A Review of the Most Commonly Used Additive Manufacturing Techniques for Improving Mandibular Resection and Reconstruction Procedures
Abstract
1. Introduction
1.1. Clinical Background and Rationale
1.2. Challenges and the Role of Additive Manufacturing
1.3. The Gap in the Literature and the Aim of the Study
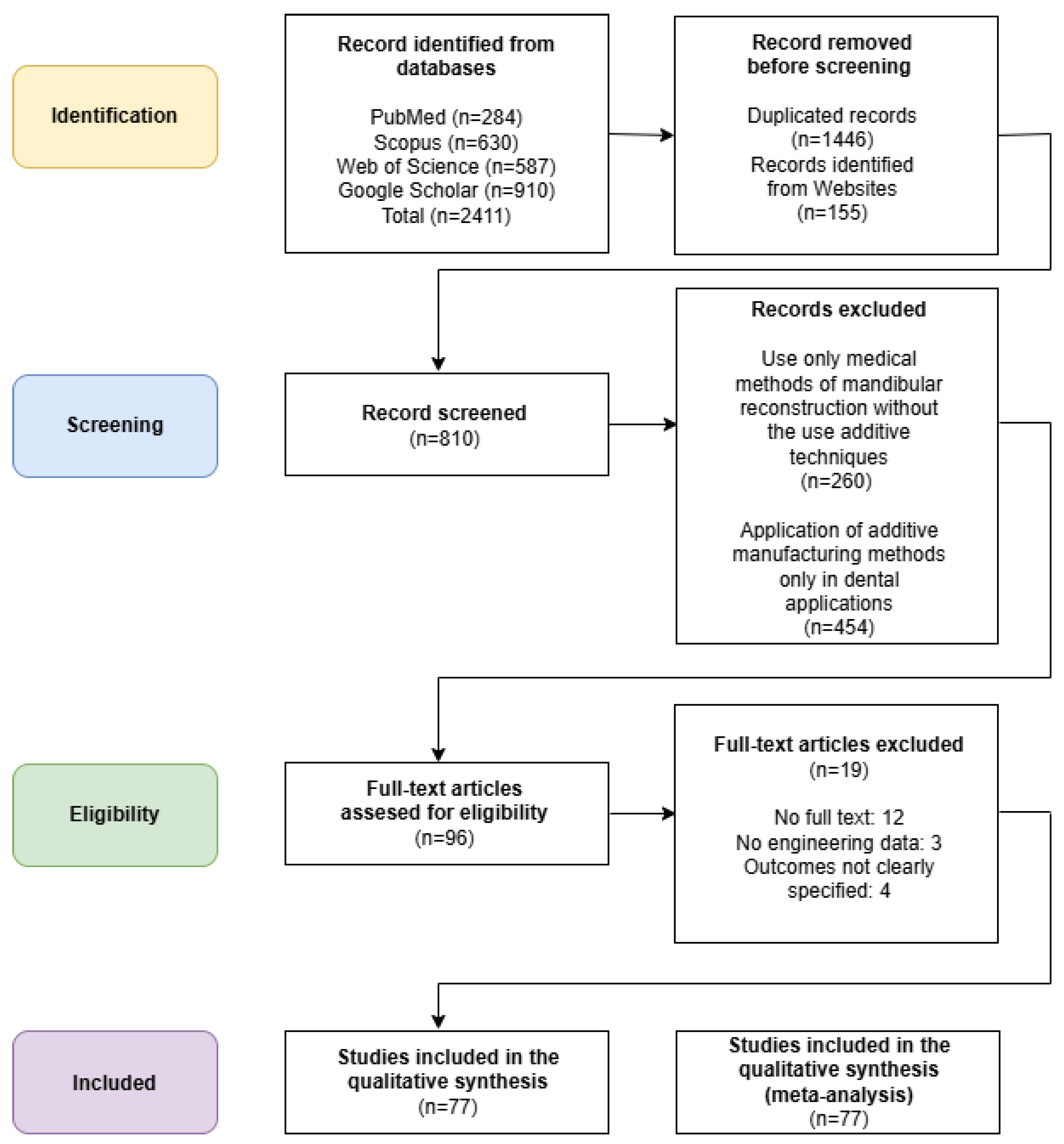
1.4. Methodology of Literature Selection
2. Reconstruction of Mandibular Geometry, CAD Modeling of Surgical Guide and Implants for Additive Manufacturing
2.1. Process of Reconstructing Mandibular Geometry from DICOM Data
2.2. Process of CAD Modeling of Surgical Guides and Implants for Additive Manufacturing
3. Results
4. The Additive Manufacturing of Anatomical Models, Surgical Templates, and Implants in the Mandible Area
4.1. Material Extrusion Technology
- MEX is instrumental in the production of customized surgical guides [42]. These guides, including implant surgical stents, are specifically designed to ensure the precise placement of implants or other surgical instruments during various procedures. The tailored nature of these guides means they can accommodate the unique anatomy of each patient, significantly improving the accuracy and efficiency of the surgical process. By facilitating proper alignment and positioning [37], MEX-generated surgical guides not only enhance surgical outcomes but also contribute to a reduction in procedural complications [36,44].
- MEX technology is not always used for the creation of implants [41]; it serves an essential role in the fabrication of temporary implants or scaffolds designed for bone regeneration [45]. Utilizing biocompatible materials, MEX can create structures that provide support and stability for healing tissues. These temporary implants are crucial in situations where immediate structural integrity is needed while the body initiates its healing processes [44].
- MEX technology is used in the development of advanced scaffolds for bone grafting procedures [38]. These scaffolds create an optimal environment for new bone growth by providing a framework that encourages cell proliferation and tissue integration [40]. The customized nature of MEX allows for the design of scaffolds that can match the specific anatomical requirements of different patients, promoting successful outcomes in bone restoration and regeneration efforts. This application of MEX not only enhances the efficacy of bone grafts but also accelerates the healing process, ultimately benefiting patient recovery.
4.2. Material Jetting Technology
- By manufacturing a physical replica of a patient’s mandible, surgeons can engage in detailed preoperative planning. This tactile model allows for a thorough assessment of the unique anatomical features and variations of the patient’s mandible [42]. Surgeons can practice complex procedures, visualize surgical options, and anticipate potential complications, ultimately enhancing surgical outcomes and reducing operative time.
- MJT can be utilized to fabricate custom prosthetics or surgical guides explicitly tailored for mandible reconstruction [42]. By capturing the intricacies of the patient’s anatomy, these models enable the design of prosthetic devices that fit securely and function effectively [48], improving patient comfort and functionality following surgical interventions.
- The highly accurate models generated via MJ are invaluable resources for both patient education and medical training [46]. They can help patients better understand their condition by providing a tangible representation of their anatomy, elucidating the surgical process, and setting realistic expectations for outcomes [39]. Additionally, these models serve as practical training tools for medical professionals, allowing them to refine their skills in a hands-on manner without the risks associated with live procedures. This approach fosters improved competence and confidence in tackling real-world clinical challenges [47].
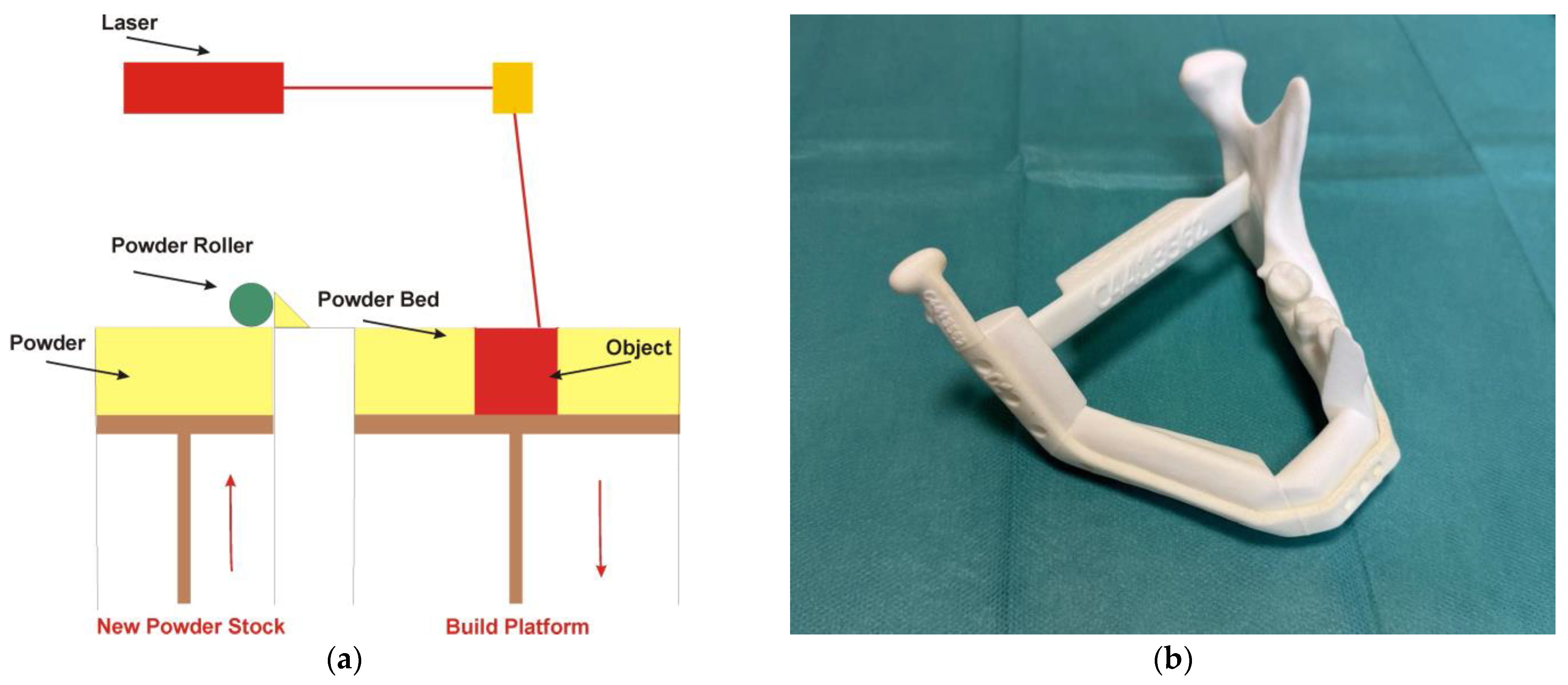
4.3. Powder Bed Fusion Technology
- One of the standout features of PBF technology is its ability to produce patient-specific implants that fit precisely within the contours of an individual’s mandible [49,50,51,52,53,54,55,56]. This level of customization is pivotal, as it not only enhances the mechanical integration of the implant with the surrounding bone but also improves the overall clinical outcomes for patients undergoing mandible reconstruction [18,58,59,60,61]. By accurately replicating the patient’s anatomical structures [63], the implants can restore both function and aesthetics more effectively than traditional implant methods [59,63].
- In addition, surgical templates are manufactured using PBF technology [57]. One of the primary functions of surgical templates is to facilitate the accurate positioning of plates, screws, and bone grafts during the intricate process of mandibular reconstruction [57,64]. The templates act as crucial guides for performing osteotomies, which are surgical cuts made to reshape or reconstruct the mandible [62]. Their incorporation into surgical practices not only enhances the precision of surgical interventions but also promotes superior patient outcomes [57,62].
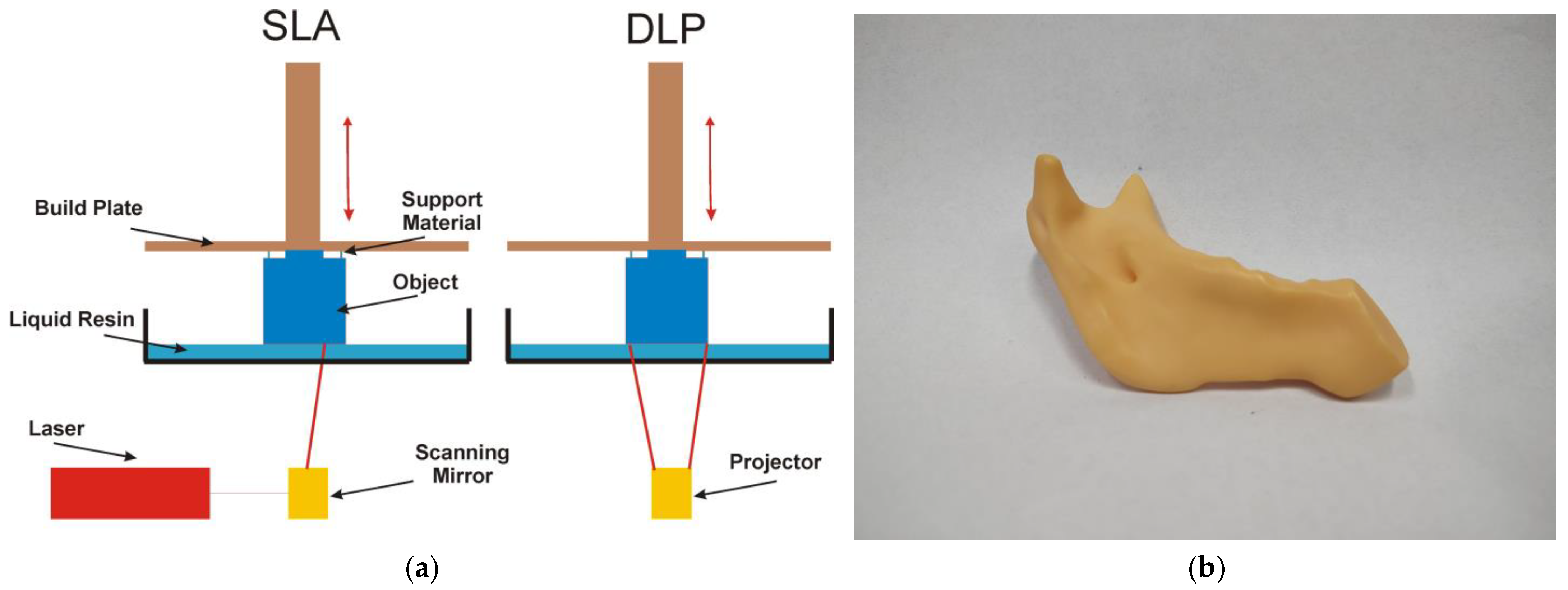
4.4. Vat Polymerization Technology
- Personalized guides that, based on a virtual surgical plan, fit perfectly to the surface of the patient’s mandible. The surgeon uses them to make precise and safe bone cuts during resection (removal of the diseased fragment) [66,69,72]. This allows cuts to be made with millimeter precision, ensuring that the graft will fit perfectly into the defect site. Additionally, they allow for the precise positioning and immobilization of the bone graft in the defect site in the mandible [67,70].
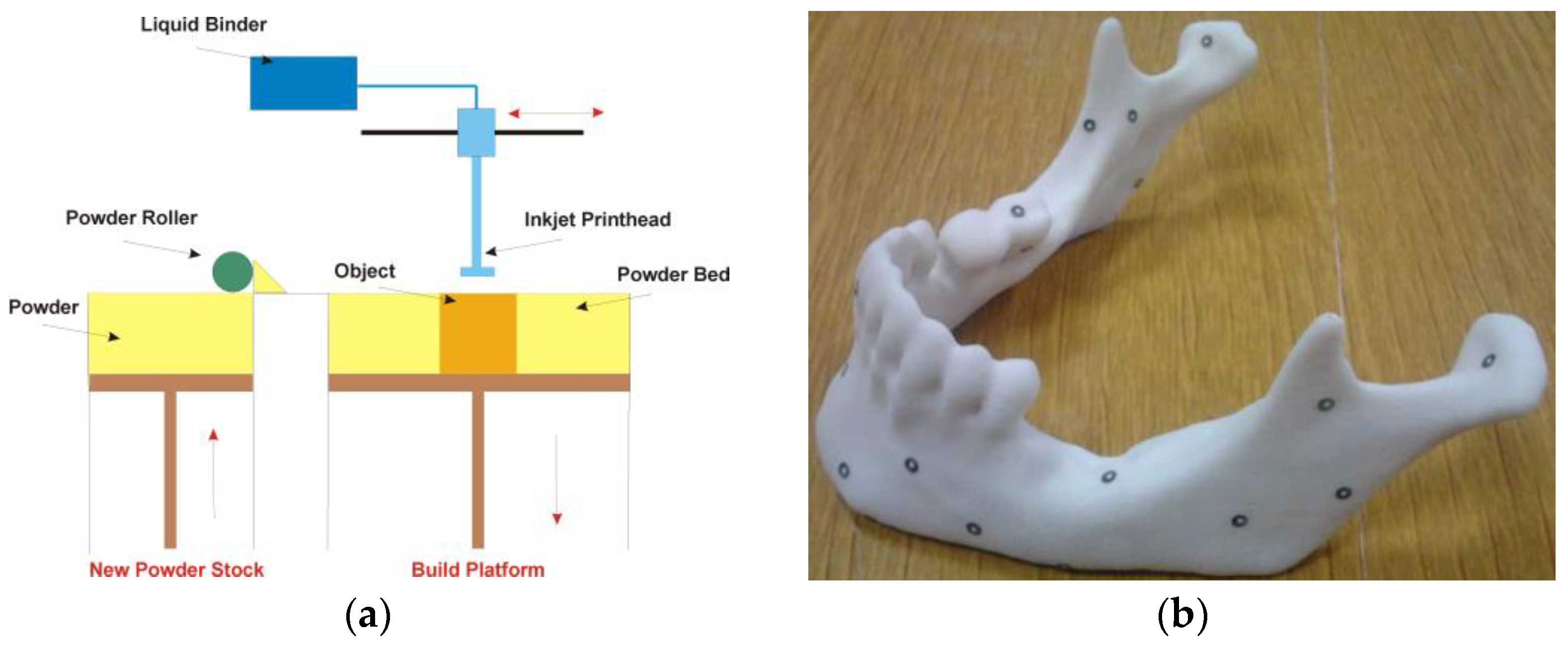
4.5. Binder Jetting Technology
5. Discussion
- It helps the surgeon prepare more effectively for the procedure;
- It increases the precision of the surgery;
- It aids in the selection of appropriate surgical tools;
- It facilitates thorough consultations with other medical professionals prior to the procedure;
- It allows for a more detailed presentation of the procedure to the patient, enabling better discussion of its course;
- It can reduce the overall surgery time under general anesthesia;
- It helps minimize blood loss during the procedure;
- It reduces the risk of intraoperative complications.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Govind, A.; Lugo, R.; Pogrel, M.A. Mandibular Reconstruction. Oral. Maxillofac. Surg. 2025, 645–672. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Gong, P.; Huang, B. The progress in reconstruction of mandibular defect caused by osteoradionecrosis. J. Oncol. 2023, 2023, 1440889. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Fichter, A.M.; Grill, F.D.; Hart, D.; Hapfelmeier, A.; Deppe, H.; Mücke, T. Bone volume change following vascularized free bone flap reconstruction of the mandible. J. Cranio-Maxillofac. Surg. 2020, 48, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Mashrah, M.A.; Aldhohrah, T.; Abdelrehem, A.; Sakran, K.A.; Ahmad, H.; Mahran, H.; Wang, L. Survival of vascularized osseous flaps in mandibular reconstruction: A network meta-analysis. PLoS ONE 2021, 16, e0257457. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.; Mazul, A.; Zenga, J.; Graboyes, E.M.; Jackson, R.; Puram, S.V.; Pipkorn, P. Complications after soft tissue with plate vs bony mandibular reconstruction: A systematic review and meta-analysis. Otolaryngol.–Head. Neck Surg. 2021, 164, 501–511. [Google Scholar] [CrossRef]
- Lilly, G.L.; Petrisor, D.; Wax, M.K. Mandibular rehabilitation: From the Andy Gump deformity to jaw-in-a-day. Laryngoscope Investig. Otolaryngol. 2021, 6, 708–720. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.W.; Stucker, B. Additive Manufacturing Technologies; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Amaya-Rivas, J.L.; Perero, B.S.; Helguero, C.G.; Hurel, J.L.; Peralta, J.M.; Flores, F.A.; Alvarado, J.D. Future trends of additive manufacturing in medical applications: An overview. Heliyon 2024, 10, e26641. [Google Scholar] [CrossRef]
- Allawi, N.I. Application of Reverse Engineering and Rapid Prototyping for Reconstruction of Human Mandible. arXiv 2021, arXiv:2106.15489. [Google Scholar] [CrossRef]
- Kumar, D.; Yadav, P.K.; Bhaskar, J. 3D Modelling of Human Joints Using Reverse Engineering for Biomedical Applications. In Advances in Manufacturing and Industrial Engineering; Springer: Singapore, 2021; pp. 865–875. [Google Scholar] [CrossRef]
- Salmi, M. Additive manufacturing processes in medical applications. Materials 2021, 14, 191. [Google Scholar] [CrossRef]
- Oren, D.; Dror, A.A.; Bramnik, T.; Sela, E.; Granot, I.; Srouji, S. The power of three-dimensional printing technology in functional restoration of rare maxillomandibular deformity due to genetic disorder: A case report. J. Med. Case Rep. 2021, 15, 197. [Google Scholar] [CrossRef]
- de Koning, S.B.; Ter Braak, T.P.; Geldof, F.; van Veen, R.L.P.; van Alphen, M.J.A.; Karssemakers, L.H.E.; Karakullukcu, M.B. Evaluating the accuracy of resection planes in mandibular surgery using a preoperative, intraoperative, and postoperative approach. Int. J. Oral Maxillofac. Surg. 2021, 50, 287–293. [Google Scholar] [CrossRef]
- Oldhoff, M.G.E.; Mirzaali, M.J.; Tümer, N.; Zhou, J.; Zadpoor, A.A. Comparison in clinical performance of surgical guides for mandibular surgery and temporomandibular joint implants fabricated by additive manufacturing techniques. J. Mech. Behav. Biomed. Mater. 2021, 119, 104512. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.A.; Liokatis, P.; Mast, G.; Ehrenfeld, M. Virtual planning for mandible resection and reconstruction. Innov. Surg. Sci. 2023, 8, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.R.; Sridevi, E.; Sankar, A.S.; Krishna, V.S.S.; Sridhar, M.; Sankar, K.S. Contemporary era of Three-dimensional printing in pediatric dentistry: An overview. J. Oral Res. Rev. 2023, 15, 72–79. [Google Scholar] [CrossRef]
- Kim, M.K.; Ham, M.J.; Kim, W.R.; Kim, H.G.; Kwon, K.J.; Kim, S.G.; Park, Y.W. Investigating the accuracy of mandibulectomy and reconstructive surgery using 3D customized implants and surgical guides in a rabbit model. Maxillofac. Plast. Reconstr. Surg. 2023, 45, 8. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, Z.C.; Li, C.; Wu, H.; Tang, C.; Wang, L.; Li, H. Application of additive manufacturing in customized titanium mandibular implants for patients with oral tumors. Oncol. Lett. 2020, 20, 51. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Qu, T.; Cheng, Y.; Li, J.; Li, Y.; Guo, J. Improving spatial resolution and diagnostic confidence with thinner slice and deep learning image reconstruction in contrast-enhanced abdominal CT. Eur. Radiol. 2023, 33, 1603–1611. [Google Scholar] [CrossRef]
- Miranda-Viana, M.; Fontenele, R.C.; Nogueira-Reis, F.; Farias-Gomes, A.; Oliveira, M.L.; Freitas, D.Q.; Haiter-Neto, F. DICOM file format has better radiographic image quality than other file formats: An objective study. Braz. Dent. J. 2023, 34, 150–157. [Google Scholar] [CrossRef]
- Dino, J.A.; Barani, S.; Poornapushpakala, S.; Subramoniam, M. Implementation of Various Filtering Technique for Noise Removal in DICOM Images of Colon Carcinoma. In Proceedings of the 2024 Global Conference on Communications and Information Technologies (GCCIT), Bangalore, India, 25–26 October 2024; pp. 1–5. [Google Scholar] [CrossRef]
- Fajar, A.; Sarno, R.; Fatichah, C.; Fahmi, A. Reconstructing and resizing 3D images from DICOM files. J. King Saud. Univ. Comput. Inf. Sci. 2022, 34, 3517–3526. [Google Scholar] [CrossRef]
- Qiu, B.; van der Wel, H.; Kraeima, J.; Glas, H.H.; Guo, J.; Borra, R.J.; van Ooijen, P.M. Automatic segmentation of mandible from conventional methods to deep learning—A review. J. Pers. Med. 2021, 11, 629. [Google Scholar] [CrossRef]
- Irshad, T.B.; Pascoletti, G.; Bianconi, F.; Zanetti, E.M. Mandibular bone segmentation from CT scans: Quantitative and qualitative comparison among software. Dent. Mater. 2024, 40, e11–e22. [Google Scholar] [CrossRef]
- Syuhada, F.; Fatichah, C.; Fabroyir, H. Image Enhancement for Visualization Jaw Bone in DICOM CT Scans using Windowing Strategies, Adaptive Denoising Bilateral Filtering, and Sharpening. In Proceedings of the 2024 7th International Seminar on Research of Information Technology and Intelligent Systems (ISRITI), Yogyakarta, Indonesia, 11–12 December 2024; pp. 564–569. [Google Scholar]
- Gruber, L.J.; Egger, J.; Bönsch, A.; Kraeima, J.; Ulbrich, M.; van den Bosch, V.; Puladi, B. Accuracy and precision of mandible segmentation and its clinical implications: Virtual reality, desktop screen and artificial intelligence. Expert. Syst. Appl. 2024, 239, 122275. [Google Scholar] [CrossRef]
- Lahoud, P.; EzEldeen, M.; Beznik, T.; Willems, H.; Leite, A.; Van Gerven, A.; Jacobs, R. Artificial intelligence for fast and accurate 3-dimensional tooth segmentation on cone-beam computed tomography. J. Endod. 2021, 47, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Belikova, K.; Rogov, O.Y.; Rybakov, A.; Maslov, M.V.; Dylov, D.V. Deep negative volume segmentation. Sci. Rep. 2021, 11, 16292. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Z.; Yang, X.; Jia, W.; Zhou, T. Three-dimensional reconstruction of jaw and dentition CBCT images based on improved marching cubes algorithm. Procedia CIRP 2020, 89, 239–244. [Google Scholar] [CrossRef]
- Chalazoniti, A.; Lattanzi, W.; Halazonetis, D.J. Shape variation and sex differences of the adult human mandible evaluated by geometric morphometrics. Sci. Rep. 2024, 14, 8546. [Google Scholar] [CrossRef]
- Zhou, L.; Miller, J.; Vezza, J.; Mayster, M.; Raffay, M.; Justice, Q.; Bernat, J. Additive manufacturing: A comprehensive review. Sensors 2024, 24, 2668. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ma, K.; Sun, J.; Bai, N.; Liu, Y. Rehabilitation of long-term mandibular defects by whole-process digital fibula flap combining with implants: A case report. J. Prosthodont. 2023, 32, 187–195. [Google Scholar] [CrossRef]
- Xu, G.; Jia, J.; Xiong, X.; Peng, L.; Bu, L.L.; Wang, X. Mandibular reconstruction with the contralateral vascularized iliac flap using individual design: Iliac crest used to reconstruct the ramus and the anterior border of the iliac wing used to reconstruct the inferior border: A case report. Front. Surg. 2022, 9, 924241. [Google Scholar] [CrossRef]
- Zhong, S.; Shi, Q.; Van Dessel, J.; Gu, Y.; Sun, Y.; Yang, S.; Politis, C. Biomechanical validation of structural optimized patient-specific mandibular reconstruction plate orienting additive manufacturing. Comput. Methods Programs Biomed. 2022, 224, 107023. [Google Scholar] [CrossRef]
- Wu, P.; Hu, L.; Li, H.; Feng, L.; Liu, Y.; Zhang, S.; Lu, R.J. Clinical application and accuracy analysis of 3D printing guide plate based on polylactic acid in mandible reconstruction with fibula flap. Ann. Transl. Med. 2021, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Moiduddin, K.; Mian, S.H.; Ameen, W.; Alkindi, M.; Ramalingam, S.; Alghamdi, O. Patient-specific surgical implant using cavity-filled approach for precise and functional mandible reconstruction. Appl. Sci. 2020, 10, 6030. [Google Scholar] [CrossRef]
- Silva, J.; Lago, C.; Soares Pereira, R.; Furtado da Costa, M.; Nascimento Martins, K.; de Luna Campos, G.; de Souza Andrade, E. The Use of 3D Model Printing for Acute Planning in Oral and Maxillofacial Traumatology. Open Dent. J. 2024, 18, e18742106326414. [Google Scholar] [CrossRef]
- Ferrari, M.; Taboni, S.; Chan, H.; Townson, J.; Gualtieri, T.; Franz, L.; Ruaro, A.; Mathews, S.; Daly, M.; Douglas, C.; et al. Hydrogel-chitosan and polylactic acid-polycaprolactone bioengineered scaffolds for reconstruction of mandibular defects: A preclinical in vivo study with assessment of translationally relevant aspects. Front. Bioeng. Biotechnol. 2024, 12, 1353523. [Google Scholar] [CrossRef]
- Baumgartner, D.; Schramel, J.P.; Kau, S.; Eberspächer-Schweda, M.C.; Unger, E.; Oberoi, G.; Peham, C. 3D printed plates based on generative design biomechanically outperform manual digital fitting and conventional systems printed in photopolymers in bridging mandibular bone defects of critical size in dogs. Front. Vet. Sci. 2023, 10, 1165689. [Google Scholar] [CrossRef]
- Slavin, B.V.; Ehlen, Q.T.; Costello, J.P.; Nayak, V.V.; Bonfante, E.A.; Jalkh, E.B.B.; Runyan, C.M.; Witek, L.; Coelho, P.G. 3D Printing Applications for Craniomaxillofacial Reconstruction: A Sweeping Review. ACS Biomater. Sci. Eng. 2023, 9, 6586–6609. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Tian, L.; Li, D.; Lu, B.; Shi, C.; Niu, Q.; Liu, F.; Kong, L.; Zhang, J. Clinical application of 3D-printed PEEK implants for repairing mandibular defects. J. Craniomaxillofac Surg. 2022, 50, 621–626. [Google Scholar] [CrossRef]
- Kim, J.-W.; Yang, B.-E.; Hong, S.-J.; Choi, H.-G.; Byeon, S.-J.; Lim, H.-K.; Chung, S.-M.; Lee, J.-H.; Byun, S.-H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837. [Google Scholar] [CrossRef]
- Katschnig, M.; Wallner, J.; Janics, T.; Burgstaller, C.; Zemann, W.; Holzer, C. Biofunctional Glycol-Modified Polyethylene Terephthalate and Thermoplastic Polyurethane Implants by Extrusion-Based Additive Manufacturing for Medical 3D Maxillofacial Defect Reconstruction. Polymers 2020, 12, 1751. [Google Scholar] [CrossRef]
- Damecourt, A.; Nieto, N.; Galmiche, S.; Garrel, R.; de Boutray, M. In-house 3D treatment planning for mandibular reconstruction by free fibula flap in cancer: Our technique, European Annals of Otorhinolaryngology. Head. Neck Dis. 2020, 137, 501–505. [Google Scholar] [CrossRef]
- Olate, S.; Uribe, F.; Huentequeo-Molina, C.; Goulart, D.R.; Sigua-Rodriguez, E.A.; Alister, J.P. Mandibular Angle Contouring Using Porous Polyethylene Stock or PEEK-based Patient Specific Implants. A Critical Analysis. J. Craniofac Surg. 2021, 32, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, S.; Gangatirkar, R.; Kandi, A.; DeLeonibus, M.; Kamel, M.; Chadalavada, S.; Gupta, R.; Munigala, H.; Tappa, K.; Kondor, S.; et al. Medical 3D Printing Using Material Jetting: Technology Overview, Medical Applications, and Challenges. Bioengineering 2025, 12, 249. [Google Scholar] [CrossRef]
- Schneider, K.H.; Oberoi, G.; Unger, E.; Janjic, K.; Rohringer, S.; Heber, S.; Agis, H.; Schedle, A.; Kiss, H.; Podesser, B.K.; et al. Medical 3D printing with polyjet technology: Effect of material type and printing orientation on printability, surface structure and cytotoxicity. 3D Print. Med. 2023, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Šimić, L.; Kopačin, V.; Mumlek, I.; Butković, J.; Zubčić, V. Improved technique of personalised surgical guides generation for mandibular free flap reconstruction using an open-source tool. Eur. Radiol. Exp. 2021, 5, 30. [Google Scholar] [CrossRef]
- Maruf, D.A.; Ren, J.; Cheng, K.; Xin, H.; Lewin, W.; Pickering, E.; Clark, J.R. Evaluation of osseointegration of plasma treated polyaryletherketone maxillofacial implants. Sci. Rep. 2025, 15, 1895. [Google Scholar] [CrossRef]
- Msallem, B.; Vavrina, J.J.; Beyer, M.; Halbeisen, F.S.; Lauer, G.; Dragu, A.; Thieringer, F.M. Dimensional Accuracy in 3D Printed Medical Models: A Follow-Up Study on SLA and SLS Technology. J. Clin. Med. 2024, 13, 5848. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Wang, C.; Yan, R.; Xiang, L.; Mu, X.; Hu, M. 3D printing titanium grid scaffold facilitates osteogenesis in mandibular segmental defects. NPJ Regen. Med. 2023, 8, 38. [Google Scholar] [CrossRef]
- Haroun, F.; Benmoussa, N.; Bidault, F.; Lassau, N.; Moya-Plana, A.; Leymarie, N.; Gorphe, P. Outcomes of mandibular reconstruction using three-dimensional custom-made porous titanium prostheses. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124, 101281. [Google Scholar] [CrossRef]
- Yang, H.J.; Oh, J.H. Reconstruction of mandibular contour defect using patient-specific titanium implant manufactured by selective laser melting method. J. Craniofacial Surg. 2022, 33, 2055–2058. [Google Scholar] [CrossRef]
- Van Kootwijk, A.; Moosabeiki, V.; Saldivar, M.C.; Pahlavani, H.; Leeflang, M.A.; Niar, S.K.; Zadpoor, A.A. Semi-automated digital workflow to design and evaluate patient-specific mandibular reconstruction implants. J. Mech. Behav. Biomed. Mater. 2022, 132, 105291. [Google Scholar] [CrossRef]
- Nemtoi, A.; Covrig, V.; Nemtoi, A.; Stoica, G.; Vatavu, R.; Haba, D.; Zetu, I. Custom-Made Direct Metal Laser Sintering Titanium Subperiosteal Implants in Oral and Maxillofacial Surgery for Severe Bone-Deficient Patients—A Pilot Study. Diagnostics 2022, 12, 2531. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.K.; Choi, Y.J.; Choi, W.C.; Song, I.S.; Lee, U.L. Reconstruction of maxillofacial bone defects using patient-specific long-lasting titanium implants. Sci. Rep. 2022, 12, 7538. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cheng, K.J.; Liu, Y.F.; Fan, Y.Y.; Wang, J.H.; Wang, R.; Dong, X.T. Experimental validation of finite element simulation of a new custom-designed fixation plate to treat mandibular angle fracture. Biomed. Eng. OnLine 2021, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Farajpour, H.; Bastami, F.; Bohlouli, M.; Khojasteh, A. Reconstruction of bilateral ramus-condyle unit defect using custom titanium prosthesis with preservation of both condyles. J. Mech. Behav. Biomed. Mater. 2021, 124, 104765. [Google Scholar] [CrossRef] [PubMed]
- Koper, D.C.; Leung, C.A.; Smeets, L.C.; Laeven, P.F.; Tuijthof, G.J.; Kessler, P.A. Topology optimization of a mandibular reconstruction plate and biomechanical validation. J. Mech. Behav. Biomed. Mater. 2021, 113, 104157. [Google Scholar] [CrossRef]
- Lang, J.J.; Bastian, M.; Foehr, P.; Seebach, M.; Weitz, J.; von Deimling, C.; Burgkart, R. Improving mandibular reconstruction by using topology optimization, patient specific design and additive manufacturing?—A biomechanical comparison against miniplates on human specimen. PLoS ONE 2021, 16, e0253002. [Google Scholar] [CrossRef]
- Msallem, B.; Sharma, N.; Cao, S.; Halbeisen, F.S.; Zeilhofer, H.-F.; Thieringer, F.M. Evaluation of the Dimensional Accuracy of 3D-Printed Anatomical Mandibular Models Using FFF, SLA, SLS, MJ, and BJ Printing Technology. J. Clin. Med. 2020, 9, 817. [Google Scholar] [CrossRef]
- Park, J.H.; Odkhuu, M.; Cho, S.; Li, J.; Park, B.Y.; Kim, J.W. 3D-printed titanium implant with pre-mounted dental implants for mandible reconstruction: A case report. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 28. [Google Scholar] [CrossRef]
- Grecchi, F.; Zecca, P.A.; Macchi, A.; Mangano, A.; Riva, F.; Grecchi, E.; Mangano, C. Full-Digital Workflow for Fabricating a Custom-Made Direct Metal Laser Sintering (DMLS) Mandibular Implant: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 2693. [Google Scholar] [CrossRef]
- Mangano, C.; Bianchi, A.; Mangano, F.G.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print. Med. 2020, 6, 1. [Google Scholar] [CrossRef]
- Moiduddin, K.; Mian, S.H.; Ahmed, N.; Ameen, W.; Al-Khalefah, H.; Mohammed, M.K.; Umer, U. Integrative and multi-disciplinary framework for the 3D rehabilitation of large mandibular defects. Int. J. Adv. Manuf. Technol. 2020, 106, 3831–3847. [Google Scholar] [CrossRef]
- Tatti, M.; Carta, F.; Bontempi, M.; Deriu, S.; Mariani, C.; Marrosu, V.; Foddis, E.; Gerosa, C.; Marongiu, G.; Saba, L.; et al. Segmental Mandibulectomy and Mandibular Reconstruction with Fibula-Free Flap Using a 3D Template. J. Pers. Med. 2024, 14, 512. [Google Scholar] [CrossRef]
- Cho, R.Y.; Byun, S.H.; Park, S.Y.; On, S.W.; Kim, J.C.; Yang, B.E. Patient-specific plates for facial fracture surgery: A retrospective case series. J. Dent. 2023, 137, 104650. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, X.; Zhang, F.; Hong, Y.; Qiu, Y.; Yang, X.; Gou, Z. Customized bioceramic scaffolds and metal meshes for challenging large-size mandibular bone defect regeneration and repair. Regen. Biomater. 2023, 10, rbad057. [Google Scholar] [CrossRef]
- Kivovics, M.; Pénzes, D.; Moldvai, J.; Mijiritsky, E.; Németh, O. A custom-made removable appliance for the decompression of odontogenic cysts fabricated using a digital workflow. J. Dent. 2022, 126, 104295. [Google Scholar] [CrossRef]
- Türker, H.; Aksoy, B.; Özsoy, K. Fabrication of Customized dental guide by stereolithography method and evaluation of dimensional accuracy with artificial neural networks. J. Mech. Behav. Biomed. Mater. 2022, 126, 105071. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-I.; Yang, B.-E.; Yi, S.-M.; Choi, H.G.; On, S.-W.; Hong, S.J.; Lim, H.-K.; Byun, S.-H. Bone Regeneration of a 3D-Printed Alloplastic and Particulate Xenogenic Graft with rhBMP-2. Int. J. Mol. Sci. 2021, 22, 12518. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, S.; Chae, Y.K.; Jung, J.; Choi, S.C.; Nam, O.H. Customized surgical guide with a bite block and retraction arm for a deeply impacted odontoma; a technical note. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 456–457. [Google Scholar] [CrossRef]
- Yousefi, F.; Shokri, A.; Farhadian, M.; Vafaei, F.; Forutan, F. Accuracy of maxillofacial prototypes fabricated by different 3-dimensional printing technologies using multi-slice and cone-beam computed tomography. Imaging Sci. Dent. 2021, 51, 41. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ren, H.; Zheng, M.; Shao, X.; Dai, T.; Wu, Y.; Liu, Y. Development of biodegradable bioactive glass ceramics by DLP printed containing EPCs/BMSCs for bone tissue engineering of rabbit mandible defects. J. Mech. Behav. Biomed. Mater. 2020, 103, 103532. [Google Scholar] [CrossRef]
- Peker Ozturk, H.; Ayyıldız, S. Comparison of different 3D printers in terms of dimensional stability by image data of a dry human mandible obtained from CBCT and CT. Int. J. Artif. Organs. 2024, 47, 49–56. [Google Scholar] [CrossRef]
- Simchi, A.; Petzoldt, F.; Hartwig, T.; Hein, S.B.; Barthel, B.; Reineke, L. Microstructural development during additive manufacturing of biomedical grade Ti-6Al-4V alloy by three-dimensional binder jetting: Material aspects and mechanical properties. Int. J. Adv. Manuf. Technol. 2023, 127, 1541–1558. [Google Scholar] [CrossRef]
- Kuah, K.X.; Salehi, M.; Huang, Z.; Zhang, S.X.; Seet, H.L.; Nai, M.L.S.; Blackwood, D.J. Surface modification with phosphate and hydroxyapatite of porous magnesium scaffolds fabricated by binder jet additive manufacturing. Crystals 2022, 12, 1850. [Google Scholar] [CrossRef]



| AM Technology | Number of Studies Identified (2020–2025) | Main Applications |
|---|---|---|
| MEX | 11 | Anatomical models, surgical guides, temporary implants/scaffolds |
| MJT | 5 | Anatomical models, surgical guides, educational/training models |
| PBF | 20 | Personalized titanium/PEEK implants, surgical guides, anatomical models |
| VPP | 11 | Surgical guides, anatomical models, bioceramic scaffolds |
| BJT | 4 | Anatomical models (in vitro) |
| DED/SHL | 0 | No relevant publications found |
| Year | AM Method and 3D Printer | Material | Application | Reference |
|---|---|---|---|---|
| 2024 | FDM | ABS | Biomodel for forming a reconstruction plate | [38] |
| 2024 | FFF/FDM Prusa i3 MK3S+/MK4 | PLA | Implantable scaffolds (rabbit studies) | [39] |
| 2023 | FFF/FDM 3DGence Industry F340 | PCL, PLA (bioceramics additives) | Biocomposite bone scaffolds | [40] |
| 2022 | FFF/FDM INTAMSYS FUNMAT HT Enhanced | PEEK | Implants | [41] |
| 2022 | FDM Not specified | PLA | Resection guides | [33] |
| 2021 | FDM Not specified | PLA | Resection guides | [35] |
| 2020 | FDM Value3D MagiX MF2000 | PLA | Creating osteotomy templates | [42] |
| 2020 | FFF/FDM HAGE3D | PET-G | Maxillofacial implants | [43] |
| 2020 | FFF/FDM Raise3D® model N2 | PLA | Models of the mandible and fibula (proximal part) | [44] |
| 2020 | FFF/FDM Apium M220 | PEEK | Mandibular angle implants of different shapes and sizes | [45] |
| 2020 | FDM Stratasys FDM machine | ABS | Surgical plate | [36] |
| Year | AM Method and 3D Printer | Material | Application | Reference | Study Type/Experimental Context |
|---|---|---|---|---|---|
| 2025 | PolyJet J5 MediJet 3D | MED610, MED615 | Planning and educational models | [46] | Educational and training models; no clinical application |
| 2023 | PolyJet Connex3 Objet500 | Vero Pure White RGD835 TangoPlus FLX930 | Mandible implants (studies conducted on dogs) | [39] | Animal study (canine model); not used in human clinical setting |
| 2023 | PolyJet Connex3 Objet500 | Vero Clear RGD810, VeroPureWhite RGD837 MED610 | Determining the quality and properties of scaffolds | [47] | In vitro mechanical testing; no clinical use |
| 2021 | PolyJet Objet30 | VeroGlaze MED620 | Mandibular osteotomy templates | [48] | Prototyping of surgical templates; no patient-based outcomes |
| 2020 | PolyJet Cubicon Lux | Photoreactive ceramic–resin composite | Creating osteotomy templates | [42] | Experimental template development; not applied clinically |
| Year | AM Method and 3D Printer | Material | Application | Reference | Study Type/Sample Size/Clinical Outcome |
|---|---|---|---|---|---|
| 2025 | SLS EOS P800 | PEEK | Implants used in mandibular bone regeneration (research conducted on a group of sheep) | [49] | Animal study (sheep); n = not specified |
| 2024 | SLS Fuse 1 | PA12 | Models for analyzing the accuracy of the reconstruction of three-dimensional anatomical models | [50] | In vitro study; model validation |
| 2023 | SLM SLM 280 | Commercialized pure titanium (CP-Ti, SLM solution) | Implant and surgical guide | [17] | Animal study (rabbits); n = not specified |
| 2023 | SLM Concept Laser | Titanium powder (not specified) | Personalized grid scaffold used in mandibular bone regeneration | [51] | Preclinical feasibility study; n = not specified |
| 2023 | SLM (not specified) | Pure titanium | Personalized implants used in mandibular bone regeneration | [52] | Clinical case series; n = few (exact n not stated) |
| 2022 | SLM MetalSys250 | Ti-6AL-4V ELI powder | Personalized implants used in mandibular bone regeneration | [53] | Case series; n = few (not stated); favorable outcome |
| 2022 | SLM SLM Solutions Group AG | Ti-6AL-4V ELI powder | Personalized implants used in mandibular bone regeneration | [54] | Case report/series; n not reported |
| 2022 | DMLS TruPrint 1000 | Ti-6AL-4V ELI powder | Personalized implants used in mandibular bone regeneration | [55] | Clinical feasibility; n ≈ small |
| 2022 | EBM Electron Beam Melting A1 Arcam | Ti-6AL-4V ELI powder | Personalized implants used in mandibular bone regeneration | [56] | Clinical case series; n = not stated |
| 2021 | SLS Sinterstation HiQ/HS | Nylon powder | Model used for creating a surgical guide | [57] | In vitro prototype only |
| 2021 | SLM SLM 280 Twin 2x400W | Ti-6AL-4V ELI powder | Personalized grid scaffold used in mandibular bone regeneration | [58] | Experimental model (clinical translation suggested) |
| 2021 | SLM AM250 | Ti-6AL-4V ELI powder | Condyle-restricting devices and fixation plates | [57] | Prototype application; no clinical trial reported |
| 2021 | SLM (not specified) | Ti-6AL-4V ELI powder | Models for analyzing the topology of the mandibular reconstruction plate | [59] | Clinical trial; n = 20; excellent fit and surgical outcome; minimal complications |
| 2021 | EBM FIT Production GmbH | Ti-6AL-4V ELI powder | Personalized implants used in mandibular bone regeneration | [60] | Case-based approach; n = not specified |
| 2020 | SLS EOSINT P 385 | PA 2200 (polyamide powder) | Models for analyzing the accuracy of the reconstruction of three-dimensional anatomical models | [61] | In vitro model comparison |
| 2020 | SLM (not specified) | Titanium alloy (not specified) | Resection guide and personalized mandibular implant | [62] | Case report; n = 1 |
| 2020 | DMLS DMP Dental100 | Ti-6AL-4V ELI powder | Personalized implants used in mandibular bone regeneration | [63] | Clinical feasibility; n not specified |
| 2020 | DMLS ProX-DMP100 3D System | Titanium alloy (not specified) | Surgical guide with subperiosteal implants | [64] | Case study; outcome not detailed |
| 2020 | EBM Arcam printer (not specified) | Ti-6AL-4V ELI powder | Personalized grid scaffold used in mandibular bone regeneration | [18] | Preclinical experiment |
| 2020 | EBM Electron Beam Melting A2 Arcam | Ti-6AL-4V ELI powder | Personalized implants with a scaffold used in mandibular bone regeneration | [65] | Clinical application; n = not stated |
| Year | AM Method and 3D Printer | Material | Application | Reference | Study Type/Sample Size/Clinical Outcome |
|---|---|---|---|---|---|
| 2024 | SLA 3D Form 2 Formlabs | Resin (not specified) | Model used for creating a surgical guide | [66] | Prototype/preclinical use |
| 2024 | SLA Form 3B+ | Standard Gray (resin)/BioMed Clear (resin) | Models for analyzing the accuracy of the reconstruction of three-dimensional anatomical models | [50] | In vitro evaluation |
| 2023 | SLA HALOT-SKY 3D | Medical grade resin | Surgical guide to assist in drilling holes for the implant plate | [67] | Clinical assistance tool, n not specified |
| 2023 | DLP Shaoxing Xunshi Technology | A mix of bioceramic powders and photosensitive acrylic | Bioceramic scaffolds used in bone tissue regeneration (research conducted on a group of rabbits) | [68] | Animal study (rabbits), n = not specified |
| 2022 | DLP RapidShape S30 | SHERprint-ortho | Surgical guide to decompress odontogenic cysts | [69] | Case-based clinical use, n = 1 |
| 2021 | SLA (not specified) | Biocompatible resin | Surgical guide for implant treatment | [70] | Clinical case study |
| 2021 | DLP Cubicon Lux | Photoreactive ceramic-resin composite | Biocompatible scaffolds to improve mandibular bone regeneration (research conducted on a group of dogs) | [71] | Animal study (dogs), n not specified |
| 2021 | DLP Nextdent 5100 | NextDent SG resin | Surgical guide to assist in the surgical extraction of a retained tooth | [72] | Clinical feasibility study |
| 2021 | DLP Planmeca Creo | Detax Freeprint model resin | Models for analyzing the accuracy of the reconstruction of three-dimensional anatomical models | [73] | In vitro validation |
| 2020 | DLP Cubicon Lux | Photoreactive ceramic-resin composite | Biocompatible scaffolds used in mandibular bone regeneration (studies conducted on a group of dogs) | [42] | Animal study (dogs), not specified |
| 2020 | DLP ACME DLP 3D | Ceramic suspensions | Bioceramic scaffolds used in bone tissue regeneration (research conducted on a group of rabbits) | [74] | Animal study (rabbits), successful integration |
| Year | AM Method and 3D Printer | Material | Application | Reference | Study Type/Sample Size/Clinical Outcome |
|---|---|---|---|---|---|
| 2024 | Polymer Binder Jetting ZCorp 3D printer | ABS150 | A manufactured anatomical model of the mandible was used to assess geometric accuracy. | [75] | In vitro accuracy validation study |
| 2023 | Binder Jetting (not specified) | Ti-6Al-4V | Manufactured scaffold structure. | [76] | Material study |
| 2022 | Binder Jetting (not specified) | Mg-Zn-Zr | Manufactured scaffold structure. | [77] | Material study after Hydroxyapatite Coating |
| 2020 | Binder Jetting (not specified) ProJet CJP 660Pro | ZP151 | A manufactured anatomical model of the mandible was used to assess geometric accuracy. | [61] | In vitro model-based study; no clinical involvement |
| Application Area | Material Extrusion (FDM/FFF) | Vat Photopolymerization (SLA/DLP) | Powder Bed Fusion (SLM/DMLS) | Binder Jetting | MEX (PEEK) |
|---|---|---|---|---|---|
| Anatomical Models |  Common (PLA/ABS) Common (PLA/ABS) |  High-resolution models High-resolution models |  Rare use (costly) Rare use (costly) |  Cost-effective Cost-effective |  Rare Rare |
| Surgical Guides |  Less common (low precision) Less common (low precision) |  Preferred (photopolymers) Preferred (photopolymers) |  Used (metal guides) Used (metal guides) |  Less precise Less precise |  Emerging Emerging |
| Reconstructive Titanium Implants |  Not applicable Not applicable |  Not applicable Not applicable |  Standard clinical choice Standard clinical choice |  Not applicable Not applicable |  Not applicable Not applicable |
| Patient-Specific PEEK Implants |  Not applicable Not applicable |  Not applicable Not applicable |  Experimental via LS Experimental via LS |  Not applicable Not applicable |  Preferred Preferred |
| Bioceramic Scaffolds (research) |  Experimental Experimental |  Experimental Experimental |  Experimental Experimental |  Used in trials Used in trials |  Not applicable Not applicable |
 —Recommended/widely used —Recommended/widely used —Limited or experimental use —Limited or experimental use —Not suitable or unsupported —Not suitable or unsupported | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turek, P.; Zaborniak, M.; Grzywacz-Danielewicz, K.; Bałuszyński, M.; Lewandowski, B.; Kluczyński, J.; Daniel, N. A Review of the Most Commonly Used Additive Manufacturing Techniques for Improving Mandibular Resection and Reconstruction Procedures. Appl. Sci. 2025, 15, 9228. https://doi.org/10.3390/app15179228
Turek P, Zaborniak M, Grzywacz-Danielewicz K, Bałuszyński M, Lewandowski B, Kluczyński J, Daniel N. A Review of the Most Commonly Used Additive Manufacturing Techniques for Improving Mandibular Resection and Reconstruction Procedures. Applied Sciences. 2025; 15(17):9228. https://doi.org/10.3390/app15179228
Chicago/Turabian StyleTurek, Paweł, Małgorzata Zaborniak, Katarzyna Grzywacz-Danielewicz, Michał Bałuszyński, Bogumił Lewandowski, Janusz Kluczyński, and Natalia Daniel. 2025. "A Review of the Most Commonly Used Additive Manufacturing Techniques for Improving Mandibular Resection and Reconstruction Procedures" Applied Sciences 15, no. 17: 9228. https://doi.org/10.3390/app15179228
APA StyleTurek, P., Zaborniak, M., Grzywacz-Danielewicz, K., Bałuszyński, M., Lewandowski, B., Kluczyński, J., & Daniel, N. (2025). A Review of the Most Commonly Used Additive Manufacturing Techniques for Improving Mandibular Resection and Reconstruction Procedures. Applied Sciences, 15(17), 9228. https://doi.org/10.3390/app15179228









