Formation of Ordered Ionic Salt Agglomerates Through Evaporative Crystallization in Hanging Drop Systems
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
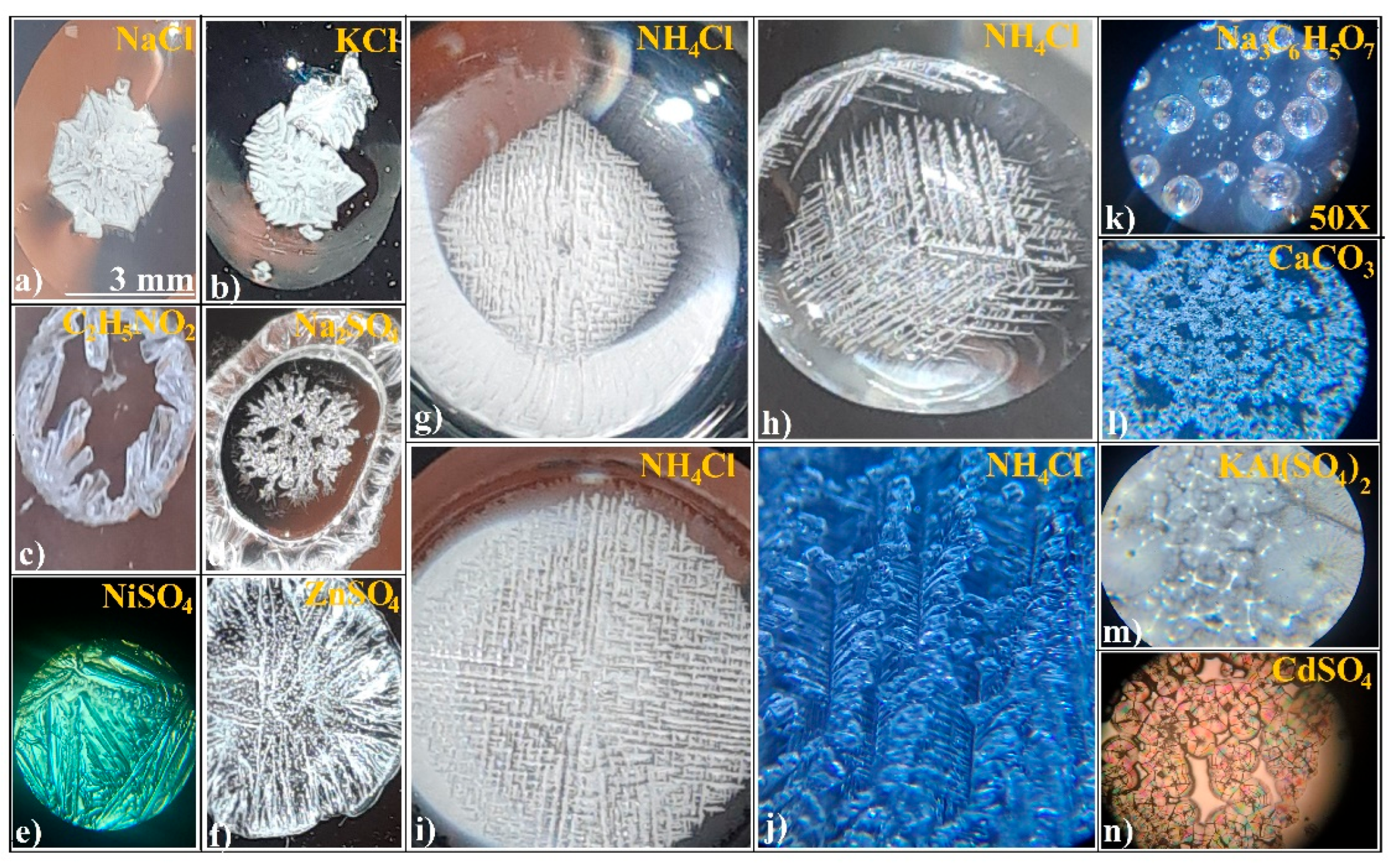
2.2.1. Preparation of Salt Solution
2.2.2. Formation of Hanging Drops
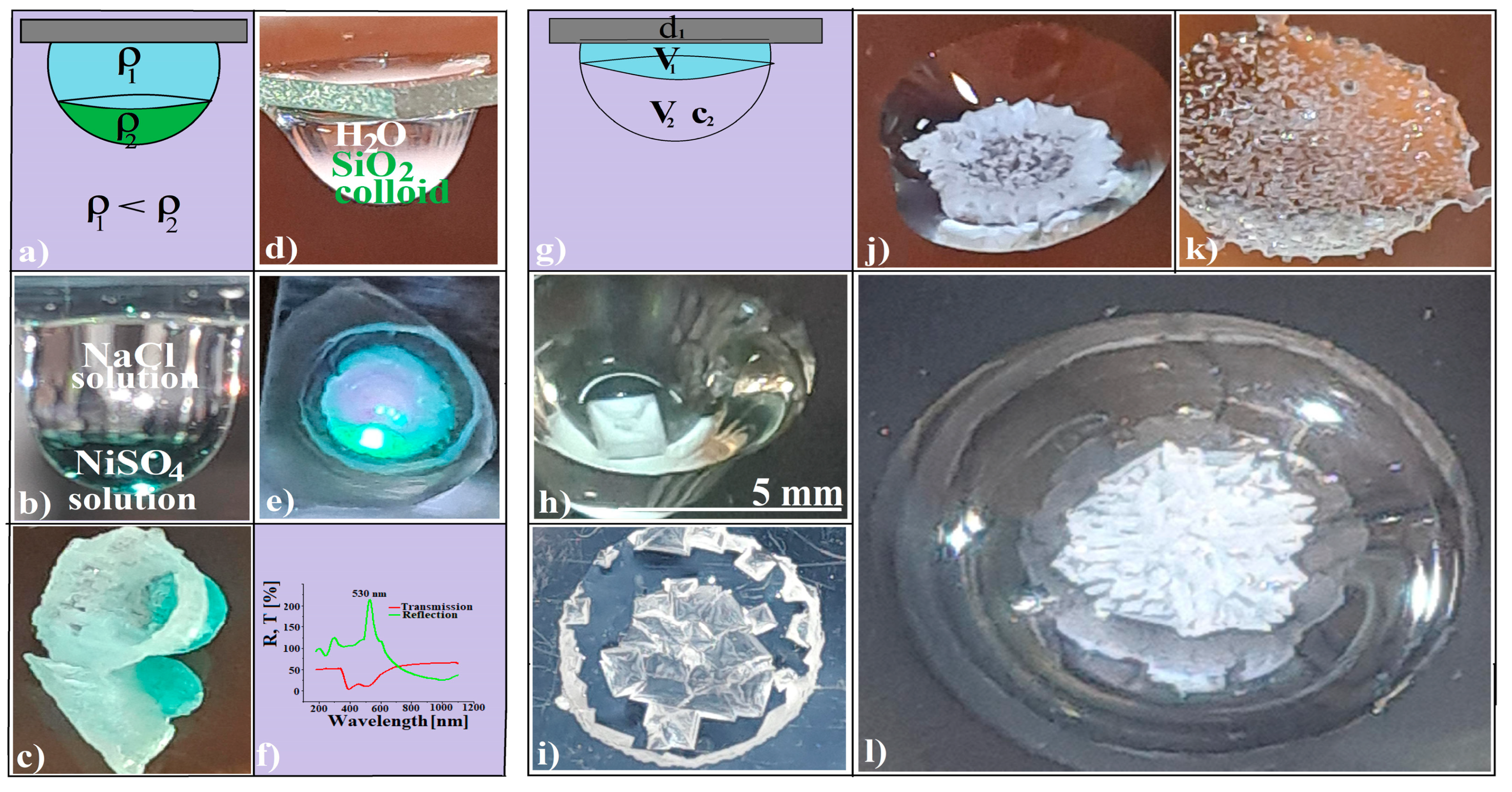
2.2.3. Layered Binary Fluid Hanging Drop Formation
2.2.4. Introducing a SiO2 Colloidal Crystal Fragment into a Hanging Drop
2.2.5. Fabrication of Faceted Convex Carbonaceous Materials
2.2.6. Fabrication of Faceted Convex Colloidal Photonic Crystals
2.2.7. Fabrication of Faceted Concave Colloidal Photonic Crystals
2.3. Investigations
3. Results and Discussion
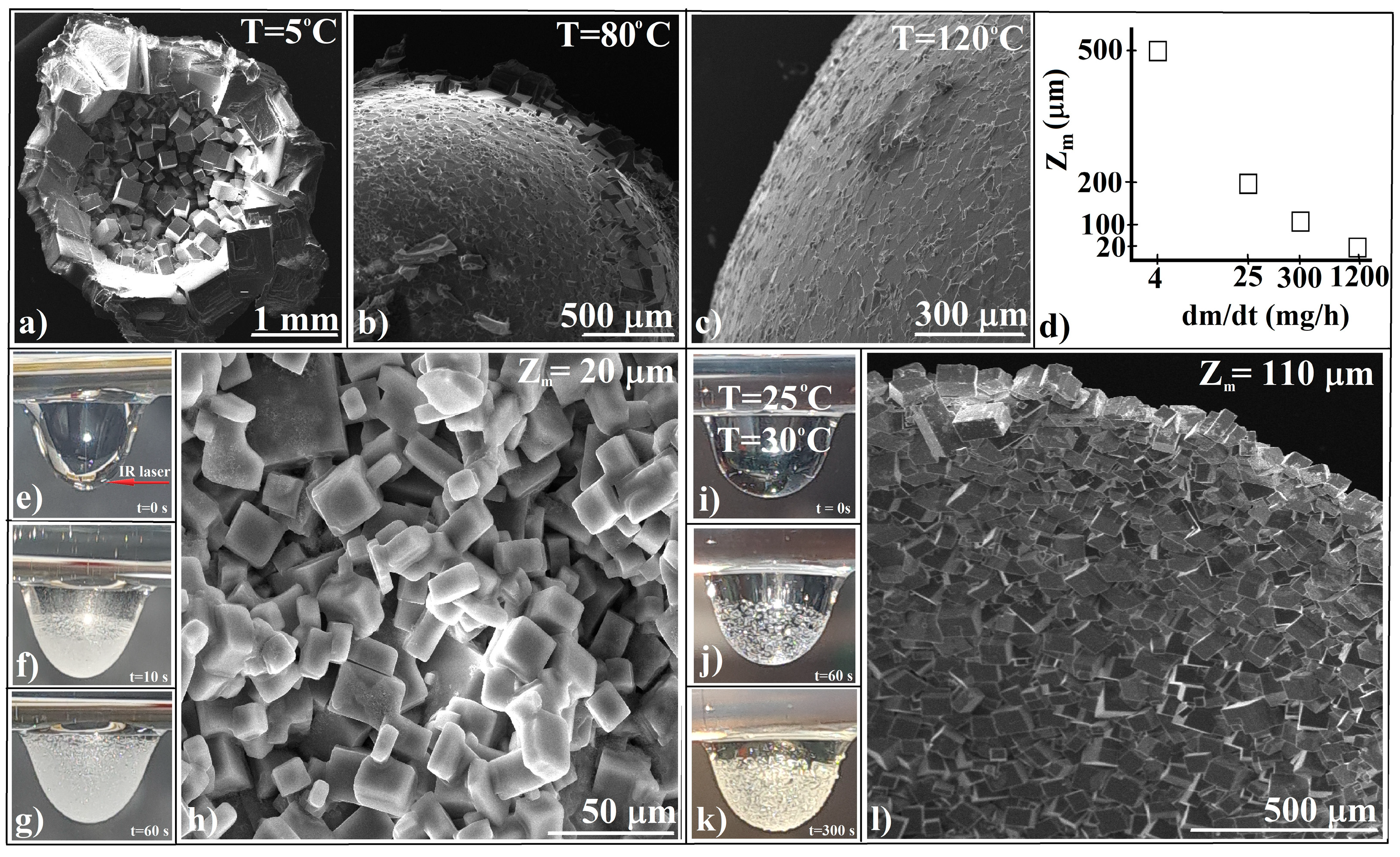
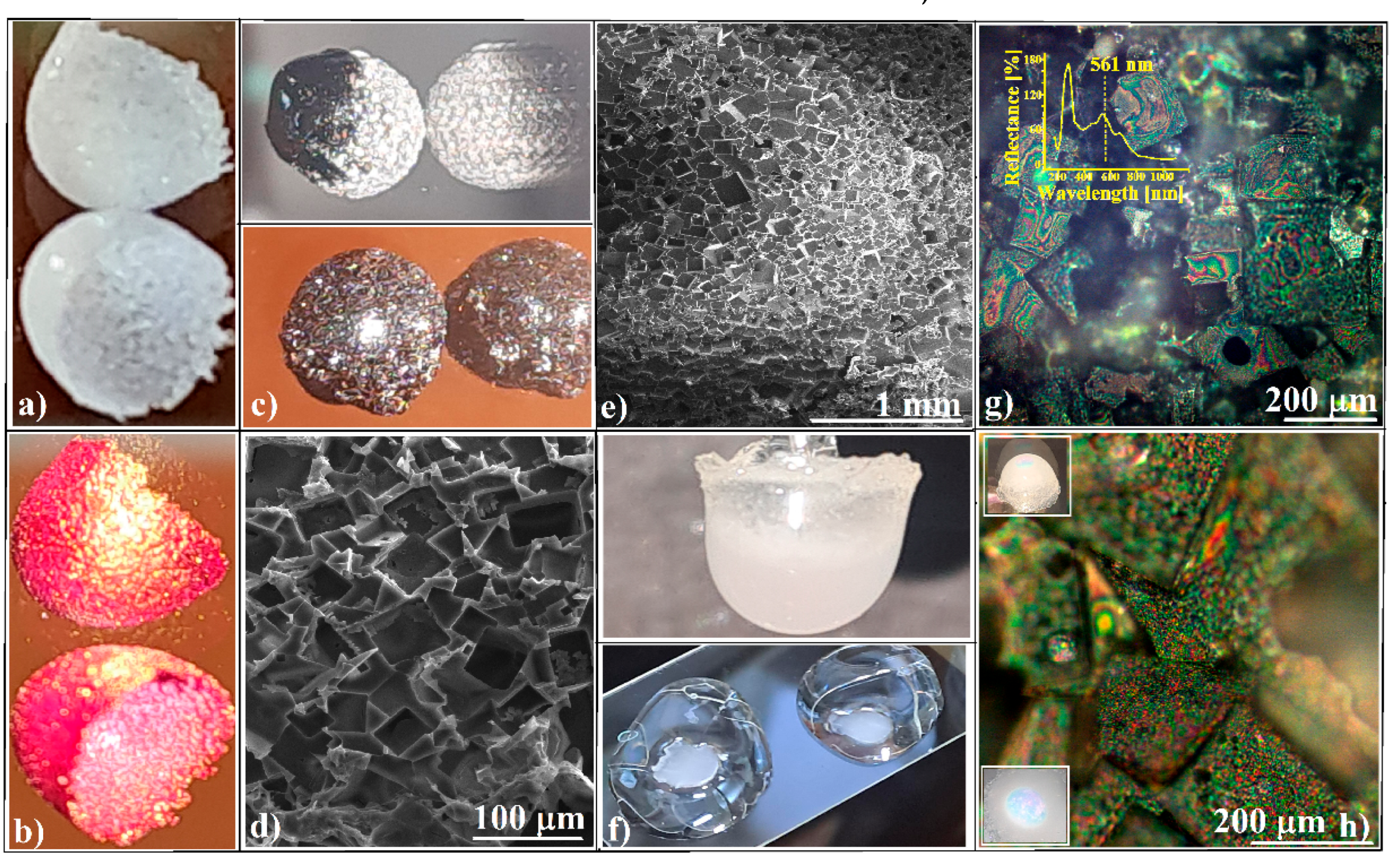
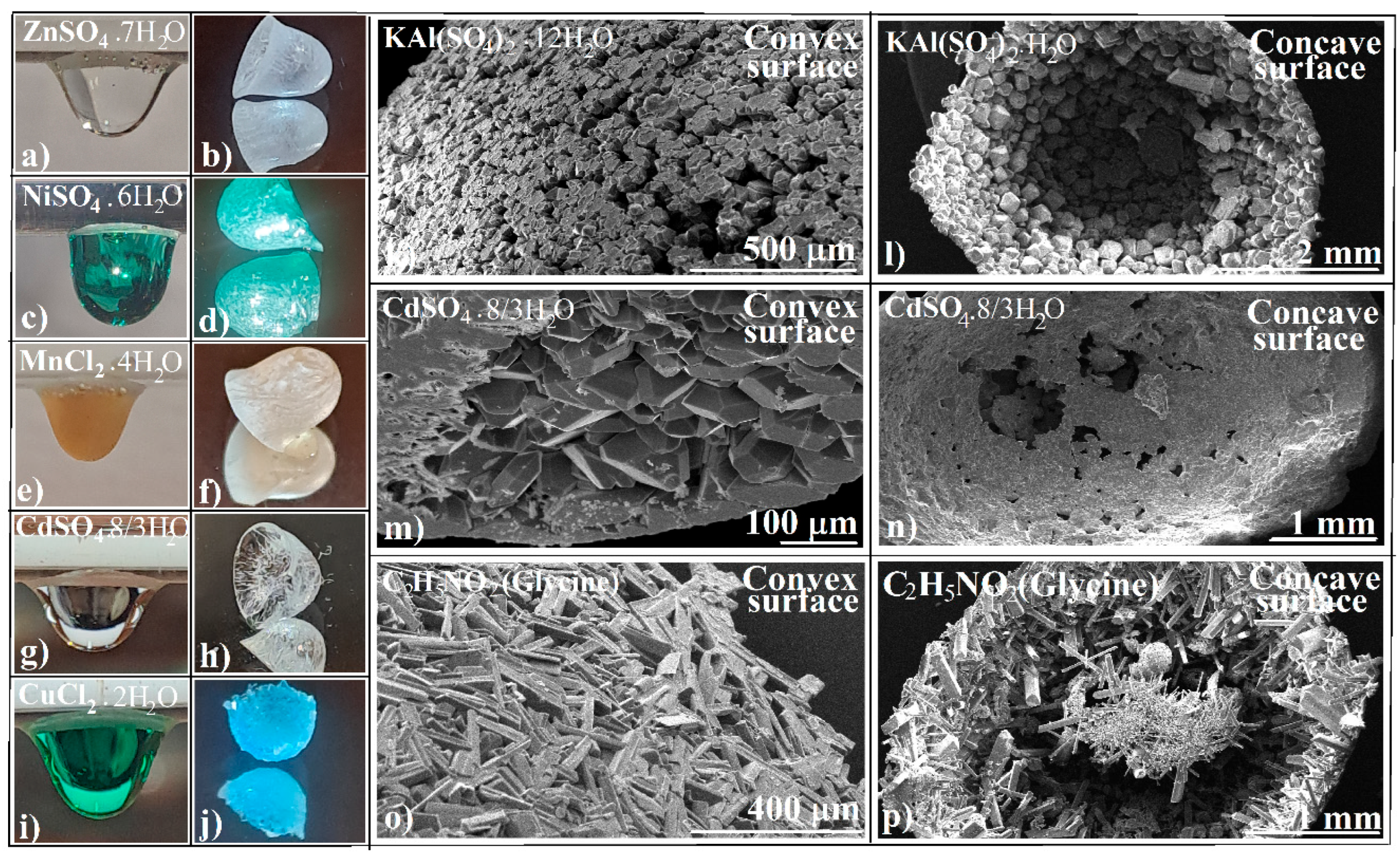
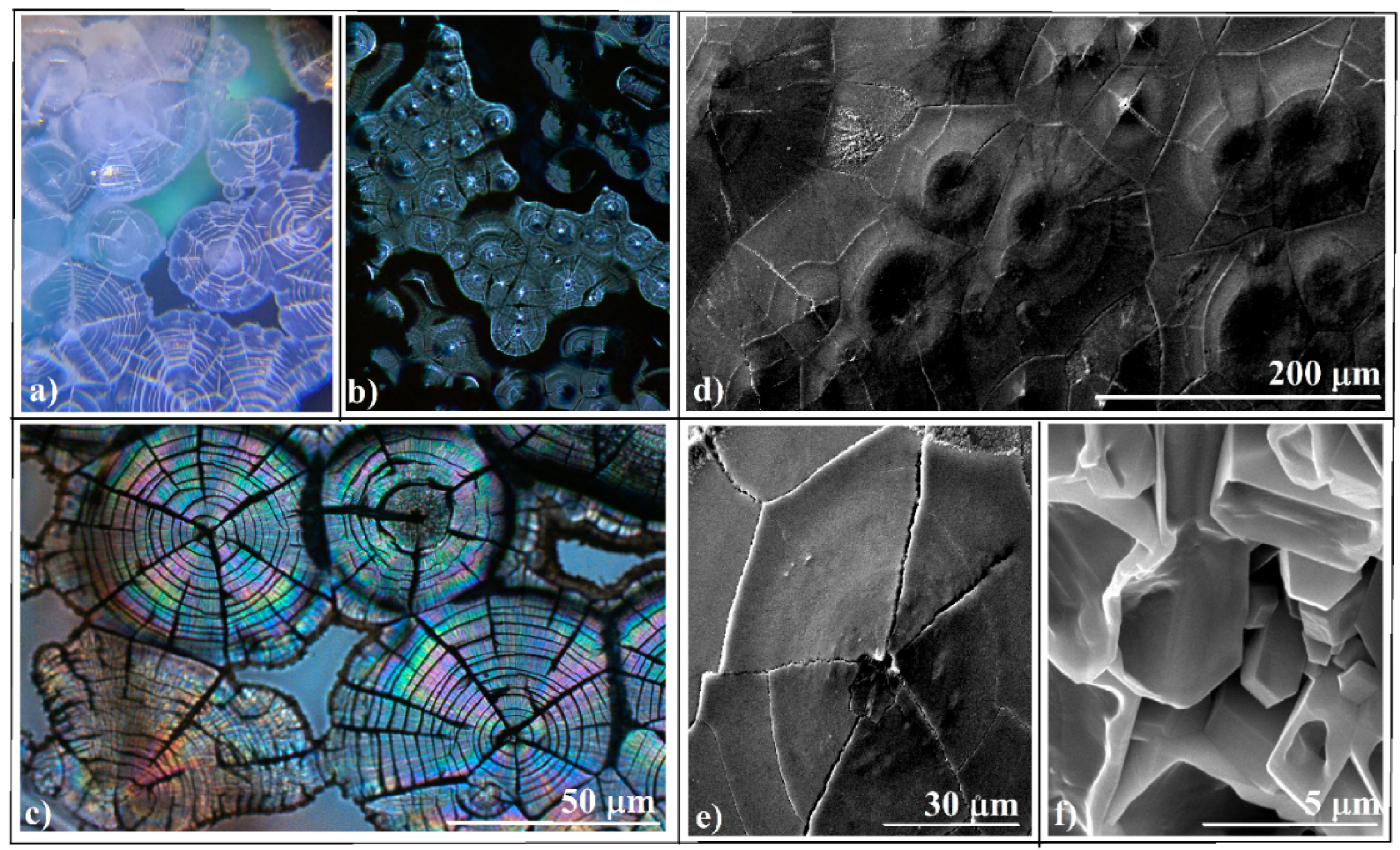
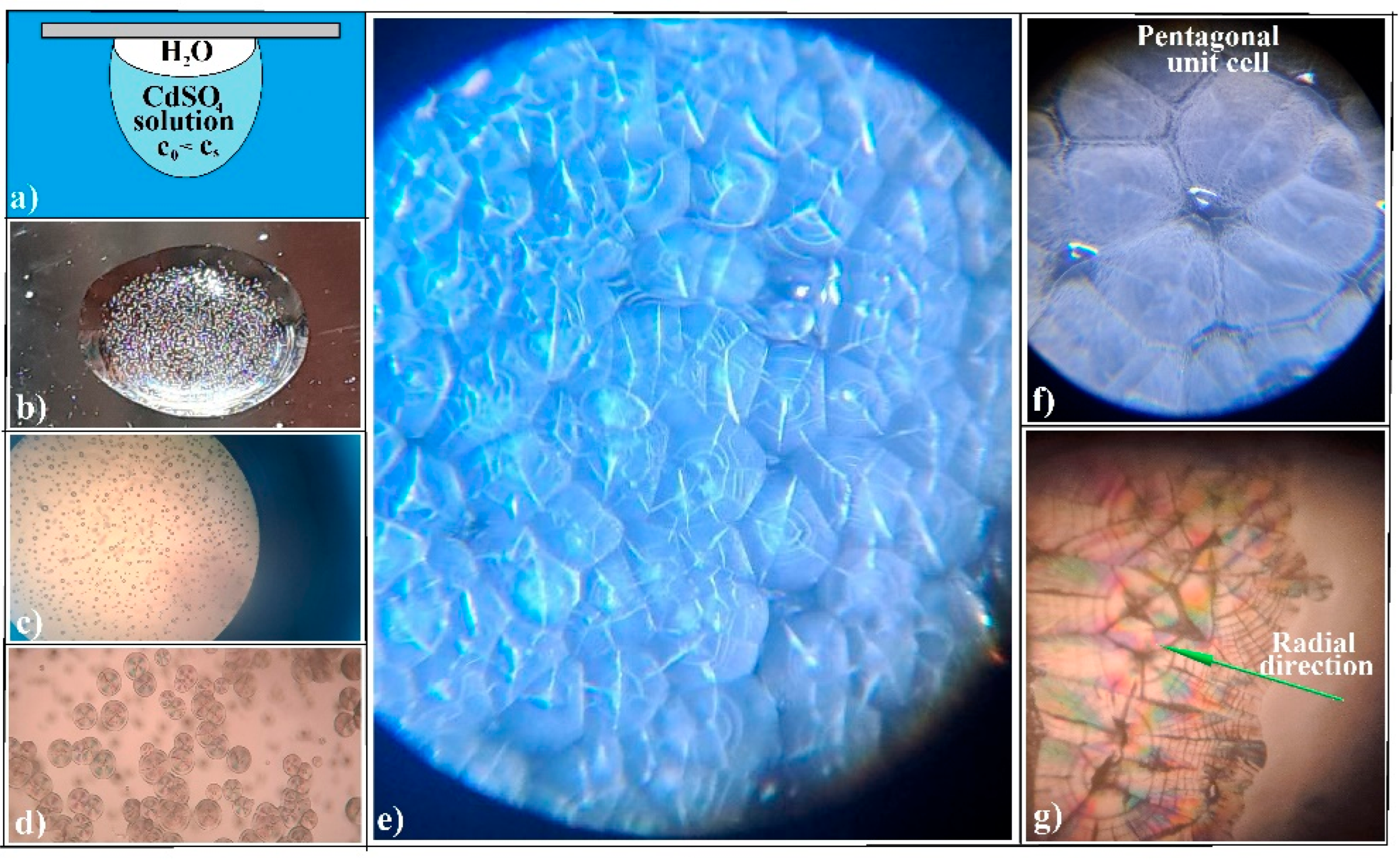
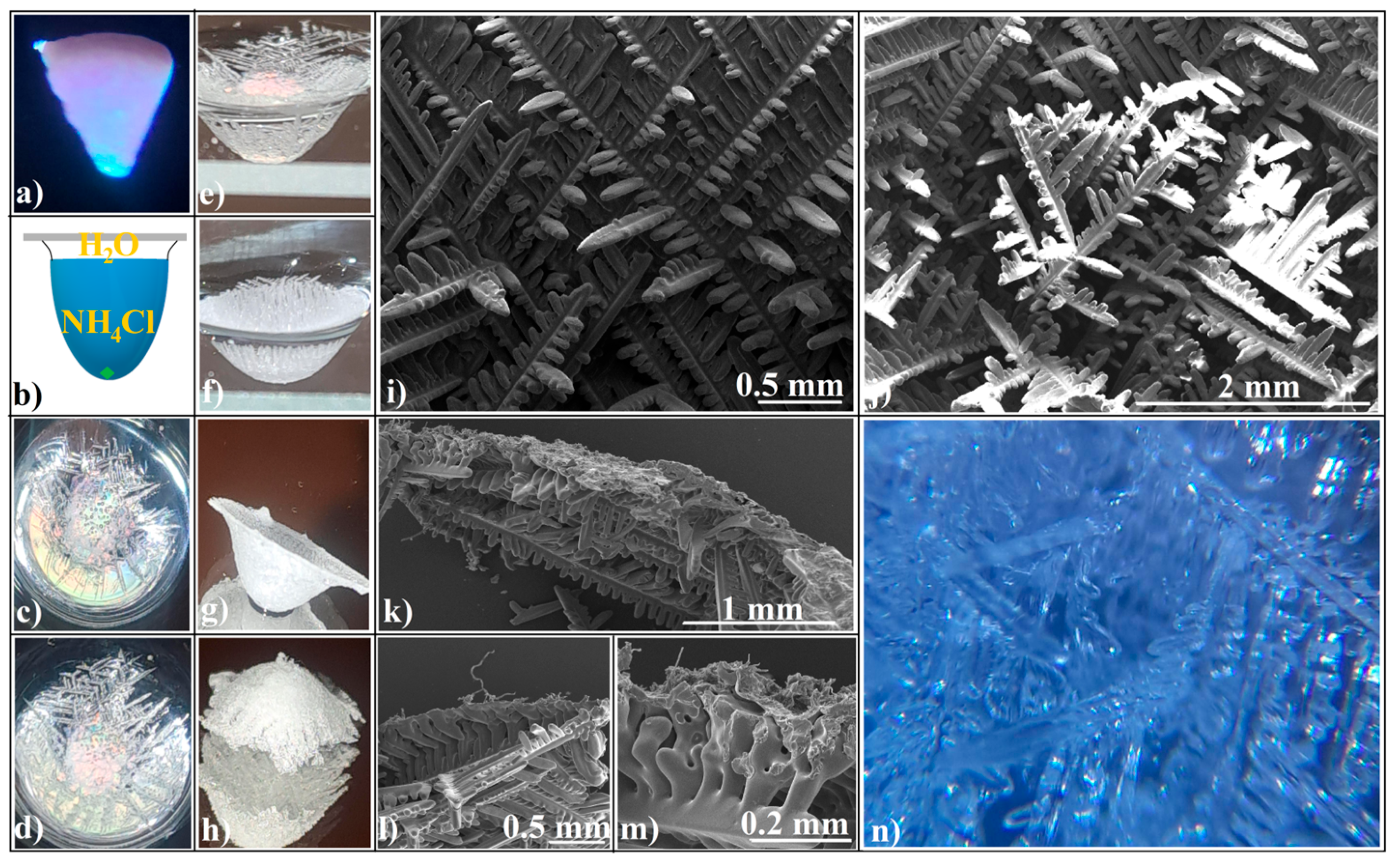
3.1. Evaporative Crystallization from a Hanging Drop
3.2. Evaporative Crystallization in a Layered Binary-Solution Hanging Drop
- (a)
- The structural units of crystalline agglomerates can undergo transformations, altering their shape and size, which adds a layer of complexity to the system. This adaptability is quite different from the static colloidal spheres typically employed in hard-sphere self-assembly processes, where the spheres maintain their shape and size throughout. It is like comparing the fluidity of a shapeshifter to the rigidity of Lego blocks. The crystallization process allows for a more flexible and adaptive structure, which can be advantageous in various applications.
- (b)
- The attachment of these structural units (ionic salt single crystals) to the agglomerate is more akin to what occurs in spin coating—a specific technique for synthesizing colloidal films—where the spheres are driven along the substrate by the centrifugal force, rather than from a wide angle as in self-assembly. This becomes even more intriguing when considering crystal agglomeration in a hanging drop, where the substrate or air/water interface is curved, not flat, as it is in spin coating.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Boncheva, M. Beyond molecules: Self-assembly of mesoscopic and macroscopic components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Wilmer, C.E.; Kim, J.; Browne, K.P.; Bishop, K.J.M. Self-assembly: From crystals to cells. Soft Matter 2009, 5, 1110–1128. [Google Scholar] [CrossRef]
- Pochan, D.; Scherman, O. Introduction: Molecular Self-Assembly. Chem. Rev. 2021, 121, 13699–13700. [Google Scholar] [CrossRef] [PubMed]
- Huie, J.C. Guided molecular self-assembly: A review of recent efforts. Smart Mater. Struct. 2003, 12, 264–271. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, R.; Zhang, R.; Huang, L.; Yuan, Q. Recent advances in supramolecular self-assembly derived materials for high-performance supercapacitors. Nanoscale Adv. 2023, 5, 2394–2412. [Google Scholar] [CrossRef]
- Shcherbina, M.A.; Chvalun, S.N. Driving Forces of the Self-Assembly of Supramolecular Systems: Partially Ordered Mesophases. Russ. J. Phys. Chem. 2018, 92, 1161–1170. [Google Scholar] [CrossRef]
- Li, Q.; Jonas, U.; Zhao, X.S.; Kapp, M. The forces at work in collodial self-assembly: A review on fundamental interactions between collodial particles. Asia-Pac. J. Chem. Eng. 2008, 3, 255–268. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Fang, F.; Fu, Y.; Yin, Z. A Review on Colloidal Self-Assembly and their Applications. Curr. Nanosci. 2016, 12, 725–746. [Google Scholar] [CrossRef]
- Thorkelsson, K.; Bai, P.; Xu, T. Self-assembly and applications of anisotropic nanomaterials: A review. Nano Today 2015, 10, 48–66. [Google Scholar] [CrossRef]
- Böker, A.; He, J.; Emrick, T.; Russell, T.P. Self-assembly of nanoparticles at interfaces. Soft Matter 2007, 3, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Gates, B.; Yin, Y.; Lu, Y. Monodispersed Colloidal Spheres: Old Materials with New Applications. Adv. Mater. 2000, 12, 693–713. [Google Scholar] [CrossRef]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef] [PubMed]
- Wieser, P.E.; Vukmanovic, Z.; Kilian, R.; Ringe, E.; Holness, M.B.; Maclennan, J.; Edmonds, M. To sink, swim, twin, or nucleate: A critical appraisal of crystal aggregation processes. Geology 2019, 47, 948–952. [Google Scholar] [CrossRef]
- Klein, C. Mineral. Britannica. Available online: https://www.britannica.com/science/mineral-chemical-compound (accessed on 5 March 2025).
- García-Ruiz, J.M.; Otálora, F. Crystal Growth in Geology: Patterns on the Rocks. In Handbook of Crystal Growth; Nishinaga, T., Rudolph, P., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume II, pp. 1–43. ISBN 9780444633033. [Google Scholar] [CrossRef]
- Ortoleva, P.; Chen, Y.; Chen, W. Agates, Geodes, Concretions and Orbicules: Self-Organized Zoning and Morphology. In Fractals and Dynamic Systems in Geoscience; Kruhl, J.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; Volume 1, pp. 283–305. ISBN 978-3-662-07304-9. [Google Scholar] [CrossRef]
- Rasmuson, Å.C. Introduction to Crystallization of Fine Chemicals and Pharmaceuticals. In Molecules: Nucleation, Aggregation and Crystallization; Sedzik, J., Riccio, P., Eds.; World Scientific Publishing: London, UK, 2009; Volume 1, pp. 145–172. ISBN 978-981-4470-49-0. [Google Scholar] [CrossRef]
- Davey, R.; Garside, J. From Molecules to Crystallizers—An Introduction to Crystallization; Oxford University Press: New York, NY, USA, 2000; Volume 1, pp. 1–96. ISBN 0198504896. [Google Scholar] [CrossRef]
- Kalikmanov, V.I. Classical Nucleation Theory. In Nucleation Theory. Lecture Notes in Physics; Springer: Dordrecht, The Netherlands, 2013; Volume 860, pp. 1–316. ISBN 978-90-481-3643-8. [Google Scholar] [CrossRef]
- Revalor, E.; Hammadi, Z.; Astier, J.-P.; Grossier, R.; Garcia, E.; Hoff, C.; Furuta, K.; Okustu, T.; Morin, R.; Veesler, S. Usual and unusual crystallization from solution. J. Cryst. Growth 2010, 312, 939–946. [Google Scholar] [CrossRef]
- Nancollas, G.H.; Purdie, N. The kinetics of crystal growth. Q. Rev. Chem. Soc. 1964, 18, 1–20. [Google Scholar] [CrossRef]
- Jackson, K.A. Crystal growth kinetics. Mater. Sci. Eng. 1984, 65, 7–13. [Google Scholar] [CrossRef]
- Heisel, S.; Holtkötter, J.; Wohlgemuth, K. Measurement of agglomeration during crystallization: Is the differentiation of aggregates and agglomerates via ultrasonic irradiation possible? Chem. Eng. Sci. 2019, 210, 115214. [Google Scholar] [CrossRef]
- David, R.; Paulaime, A.-M.; Espitalier, F.; Rouleau, L. Modelling of multiple-mechanism agglomeration in a crystallization process. Powder Technol. 2003, 130, 338–344. [Google Scholar] [CrossRef]
- Shahidzadeh-Bonn, N.; Rafaï, S.; Bonn, D.; Wegdam, G. Salt Crystallization during Evaporation: Impact of Interfacial Properties. Langmuir 2008, 24, 8599–8605. [Google Scholar] [CrossRef]
- Barrett, M.; McNamara, M.; Hao, H.X.; Barrett, P.; Glennon, B. Supersaturation tracking for the development, optimization and control of crystallization processes. Chem. Eng. Res. Des. 2010, 88, 1108–1119. [Google Scholar] [CrossRef]
- Kim, K.-J.; Mersmann, A. Estimation of metastable zone width in different nucleation processes. Chem. Eng. Sci. 2001, 56, 2315–2324. [Google Scholar] [CrossRef]
- Misyura, S.Y. Different modes of heat transfer and crystallization in a drop of NaCl solution: The influence of key factors on the crystallization rate and the heat transfer coefficient. Int. J. Therm. Sci. 2021, 159, 106602. [Google Scholar] [CrossRef]
- Yang, H.; Florence, A.J. Relating induction time and metastable zone width. CrystEngComm 2017, 19, 3966–3978. [Google Scholar] [CrossRef]
- Misyura, S. The dependence of evaporation and crystallization kinetics on dynamic and thermal background. AIChE J. 2020, 66, e16282. [Google Scholar] [CrossRef]
- Qu, J.; Escobar, L.; Li, J.; Rao, Z.; Xu, B. Experimental study of evaporation and crystallization of brine droplets under different temperatures and humidity levels. Int. Commun. Heat Mass Transf. 2020, 110, 104427. [Google Scholar] [CrossRef]
- Ancheyta-Palacios, M.; Sánchez-Santiago, K.; Escalera Santos, G.J.; Carreón, Y.P.; González-Gutiérrez, J. Gravity-driven crystallization in protein and NaCl droplets. Colloids Surf. A Physicochem. Eng. Asp. 2025, 707, 135821. [Google Scholar] [CrossRef]
- Fontana, P.; Schefer, J.; Pettit, D. Characterization of sodium chloride crystals grown in microgravity. J. Cryst. Growth 2011, 324, 207–211. [Google Scholar] [CrossRef]
- Bunio, L.B.; Wang, J.; Kannaiyan, R.; Gates, I.D. Evaporation and crystallization of NaCl-water droplets suspended in air by acoustic levitation. Chem. Eng. Sci. 2022, 251, 117441. [Google Scholar] [CrossRef]
- Page, A.J.; Sear, R.P. Crystallization controlled by the geometry of a surface. J. Am. Chem. Soc. 2009, 131, 17550–17551. [Google Scholar] [CrossRef]
- Mann, S.; Archibald, D.D.; Didymus, J.M.; Douglas, T.; Heywood, B.R.; Meldrum, F.C.; Reeves, N.J. Crystallization at Inorganic-organic Interfaces: Biominerals and Biomimetic Synthesis. Science 1993, 261, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Krog, N.; Larsson, K. Crystallization at Interfaces in Food Emulsions—A General Phenomenon. Eur. J. Lipid Sci. Technol. 1992, 94, 55–57. [Google Scholar] [CrossRef]
- Ford, I.J. Models of Crystallisation in Evaporating Droplets. MRS Online Proc. Libr. 1995, 398, 637–642. [Google Scholar] [CrossRef]
- Armstrong Green, N.C.; Haddrell, A.E.; Gregson, F.K.A.; Lewis, D.; Church, T.; Reid, J.P. Studies of the Crystallization and Dissolution of Individual Suspended Sodium Chloride Aerosol Particles. J. Phys. Chem. A 2024, 128, 4315–4323. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamanaka, S.; Shirakawa, Y.; Shimosaka, A.; Hidaka, J. Preparation of porous particles by liquid–liquid interfacial crystallization. Adv. Powder Technol. 2011, 22, 125–130. [Google Scholar] [CrossRef]
- Coupland, J.N. Crystallization in emulsions. Curr. Opin. Colloid Interface Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Kumar, V.; Dash, S. Patterns during Evaporative Crystallization of a Saline Droplet. Langmuir 2022, 38, 10265–10273. [Google Scholar] [CrossRef]
- Sperling, M.; Gradzielski, M. Droplets, Evaporation and a Superhydrophobic Surface: Simple Tools for Guiding Colloidal Particles into Complex Materials. Gels 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, D.H.; Marshall, W.R., Jr. Evaporation from drops containing dissolved solids. AIChE J. 1960, 6, 9–23. [Google Scholar] [CrossRef]
- Fontana, P.; Pettit, D.; Cristoforetti, S. Sodium chloride crystallization from thin liquid sheets, thick layers, and sessile drops in microgravity. J. Cryst. Growth 2015, 428, 80–85. [Google Scholar] [CrossRef]
- Bormashenko, E.; Roy, P.K.; Shoval, S.; Legchenkova, I. Interfacial Crystallization within Liquid Marbles. Condens. Matter 2020, 5, 62. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Guo, H.; Zhao, J.; Luo, H.; Huang, X. A perspective view of salt crystallization from solution in porous media: Morphology, mechanism, and salt efflorescence. Sci. Rep. 2024, 14, 23510. [Google Scholar] [CrossRef] [PubMed]
- Desarnaud, J.; Derluyn, H.; Carmeliet, J.; Bonn, D.; Shahidzadeh, N. Metastability Limit for the Nucleation of NaCl Crystals in Confinement. J. Phys. Chem. Lett. 2014, 5, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Naillon, A.; Duru, P.; Marcoux, M.; Prat, M. Evaporation with sodium chloride crystallization in a capillary tube. J. Cryst. Growth 2015, 422, 52–61. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate Science: From Structures to Properties. Adv. Mater. 2020, 32, 2001457. [Google Scholar] [CrossRef]
- Bika, D.G.; Gentzler, M.; Michaels, J.N. Mechanical properties of agglomerates. Powder Technol. 2001, 117, 98–112. [Google Scholar] [CrossRef]
- Sandu, I.; Urzica, I.; Dumitru, M.; Banici, A.; Fleaca, C.; Dumitrache, F. Crystallization from Thin Films on a Hot Plate: A New Approach of Solid Surface Pattering. J. Mater. Sci. Chem. Eng. 2019, 7, 9–18. [Google Scholar] [CrossRef]
- Sandu, I.; Urzica, I.; Niculescu, A.M.; Fleaca, C.T.; Dumitrache, F.; Badiceanu, M. Self-organisation of single-crystals as ripple patterns through laser ablation of ionic salt solutions. Appl. Surf. Sci. 2017, 417, 160–164. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Ji, X.; Banks, C.E.; Song, J. Flower-like agglomerates of hydroxyapatite crystals formed on an egg-shell membrane. Colloids Surf. B 2011, 82, 490–496. [Google Scholar] [CrossRef]
- Shahidzadeh, N.; Schut, M.; Desarnaud, J.; Prat, M.; Bonn, D. Salt stains from evaporating droplets. Sci. Rep. 2015, 5, 10335. [Google Scholar] [CrossRef]
- Giri, A.; Dutta Choudhury, M.; Dutta, T.; Tarafdar, S. Multifractal Growth of Crystalline NaCl Aggregates in a Gelatin Medium. Cryst. Growth Des. 2013, 13, 341–345. [Google Scholar] [CrossRef]
- Pathak, B.; Christy, J.; Sefiane, K.; Gozuacik, D. Complex Pattern Formation in Solutions of Protein and Mixed Salts Using Dehydrating Sessile Droplets. Langmuir 2020, 36, 9728–9737. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J. Continuous Production of NaCl Spherical Particles via Emulsion Crystallization in Microfluidic Devices. Crystal Growth Des. 2023, 23, 8918–8928. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.; Garcia-Ruiz, J.M. Model of textural development of layered crystal aggregates. Eur. J. Mineral. 2000, 12, 609–614. [Google Scholar] [CrossRef]
- Mailleur, A.; Pirat, C.; Simon, G.; Fulcrand, R.; Colombani, J. Ring shells obtained from pure water drops evaporating on a soluble substrate. Colloids Surf. A 2022, 651, 129724. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Wang, Z.; Wang, N.; Zang, D. Evaporation of Saline Droplets on a Superhydrophobic Substrate: Formation of Crystal Shell and “Legs”. Materials 2023, 16, 5168. [Google Scholar] [CrossRef]
- Cölfen, H.; Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 2005, 44, 5576–5591. [Google Scholar] [CrossRef]
- Sandu, I.; Dumitru, M.; Fleaca, C.T.; Dumitrache, F. Rise and side infiltration in opals and porous materials for their skin-free replica synthesis. Mater. Res. Express 2019, 6, 046201. [Google Scholar] [CrossRef]
- Hetherington, N.B.J.; Kulak, A.N.; Kim, Y.-Y.; Noel, E.H.; Snoswell, D.; Butler, M.; Meldrum, F.C. Porous Single Crystals of Calcite from Colloidal Crystal Templates: ACC Is Not Required for Nanoscale Templating. Adv. Funct. Mater. 2011, 21, 948–954. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Pan, H.; Tang, R. Biomineralization inspired crystal growth for biomimetic materials preparation. J. Cryst. Growth 2023, 603, 127029. [Google Scholar] [CrossRef]
- Qin, K.; Zheng, Z.; Wang, J.; Pan, H.; Tang, R. Biomineralization strategy: From material manufacturing to biological regulation. Giant 2024, 19, 100317. [Google Scholar] [CrossRef]
- Kato, M.; Ito, H.; Hasegawa, M.; Ishii, K. Soft crystals-flexible response systems with high structural order. Chem. A Eur. J. 2019, 25, 5099–5329. [Google Scholar] [CrossRef] [PubMed]
- Rapis, E. A change in the physical state of a non-equilibrium blood plasma protein film in patients with carcinoma. Tech. Phys. 2002, 47, 510–512. [Google Scholar] [CrossRef]
- Li, L.; Xia, Y.; Zeng, M.; Fu, L. Facet engineering of ultrathin two-dimensional materials. Chem. Soc. Rev. 2022, 51, 7327–7343. [Google Scholar] [CrossRef]
- Ejima, H.; Yanai, N.; Best, J.P.; Sindoro, M.; Granick, S.; Caruso, F. Near-incompressible faceted polymer microcapsules from metal-organic framework templates. Adv. Mater. 2013, 25, 5767–5771. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chang, J.-Y.; Chi, G.-C. Design of GaN convex diffractive microlenses. Opt. Laser Technol. 2002, 34, 569–573. [Google Scholar] [CrossRef]
- Churin, E.; Bayvel, P.; Smirnitskii, V.; Timofeev, F.; Midwinter, J. Concave grating and convex mirror double dispersion spectrograph for optical network applications. Appl. Opt. 1997, 36, 7822–7825. [Google Scholar] [CrossRef]
- Sandu, I.; Antohe, I.; Fleaca, C.T.; Dumitrache, F.; Urzica, I.; Brajnicov, S.; Iagaru, R.; Sava, B.A.; Dumitru, M. Shaping in the Third Direction: Self-Assembly of Convex Colloidal Photonic Crystals on an Optical Fiber Tip by Hanging Drop Method. Polymers 2024, 16, 33. [Google Scholar] [CrossRef]
- Sandu, I.; Antohe, I.; Fleaca, C.T.; Dumitrache, F.; Urzica, I.; Dumitru, M. Shaping in the Third Direction: Colloidal Photonic Crystals with Quadratic Surfaces Self-Assembled by Hanging-Drop Method. Polymers 2024, 16, 1931. [Google Scholar] [CrossRef]
- Sandu, I.; Dumitru, M.; Fleaca, C.T.; Dumitrache, F. Hanging colloidal drop: A new photonic crystal synthesis route. Photonics Nanostruct. Fundam. Appl. 2018, 29, 42–48. [Google Scholar] [CrossRef]
- Li, Y.; Diddens, C.; Lv, P.; Wijshoff, H.; Versluis, M.; Lohse, D. Gravitational effect in evaporating binary microdroplets. Phys. Rev. Lett. 2019, 122, 114501. [Google Scholar] [CrossRef]
- Ma, X.; Sefiane, K.; Bennacer, R.; Lapert, X.; Bakir, F. Solutal and Gravitational Effects during Binary Mixture Droplets Evaporation. Microgravity Sci. Technol. 2024, 36, 17. [Google Scholar] [CrossRef]
- Sandu, I.; Iordache, I.; Fleaca, C.; Dumitrache, F.; Niculescu, A. The Influence of Gravity on Single Crystallization in Large Volume Drops. J. Crystalliz. Proc. Technol. 2014, 4, 206–211. [Google Scholar] [CrossRef]
- Çelikbilek, M.; Ersundu, A.E.; Aydın, S. Crystallization Kinetics of Amorphous Materials. In Advances in Crystallization Processes; Mastai, Y., Ed.; InTech Europe: Rijeka, Croatia, 2012; Volume 1, pp. 127–162. ISBN 978-953-51-4297-3. [Google Scholar] [CrossRef]
- Pettit, D.; Fontana, P. Comparison of sodium chloride hopper cubes grown under microgravity and terrestrial conditions. npj Microgravity 2019, 5, 25. [Google Scholar] [CrossRef]
- Gielen, B.; Jordens, J.; Thomassen, L.C.J.; Braeken, L.; Van Gerven, T. Agglomeration Control during Ultrasonic Crystallization of an Active Pharmaceutical Ingredient. Crystals 2017, 7, 40. [Google Scholar] [CrossRef]
- Im, S.H.; Park, O.O. Effect of Evaporation Temperature on the Quality of Colloidal Crystals at the Water—Air Interface. Langmuir 2002, 18, 9642–9646. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wang, Z.S.; Ji, Y.H. Influence of growth parameters on the fabrication of high-quality colloidal crystals via a controlled evaporation self-assembly method. Thin Solid Film. 2010, 518, 5083–5090. [Google Scholar] [CrossRef]
- Chen, R.-Q.; Lu, Q.-Q.; Cheng, Q.-D.; Ao, L.-B.; Zhang, C.-Y.; Hou, H.; Liu, Y.-M.; Li, D.-W.; Yin, D.-C. An ignored variable: Solution preparation temperature in protein crystallization. Sci. Rep. 2015, 5, 7797. [Google Scholar] [CrossRef]
- Astier, J.-P.; Veesler, S. Using Temperature To Crystallize Proteins: A Mini-Review. Cryst. Growth Des. 2008, 8, 4215–4219. [Google Scholar] [CrossRef]
- Quilaqueo, M.; Aguilera, J.M. Crystallization of NaCl by fast evaporation of water in droplets of NaCl solutions. Food Res. Int. 2016, 84, 143–149. [Google Scholar] [CrossRef]
- Sassen, K. Infrared (10.6-μm) radiation induced evaporation of large water drops. J. Opt. Soc. Am. 1981, 71, 887–891. [Google Scholar] [CrossRef]
- Park, B.S.; Armstrong, R.L. Laser droplet heating: Fast and slow heating regimes. Appl Opt. 1989, 28, 3671–3680. [Google Scholar] [CrossRef]
- Ferraz-Albani, L.A.; Baldelli, A.; Knapp, C.J.; Jäger, W.; Vehring, R.; Nobes, D.S.; Olfert, J.S.; Kostiuk, L.W. Enhanced Evaporation of Microscale Droplets With an Infrared Laser. J. Heat Transf. 2017, 139, 011503. [Google Scholar] [CrossRef]
- Kaplunov, I.; Kropotov, G.; Rogalin, V.; Shahmin, A. Optical properties of alkali halide crystals. J. Phys. Conf. Ser. 2020, 1697, 012253. [Google Scholar] [CrossRef]
- Nistor, S.V.; Nistor, L.C.; Teodorescu, S.V.; Ciucur, G.; Chis, I.; Drăgulinescu, D.; Grigorescu, D.; Grigoriu, C. Output couplers of ultra-transparent KCl and NaCl in a pulsed CO2 laser. Opt. Laser Technol. 1989, 21, 377–380. [Google Scholar] [CrossRef]
- Chen, L.; Ren, G.; Liu, L.; Guo, P.; Wang, E.; Zhu, Z.; Yang, J.; Shen, J.; Zhang, Z.; Zhou, L.; et al. Probing NaCl hydrate formation from aqueous solutions by terahertz time-domain spectroscopy. Phys. Chem. Chem. Phys. 2020, 22, 17791–17797. [Google Scholar] [CrossRef]
- Ajito, K.; Ueno, Y.; Kim, J.Y.; Sumikama, T. Capturing the Freeze-Drying Dynamics of NaCl Nanoparticles Using THz Spectroscopy. J. Am. Chem. Soc. 2018, 140, 13793–13797. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Engel, T.; Flury, M.; Visser, M. Faceted structure: A design for desired illumination and manufacture using 3D printing. OSA Contin. 2018, 1, 26–39. [Google Scholar] [CrossRef]
- Joung, D.; Nemilentsau, A.; Agarwal, K.; Dai, C.; Liu, C.; Su, Q.; Li, J.; Low, T.; Koester, S.J.; Cho, J.H. Self-Assembled Three-Dimensional Graphene-Based Polyhedrons Inducing Volumetric Light Confinement. Nano Lett. 2017, 17, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, H.; Li, W.; Haghi-Ashtiani, P.; Lejay, P.; Bai, J. Growth of carbon nanotubes in six orthogonal directions on spherical alumina microparticles. Carbon 2011, 49, 2273–2286. [Google Scholar] [CrossRef]
- Wang, B.; Jin, P.; Yue, Y.; Ji, S.; Li, Y.; Luo, H. Synthesis of NaCl single crystals with defined morphologies as templates for fabricating hollow nano/micro-structures. RSC Adv. 2015, 5, 5072–5076. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, J.; Jiang, Y.; Liu, P.; Zhang, Q.; Wei, R.; Chen, X.; Feng, J. Self-Sacrificial Salt Templating: Simple Auxiliary Control over the Nanoporous Structure of Porous Carbon Monoliths Prepared through the Solvothermal Route. Nanomaterials 2018, 8, 255. [Google Scholar] [CrossRef]
- Li, J.; Lu, D.; Kondamareddy, K.K.; Gu, W.; Liu, Y.; Su, Y.; You, Z.; Fan, H.; Ho, W. Fabrication of Hexagonal Prism-Shaped Double S-Scheme Cu2O@CdS/ZnS Heterojunctions Utilizing Zeolite Templates: Enhanced Photocatalytic Hydrogen Production and Mechanism Insight. Cryst. Growth Des. 2024, 24, 8769–8781. [Google Scholar] [CrossRef]
- Jalaly, M.; Hosseini, R.; Bakhshi, A.; Chehelamirani, M. Self-assembly synthesis of 3D graphene/nano-Fe3O4 hybrid aerogels with improved mechanical and thermal properties. J. Alloys Cpds. 2022, 902, 163718. [Google Scholar] [CrossRef]
- Sögütoglu, L.-C.; Steiger, M.; Houben, J.; Biemans, D.; Fischer, H.R.; Donkers, P.; Huinink, H.; Adan, O.C.G. Understanding the Hydration Process of Salts: The Impact of a Nucleation Barrier. Cryst. Growth Des. 2019, 19, 2279–2288. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Schmidt, T.; Ruck, W. Hydration and dehydration of salt hydrates and hydroxides for thermal energy storage—Kinetics and energy release. Energy Procedia 2012, 30, 362–369. [Google Scholar] [CrossRef]
- Tian, F.; Qu, H.; Zimmermann, A.; Munk, T.; Jørgensen, A.C.; Rantanen, J. Factors affecting crystallization of hydrates. J. Pharm. Pharmacol. 2010, 62, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Gránásy, L.; Pusztai, T.; Tegze, G.; Warren, J.A.; Douglas, J.F. Growth and form of spherulites. Phys. Rev. E. 2005, 72, 011605. [Google Scholar] [CrossRef]
- Liu, X.; Contal, C.; Schmutz, M.; Krafft, M.P. Two-Dimensional Radial or Ring-Banded Nonbirefringent Spherulites of Semifluorinated Alkanes Coexistent with Close-Packed Self-Assembled Surface Nanodomains. Langmuir 2018, 34, 15126–15133. [Google Scholar] [CrossRef]
- Beck, R.; Andreassen, J.-P. Spherulitic Growth of Calcium Carbonate. Cryst. Growth Des. 2010, 10, 2934–2947. [Google Scholar] [CrossRef]
- Raimo, M. An optical test to unveil twisting of birefringent crystals in spherulites. R. Soc. Open Sci. 2019, 6, 181215. [Google Scholar] [CrossRef]
- Clough, S.; Van Aartsen, J.J.; Stein, R.S. Scattering of Light by Two-Dimensional Spherulites. J. Appl. Phys. 1965, 36, 3072–3085. [Google Scholar] [CrossRef]
- Edwards, A.M.J.; Atkinson, P.S.; Cheung, C.S.; Liang, H.; Fairhurst, D.J.; Ouali, F.F. Density-driven flows in evaporating binary liquid droplets. Phys. Rev. Lett. 2018, 121, 184501. [Google Scholar] [CrossRef] [PubMed]
- Koldeweij, R.B.J.; van Capelleveen, B.F.; Lohse, D.; Visser, C.W. Marangoni-driven spreading of miscible liquids in the binary pendant drop geometry. Soft Matter 2019, 15, 8525–8531. [Google Scholar] [CrossRef] [PubMed]
- Pahlavan, A.A.; Yang, L.; Bain, C.D.; Stone, H.A. Evaporation of binary-mixture liquid droplets. The formation of picoliter pancake shapes. Phys. Rev. Lett. 2021, 127, 024501. [Google Scholar] [CrossRef]
- Phillips, O.M. On flow induced by diffusion in a stably stratified fluid. Deep-Sea Res. 1970, 17, 435–443. [Google Scholar] [CrossRef]
- Stewart, R.W. The problem of diffusion on a stratified fluid. Adv. Geophys. 1959, 6, 303–311. [Google Scholar] [CrossRef]
- Zhang, Q.; Amooie, A.; Bazant, M.Z.; Bischofberger, I. Growth morphology and symmetry selection of interfacial instabilities in anisotropic environments. Soft Matter 2021, 17, 1202–1209. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Zhang, L.; Fu, B.; Tao, P.; Song, C.; Shang, W.; Deng, T. Self-assembly in Hopper shaped crystals. Adv. Funct. Mater. 2020, 30, 1908108. [Google Scholar] [CrossRef]
- van der Voort, E.; Hartman, P. The role of water in the habit change from cube to octahedron of alkali halide crystals. J. Cryst. Growth 1990, 104, 450–456. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, D.; Zhou, J. DNA action on the growth habit modification of NaCl crystals. CrystEngComm 2017, 19, 5356–5360. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Ganguly, B. Crystal habit modification of sodium chloride using habit modifiers: A dive into more than 50 years of research & development on crystal habit. CrystEngComm 2025, 27, 2439–2451. [Google Scholar] [CrossRef]
- Desarnaud, J.; Derluyn, H.; Carmeliet, J.; Bonn, D.; Shahidzadeh, N. Hopper growth of salt crystals. J. Phys. Chem. Lett. 2018, 9, 2961–2966. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Wang, Z.; Zhang, Z.; Wang, S.; Wang, S. Hopper-like single crystals of sodium chloride grown at the interface of metastable water droplets. Angew. Chem. Int. Ed. 2011, 50, 6044–6047. [Google Scholar] [CrossRef]
- Roy, J.G.; John, L.H.; Theodor, W.H. Transcript from an Interview with the 2005 Physics Laureates. Available online: https://www.nobelprize.org/prizes/physics/2005/glauber/225060-2005-physics-laureates-interview-transcript (accessed on 7 March 2025).

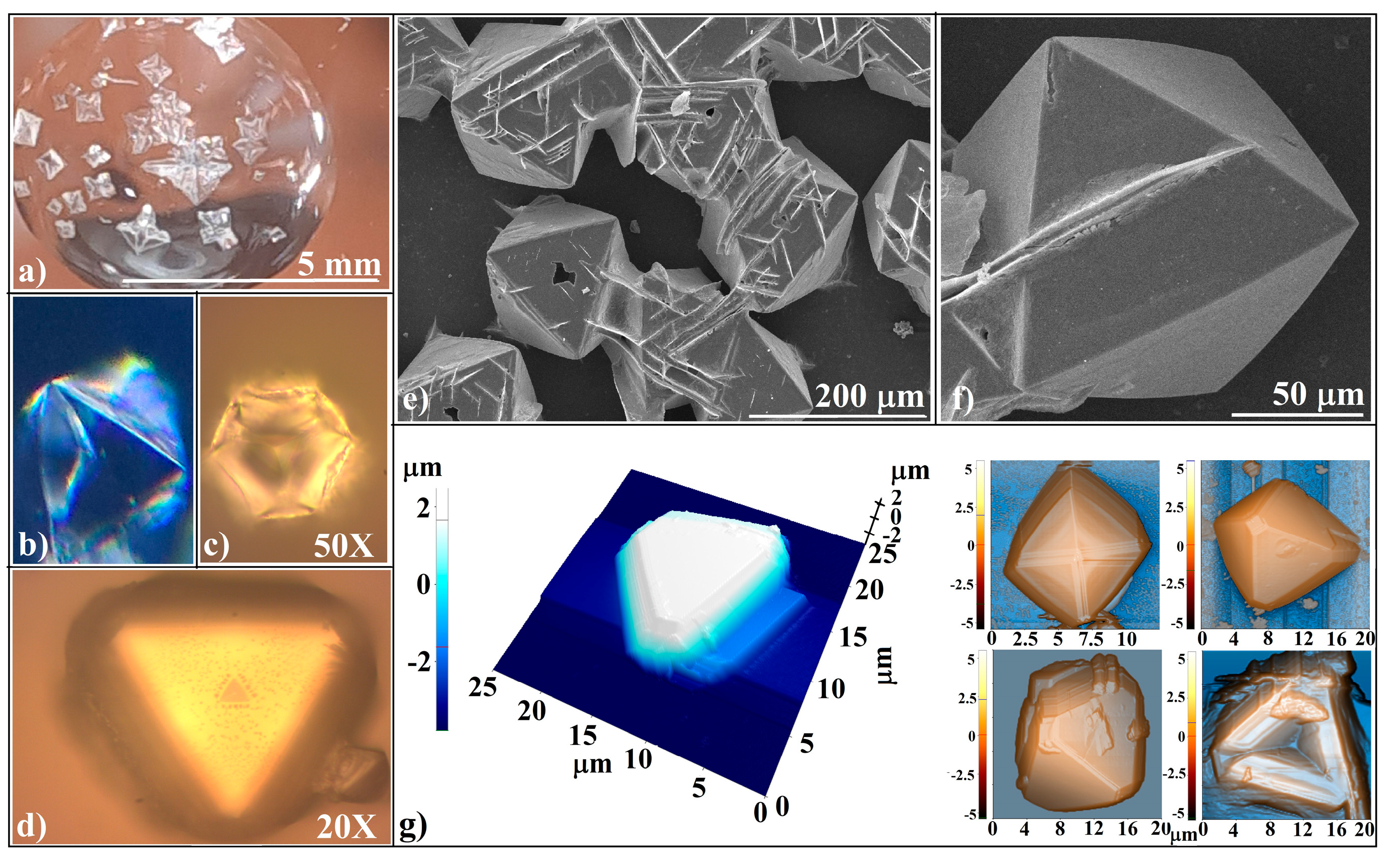
| T = 25 °C RH = 60% | V1 [μL] | V2 [μL] | c1 [% wt.] | d1 [mm] | C2 [% wt.] |
|---|---|---|---|---|---|
| Single crystal (Figure 6h) | 0 ÷ 10 | 150–170 | 0 | 7 | 95 |
| Aggregate with few crystals (Figure 6i) | 0 ÷ 10 | 50–60 | 0 | 7 | 95 |
| Ring (Figure 6j) | 10 ÷ 20 | 15–30 | 0 | 7 | 95 |
| Single layer (Figure 6k) | 10 ÷ 20 | 50–60 | 0 | 7 | 95 |
| Dendrite (Figure 6l) | 20 ÷ 30 | 60–150 | 0 | 7 | 95 |
| Lack of reproducibility and control | >30 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandu, I.; Fleaca, C.T.; Antohe, I.; Dumitrache, F.; Urzica, I.; Brajnicov, S.; Popescu, I.; Dumitru, M. Formation of Ordered Ionic Salt Agglomerates Through Evaporative Crystallization in Hanging Drop Systems. Appl. Sci. 2025, 15, 9280. https://doi.org/10.3390/app15179280
Sandu I, Fleaca CT, Antohe I, Dumitrache F, Urzica I, Brajnicov S, Popescu I, Dumitru M. Formation of Ordered Ionic Salt Agglomerates Through Evaporative Crystallization in Hanging Drop Systems. Applied Sciences. 2025; 15(17):9280. https://doi.org/10.3390/app15179280
Chicago/Turabian StyleSandu, Ion, Claudiu Teodor Fleaca, Iulia Antohe, Florian Dumitrache, Iuliana Urzica, Simona Brajnicov, Iustina Popescu, and Marius Dumitru. 2025. "Formation of Ordered Ionic Salt Agglomerates Through Evaporative Crystallization in Hanging Drop Systems" Applied Sciences 15, no. 17: 9280. https://doi.org/10.3390/app15179280
APA StyleSandu, I., Fleaca, C. T., Antohe, I., Dumitrache, F., Urzica, I., Brajnicov, S., Popescu, I., & Dumitru, M. (2025). Formation of Ordered Ionic Salt Agglomerates Through Evaporative Crystallization in Hanging Drop Systems. Applied Sciences, 15(17), 9280. https://doi.org/10.3390/app15179280






.jpg)

