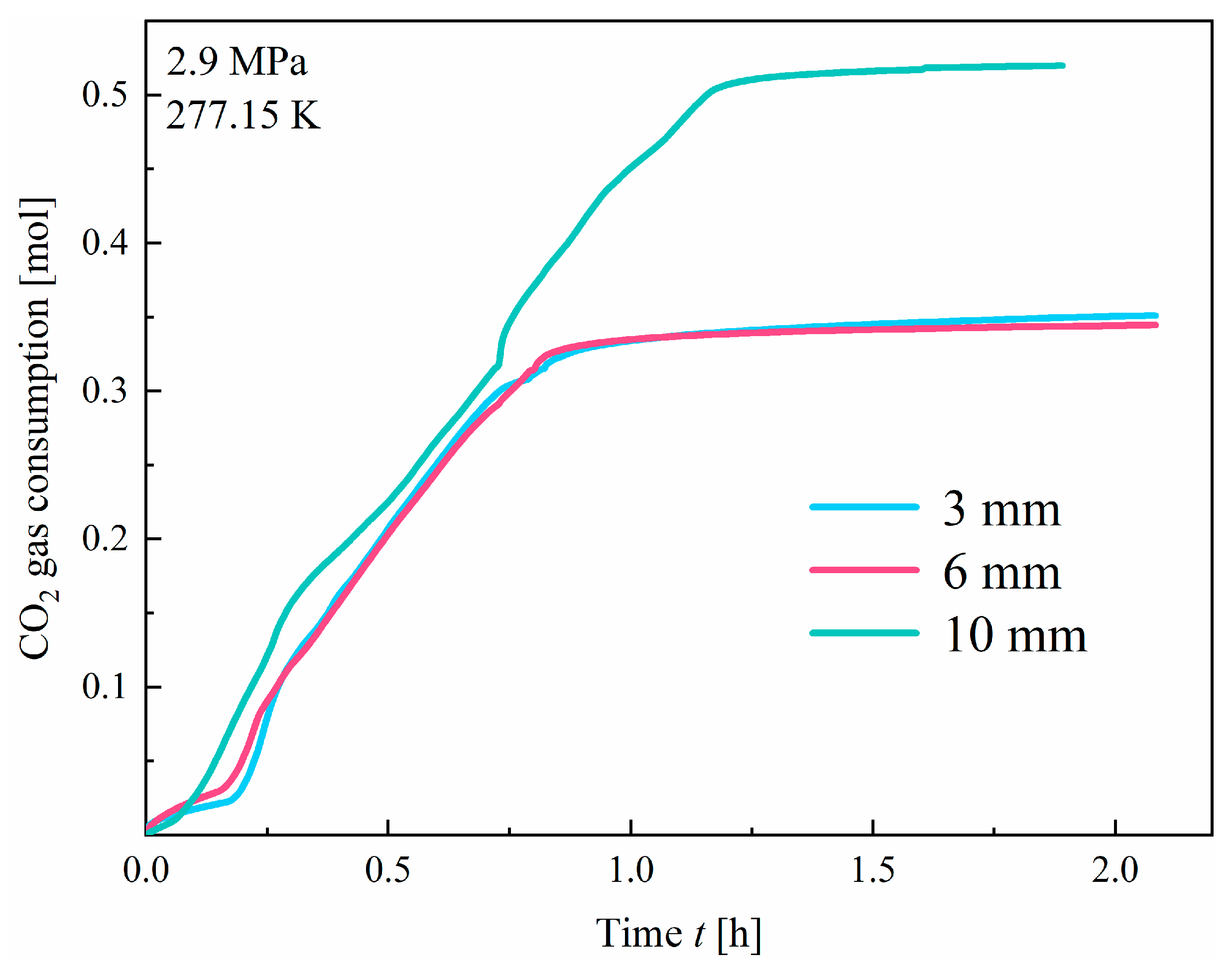
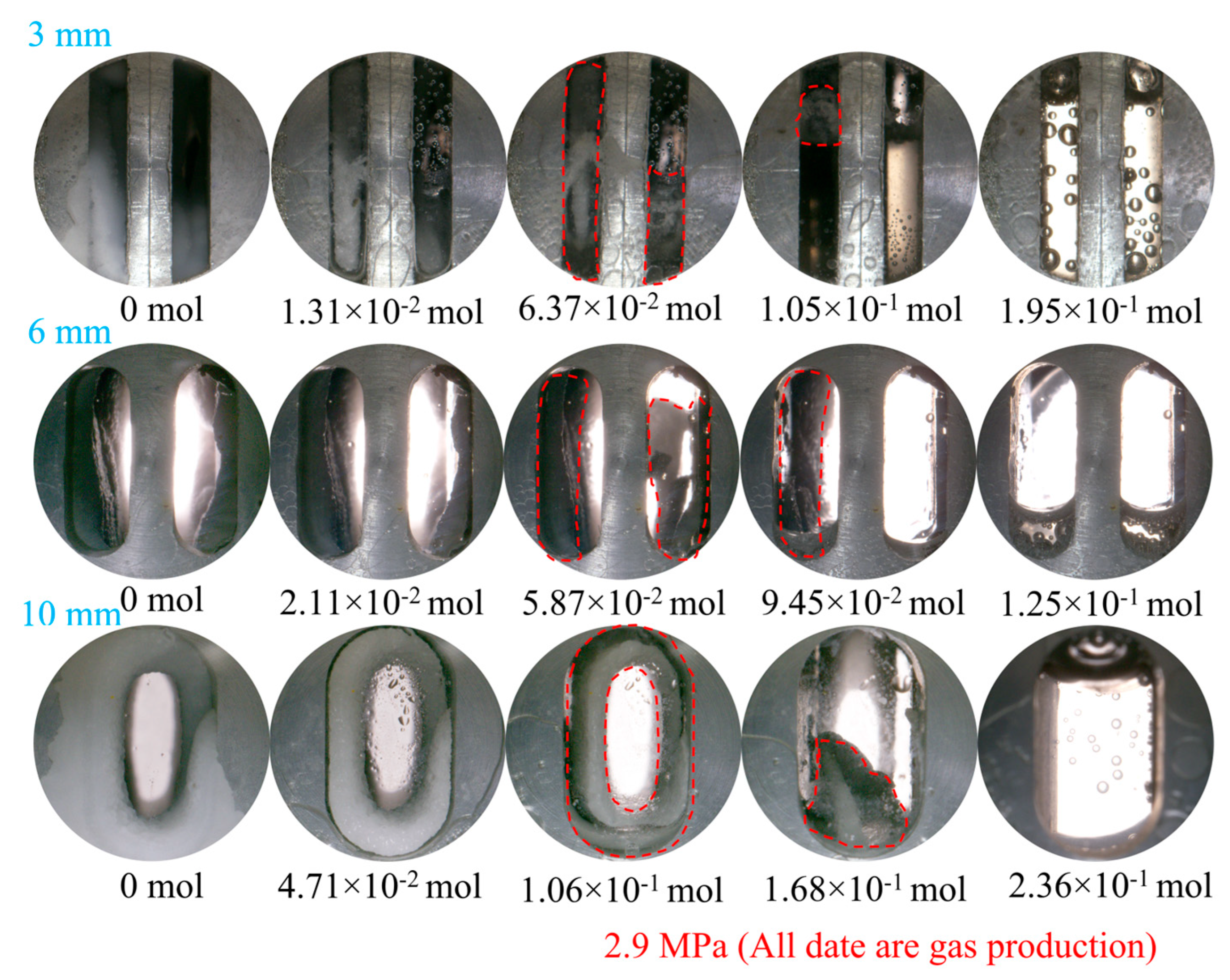
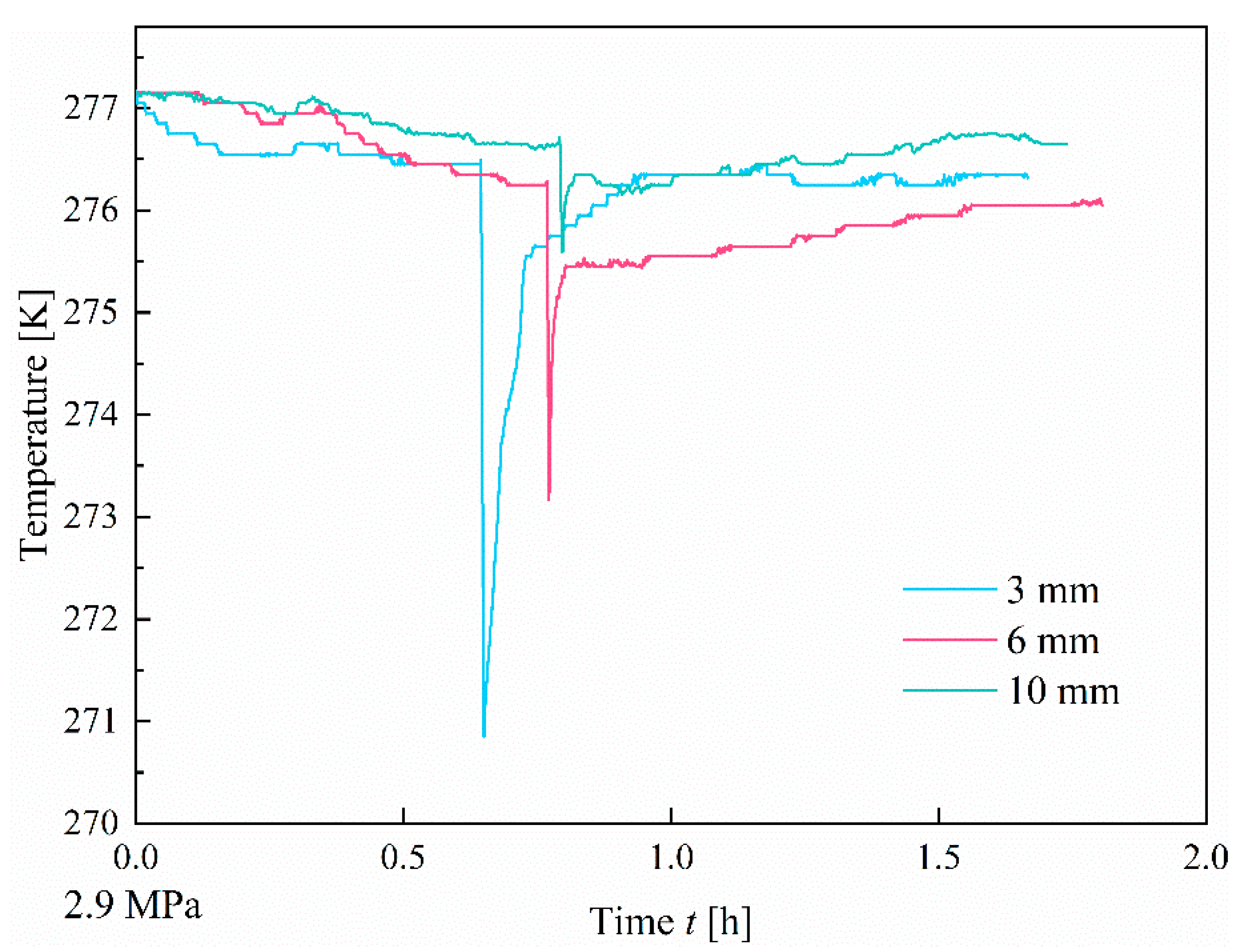
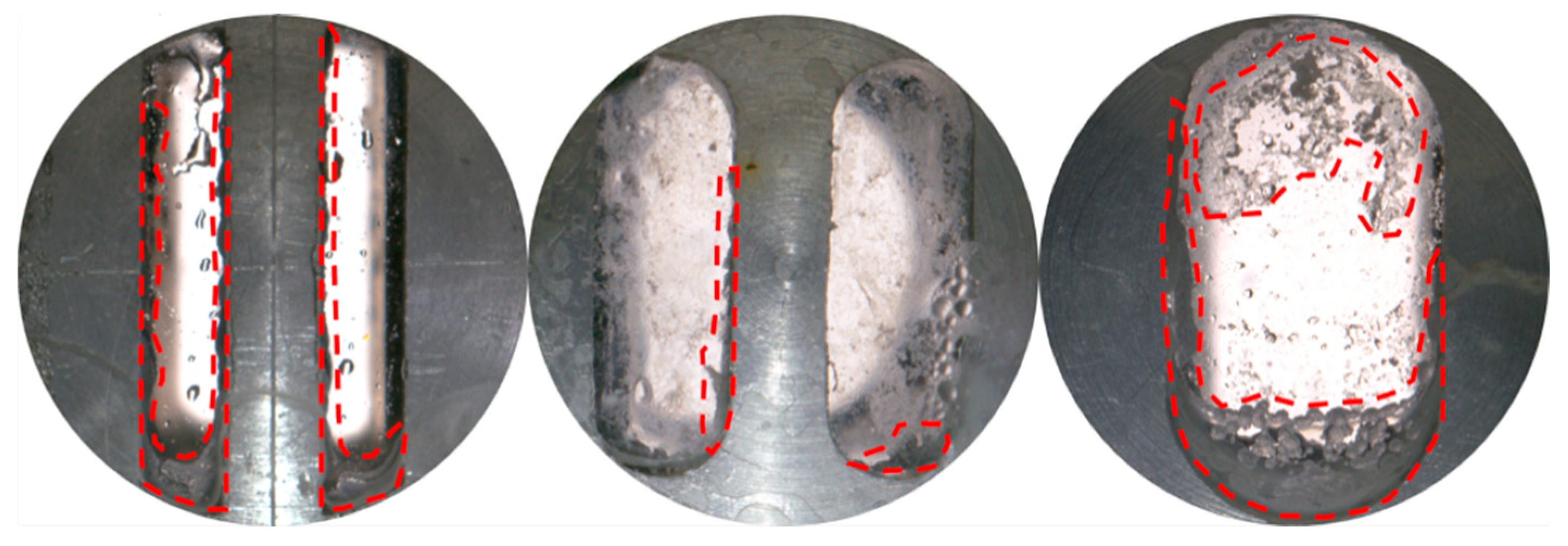
3.1.1. Effect of Fracture Width
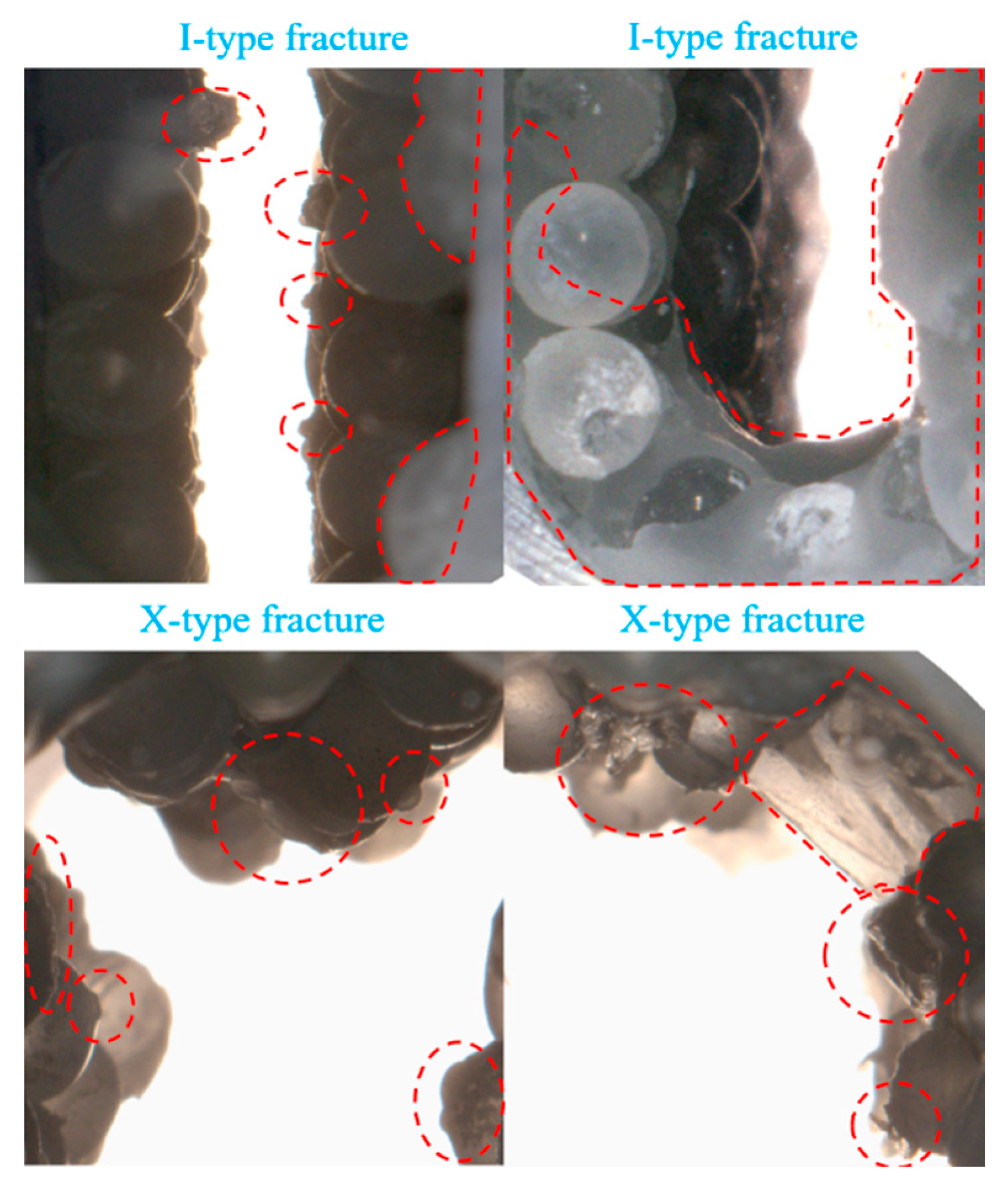
3.1.2. Effect of Fracture Type
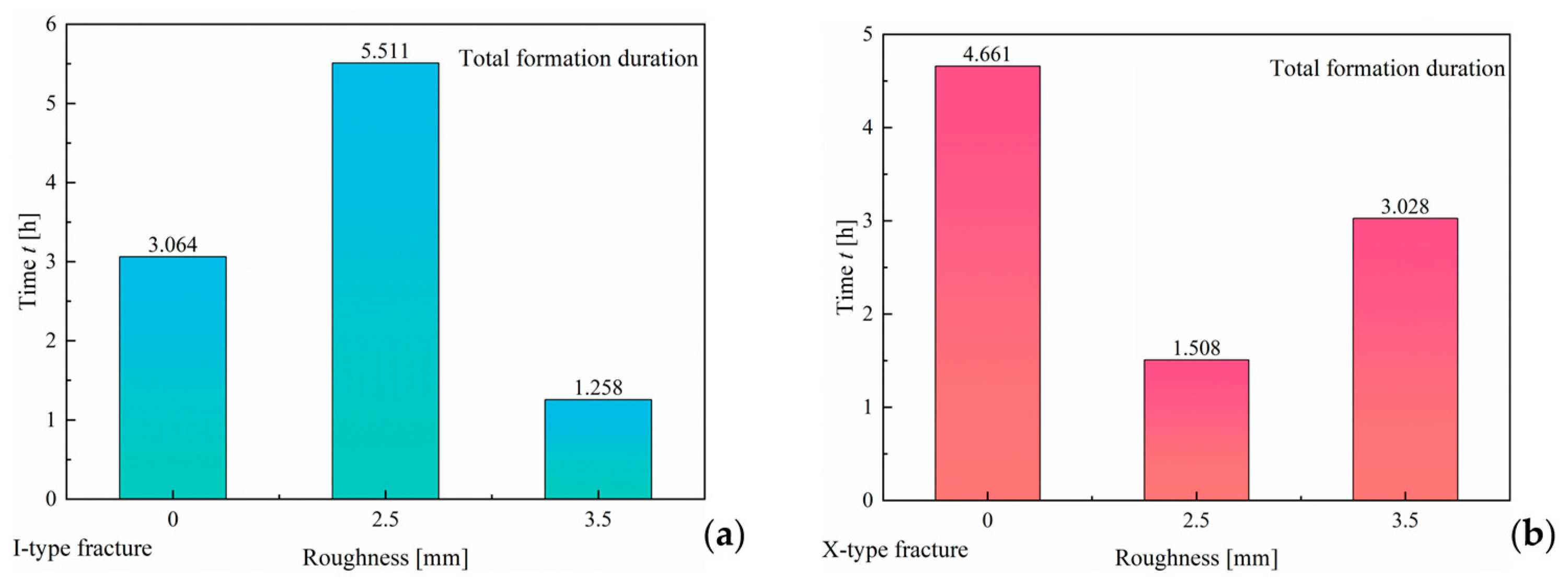
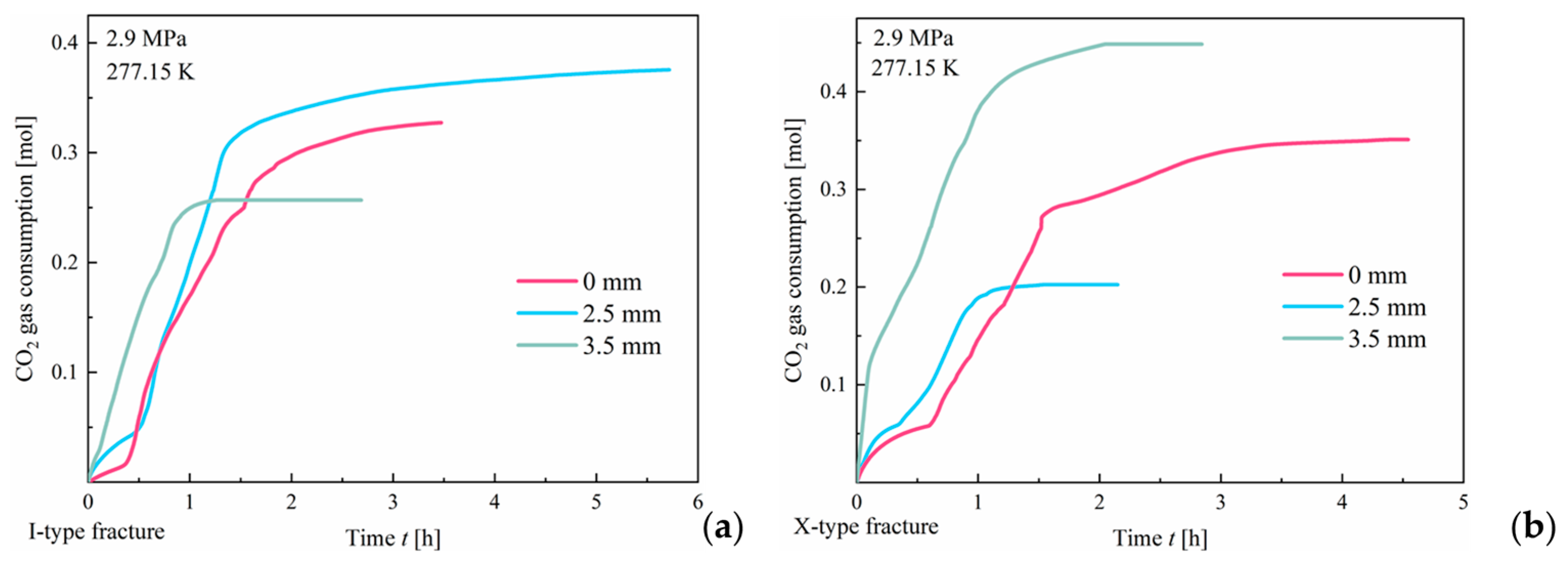
3.1.3. Effect of Roughness
3.2. Dissociation Kinetics and Morphological Characteristics of CO2 Hydrates Within Fractures
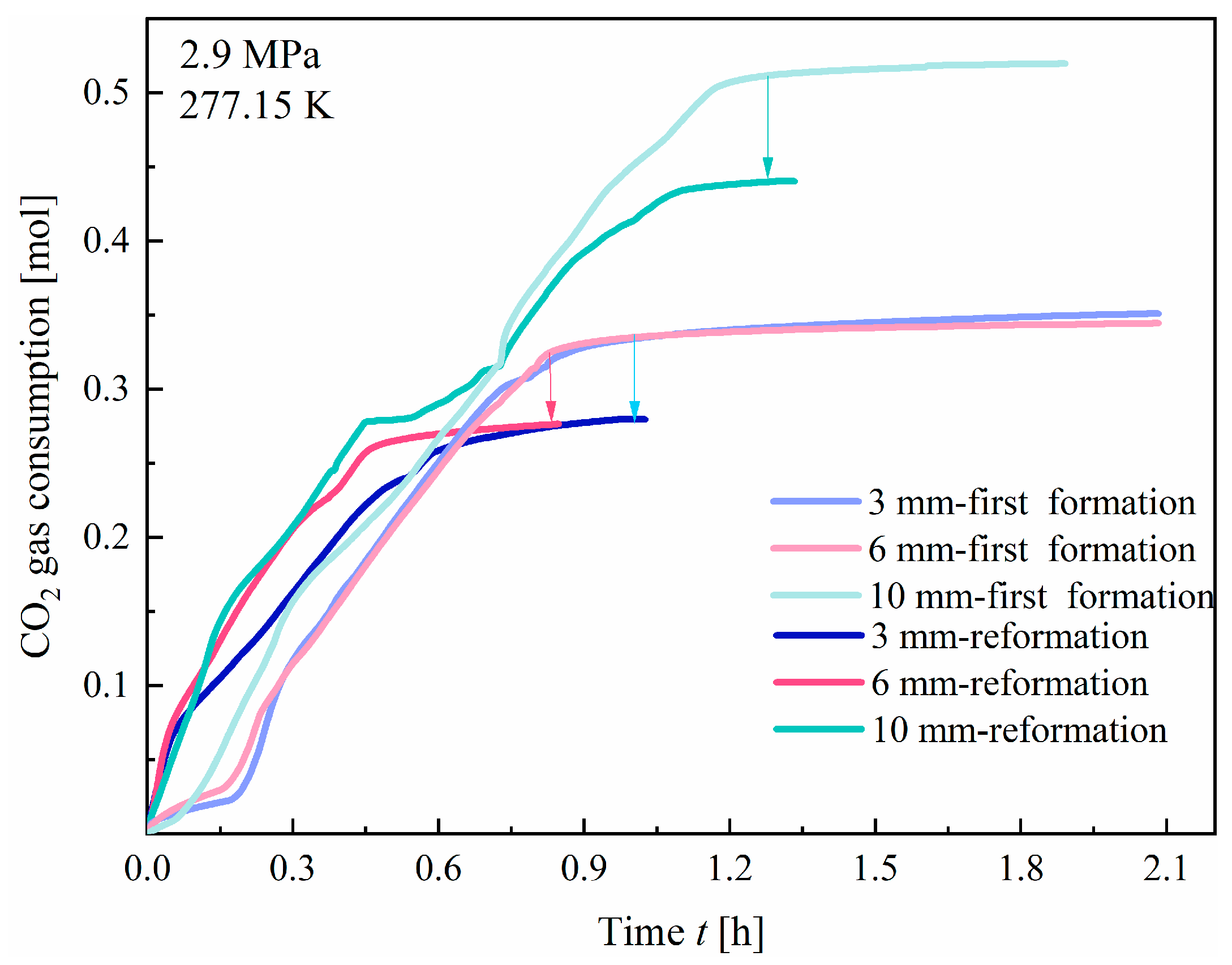
3.3. Hydrate Reformation
4. Conclusions
- (1)
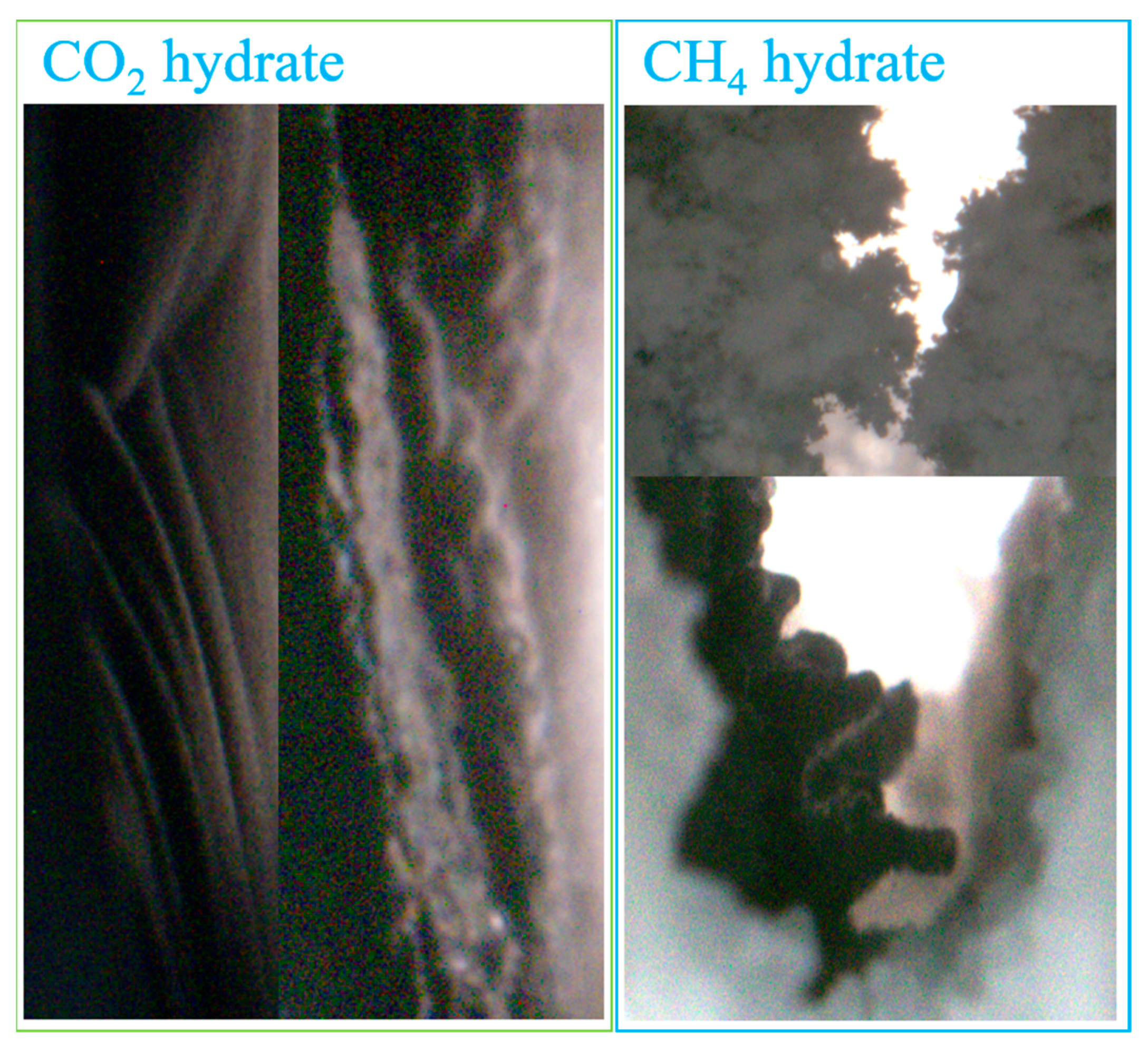
- At a pressure of 2.9 MPa, the induction time of CO2 hydrates is inversely correlated with fracture width. In comparison to the other two width sizes, the 10 mm width fracture exhibits the shortest induction time, the longest formation duration, and the highest hydrate yield (approximately 0.52 mol). In contrast to the irregular, flocculent morphology of CH4 hydrates, CO2 hydrates predominantly exhibit a smooth, flat, thin-wall structure. This morphological characteristic remains consistent, regardless of variations in fracture width;
- (2)
- The X-type fracture facilitates gas flow, enabling CO2 dissolution and hydrate induction to occur nearly simultaneously. Hydrate formation is controlled by both the formation rate and the total available space. In comparison to the I-type fracture, the X-type fracture exhibits a shorter hydrate formation duration. Furthermore, due to interface clogging, the final hydrate yield in X-type fractures is ultimately limited;
- (3)
- An increase in fracture surface roughness enhances the number of nucleation sites for hydrate formation. In both fracture types, the induction time for CO2 hydrate formation is nearly negligible. However, a significant difference is observed in the progression of formation duration under varying roughness conditions. In I-type fractures, the hydrate formation duration gradually increases with increasing surface roughness. In contrast, in X-type fractures, the formation duration decreases as the roughness increases. However, in both fracture types, an increase in roughness does not significantly affect the final hydrate yield;
- (4)
- Hydrate dissociation exhibits a diffusion pattern from the fracture surface toward the interior, with residual hydrates displaying an uneven, wavy morphology. Gas production is influenced by fracture width. Among the tested widths, the 10 mm fracture yielded the highest gas volume, reaching approximately 0.24 mol;
- (5)
- The reformation of hydrates exhibits a “memory effect” that significantly reduces the induction time compared to that of the initial formation. However, due to the high solubility of CO2 in water, the hydrate yield during reformation remains consistently lower than that of the first formation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment Programme. Emissions Gap Report 2023: Broken Record–Temperatures Hit New Highs, yet World Fails to Cut Emissions (Again); Knowledge Repository–UNEP: Nairobi, Kenya, 2023. [Google Scholar]
- Ma, Z.W.; Zhang, P.; Bao, H.S.; Deng, S. Review of Fundamental Properties of CO2 Hydrates and CO2 Capture and Separation Using Hydration Method. Renew. Sust. Energ. Rev. 2016, 53, 1273–1302. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon Dioxide Sequestration via Gas Hydrates: A Potential Pathway toward Decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., III; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American heart association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- Amstrup, S.C.; DeWeaver, E.T.; Douglas, D.C.; Marcot, B.G.; Durner, G.M.; Bitz, C.M.; Bailey, D.A. Greenhouse gas mitigation can reduce sea-ice loss and increase polar bear persistence. Nature 2010, 468, 955–958. [Google Scholar] [CrossRef]
- Xu, R. Global CO2 Emissions Reach a Record High; China Science Daily: Beijing, China, 2024. [Google Scholar] [CrossRef]
- Karayannis, V.; Charalampides, G.; Lakioti, E. Socio-economic aspects of CCS technologies. Procedia Econ. Financ. 2014, 14, 295–302. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-combustion CO2 capture with chemical absorption: A state-of-the-art review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Wood, D. Carbon dioxide (CO2) handling and carbon capture utilization and sequestration (CCUS) research relevant to natural gas: A collection of published research (2009–2015). J. Nat. Gas. Sci. Eng. 2015, 25, A1–A9. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Elaouzy, Y.; Zaabout, A. Carbon capture, utilization and storage in buildings: Analysis of performance, social acceptance, policy measures, and the role of artificial intelligence. Build. Environ. 2025, 275, 112817. [Google Scholar] [CrossRef]
- Davoodi, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S.; Minaev, K.M. Review of technological progress in carbon dioxide capture, storage, and utilization. Gas. Sci. Eng. 2023, 117, 205070. [Google Scholar] [CrossRef]
- Lu, S.; Hu, C.; Wang, X.; Quaye, J.A.; Deng, L. Carbon dioxide storage in clastic rocks: Review and perspectives. Renew. Sust. Energ. Rev. 2025, 213, 115487. [Google Scholar] [CrossRef]
- Ma, C.; Hu, X.; Si, H.; Luo, T.; Pan, J.; Han, T.; Zhu, Y.; Yang, W.; Song, Y. Gas Phase Permeability of CO2 Hydrate-Bearing Silty-Clayey Sediments. Energy Fuels 2025, 39, 14294–14307. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, T.; Wu, Z.; Sun, P.; Chen, Y.; Ding, Y.; Xue, Y.; Zhao, Y.; Yang, W.; N, M.B.; et al. Experimental study on the permeability of hydrate-bearing silty-clayey sediments with grain-cementing and pore-filling hydrate morphologies. Energy Fuels 2024, 38, 3118–3130. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, T.; Xue, Y.; Wang, A.; Zhao, Y.; Yang, W.; Han, T.; Madhusudhan, B.; Zhang, Y.; Song, Y. Geophysical Properties of Hydrate-Bearing Silty-Clayey Sediments with Different Compaction Patterns. Energy Fuels 2024, 38, 20607–20617. [Google Scholar] [CrossRef]
- Priest, J.A.; Rees, E.V.; Clayton, C.R. Influence of gas hydrate morphology on the seismic velocities of sands. J. Geophys. Res. Solid Earth 2009, 114, B11205. [Google Scholar] [CrossRef]
- Yodpetch, V.; Zhang, Y.; Zheng, J.; Kulprathipanja, S.; Linga, P.; Rangsunvigit, P. Experimental study on CO2 hydrate formation in clay-rich sediments for sub-seafloor CO2 sequestration. Chem. Eng. J. 2025, 507, 160533. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, G.; Liu, L.; Liu, C.; Wan, Y.; Bu, Q.; Ji, Y.; Zhang, Z.; Kong, L. Mechanical properties of gas hydrate-bearing sediments: Research progress, challenges and perspectives. Earth Sci. Rev. 2025, 262, 105058. [Google Scholar] [CrossRef]
- Borrero, A.; Díaz-Acosta, A.; Blazquez, S.; Zerón, I.M.; Algaba, J.; Conde, M.M.; Blas, F.J. Three-Phase Equilibria of CO2 Hydrate from Computer Simulation in the Presence of NaCl. Energy Fuels 2025, 39, 5522–5533. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, W.; Mu, J.; An, X.; Zhang, Z.; Guo, L. Research Progress on Hydrate-based Technology for CO2 Marine Sequestration under the Background of “Dual Carbon”. Chem. Eng. Manag. 2024, 16, 96–100. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Shan, T.; Yuan, Q.; Yin, S.; Li, J.; Wu, Q.; Zhang, P. Molecular dynamics study of the influence of water molecular phase state on the replacement of CO2–CH4 hydrate in porous media. J. Mol. Liq. 2023, 391, 123401. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, C.; Luo, T.; Wang, A.; Ma, C.; Song, Y.; Yang, W. Triaxial Shearing Characteristics of Hydrate-Bearing Silty-Clayey Sediments under Deviatoric Consolidation. Energy Fuel 2025, 39, 6865–6880. [Google Scholar] [CrossRef]
- Nagashima, H.D.; Miyagi, T.; Yasuda, K.; Ohmura, R. Clathrate hydrates at temperatures below the freezing point of water: A review. Fluid Ph. Equilib. 2020, 517, 112610. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, Y.T. Review on CO2 hydrate formation/dissociation and its cold energy application. Renew. Sustain. Energ. Rev. 2016, 62, 478–494. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Dissociation kinetics of methane hydrate and CO2 hydrate for different granular composition. Fuel 2020, 262, 116614. [Google Scholar] [CrossRef]
- Teng, Y.; Li, Y.; Huang, T.; Chen, Y.; Wang, P.; Wang, B.; An, S.; Li, Y.; Han, S.; Zhu, J.; et al. Hydrogen purification via hydrate-based methods: Insights into H2-CO2-CO hydrate structures, thermodynamics, and kinetics. Gas Sci. Eng. 2024, 131, 205484. [Google Scholar] [CrossRef]
- Zheng, J.; Bhatnagar, K.; Khurana, M.; Zhang, P.; Zhang, B.Y.; Linga, P. Semiclathrate based CO2 capture from fuel gas mixture at ambient temperature: Effect of concentrations of tetra-n-butylammonium fluoride (TBAF) and kinetic additives. Appl. Energy 2018, 217, 377–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; He, Y.; Wang, F. Enhanced CO2 hydrate formation via biopromoter coupled with initial stirring activation. Fuel 2022, 330, 125713. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F. Experimental study on the effect of SDS and micron copper particles mixture on carbon dioxide hydrates formation. Energies 2022, 15, 6540. [Google Scholar] [CrossRef]
- Naeiji, P.; Varaminian, F. Kinetic study of carbon dioxide hydrate formation by thermal analysis in the presence of two surfactants: Sodium dodecyl sulfate (SDS) and lauryl alcohol ethoxylate (LAE). J. Mol. Liq. 2018, 254, 120–129. [Google Scholar] [CrossRef]
- Chen, H.N.; Sun, Y.F.; Pang, W.X.; Wang, M.L.; Wang, M.; Zhong, J.R.; Ren, L.-L.; Cao, B.-J.; Rao, D.; Sun, C.-Y.; et al. Quantitative evaluation of hydrate-based CO2 storage in unsealed marine sediments: Viewpoint from the driving force of hydrate formation and CO2-water contact ability. Fuel 2024, 376, 132682. [Google Scholar] [CrossRef]
- Rehman, A.N.; Bavoh, C.B.; Pendyala, R.; Lal, B. Research advances, maturation, and challenges of hydrate-based CO2 sequestration in porous media. ACS Sustain. Chem. Eng. 2021, 9, 15075–15108. [Google Scholar] [CrossRef]
- Mekala, P.; Busch, M.; Mech, D.; Patel, R.; Sangwai, J.S. Effect of silica sand size on the formation kinetics of CO2 hydrate in porous media in the presence of pure water and seawater relevant for CO2 sequestration. Pet. Sci. Eng. 2014, 122, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Wu, Q.; Wang, C.; Nan, J. Experimental study on the characteristics of CO2 hydrate formation in porous media below freezing point. China Pet. Process. Petrochem. 2015, 17, 32–38. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Dong, S.; Zheng, J.N.; Lei, X.; Chen, C.; Song, Y. Fractal analysis on CO2 hydrate-bearing sands during formation and dissociation processes with NMR. Stoten 2023, 859, 160326. [Google Scholar] [CrossRef]
- Chen, X.; Espinoza, D.N. Surface area controls gas hydrate dissociation kinetics in porous media. Fuel 2018, 234, 358–363. [Google Scholar] [CrossRef]
- Wu, C.; Fan, S.; Wang, Y.; Lang, X. Morphology observation on formation and dissociation cycles of methane hydrate in stacked quartz sandy sediments. J. Nat. Gas. Sci. Eng. 2022, 98, 104382. [Google Scholar] [CrossRef]
- Dutkiewicz, A.; Müller, R.D. Deep-sea hiatuses track the vigor of Cenozoic ocean bottom currents. Geology 2022, 50, 710–715. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, D.; Wu, H.; Liu, P.; Cai, M.; Zhang, C.; Tang, J. Numerical Simulation Study on the Influence of Fracture on Borehole Wave Modes: Insights from Fracture Width, Filling Condition, and Acoustic Frequency. Sensors 2024, 24, 3955. [Google Scholar] [CrossRef]
- Rees, E.V.L.; Priest, J.A.; Clayton, C.R.I. The structure of methane gas hydrate bearing sediments from the Krishna–Godavari Basin as seen from Micro-CT scanning. Mar. Pet. Geol. 2011, 28, 1283–1293. [Google Scholar] [CrossRef]
- Ma, C.; Hu, X.; Si, H.; Wang, J.; Pan, J.; Luo, T.; Han, T.; Wang, A. Formation Kinetics and Morphology Characteristics of Natural Gas Hydrates in Sandstone Fractures. Appl. Sci. 2025, 15, 7399. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Guo, C.; Song, Y.; Liu, W.; Cheng, C.; Ye, C.; Xue, K. Memory Effects of Structure I and II Gas Hydrates. Acta Phys. Chim. Sin. 2011, 27, 1305–1311. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, L.; Liu, B.; Ban, S.; Chen, G. A molecular dynamic simulation on the memory effect of methane hydrate. J. Mol. Liq. 2022, 363, 119831. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Liu, W.; Zhao, J.; Song, Y. Investigation on the induction time of methane hydrate formation in porous media under quiescent conditions. Pet. Sci. Eng. 2016, 145, 565–572. [Google Scholar] [CrossRef]


| Number | Pressure | Fracture Type | Fracture Width [mm] | Roughness [mm] | Note |
|---|---|---|---|---|---|
| 1 | 2.9 MPa | I | 3/6/10 | / | 3 groups |
| 2 | X | 3/6/10 | / | 3 groups | |
| 3 | I | 10 | 2.5/3.5 | 2 groups | |
| 4 | X | 10 | 2.5/3.5 | 2 groups |
| Pressure [MPa] | Fracture Type | Fracture Width [mm] | Total Formation Duration [min] |
|---|---|---|---|
| 2.9 MPa | I | 3 | 81 |
| 6 | 82.17 | ||
| 10 | 103.5 | ||
| X | 3 | 57.83 | |
| 6 | 71.33 | ||
| 10 | 46.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Si, H.; Wang, J.; Luo, T.; Han, T.; Dong, Z.; Ma, C. Kinetics and Morphological Characteristics of CO2 Hydrate Formation Within Sandstone Fractures. Appl. Sci. 2025, 15, 9440. https://doi.org/10.3390/app15179440
Ma C, Si H, Wang J, Luo T, Han T, Dong Z, Ma C. Kinetics and Morphological Characteristics of CO2 Hydrate Formation Within Sandstone Fractures. Applied Sciences. 2025; 15(17):9440. https://doi.org/10.3390/app15179440
Chicago/Turabian StyleMa, Chuanhe, Hongxiang Si, Jiyao Wang, Tingting Luo, Tao Han, Ziyang Dong, and Chaozheng Ma. 2025. "Kinetics and Morphological Characteristics of CO2 Hydrate Formation Within Sandstone Fractures" Applied Sciences 15, no. 17: 9440. https://doi.org/10.3390/app15179440
APA StyleMa, C., Si, H., Wang, J., Luo, T., Han, T., Dong, Z., & Ma, C. (2025). Kinetics and Morphological Characteristics of CO2 Hydrate Formation Within Sandstone Fractures. Applied Sciences, 15(17), 9440. https://doi.org/10.3390/app15179440







